| Research Article | ||
Open Vet. J.. 2024; 14(10): 2618-2627 Open Veterinary Journal, (2024), Vol. 14(10): 2618–2627 Research Article Comparative efficacy of eugenol, eugenol nanoemulsion, and metronidazole against Trichomonas gallinae: An experimental studyAbdollah Khaki1, Mohamad Reza Youssefi2* and Nadia Taiefi Nasrabadi11Department of Veterinary Parasitology, Karaj Branch, Islamic Azad University, Karaj, Iran 2Department of Veterinary Parasitology, Babol Branch, Islamic Azad University, Babol, Iran *Corresponding Author: Mohamad Reza Youssefi. Department of Veterinary Parasitology, Babol Branch, Islamic Azad University, Babol, Iran. Email: youssefi929 [at] hotmail.com Submitted: 03/07/2024 Accepted: 29/09/2024 Published: 31/10/2024 © 2024 Open Veterinary Journal
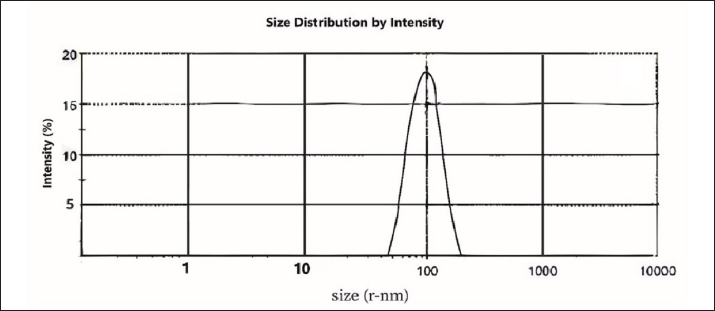
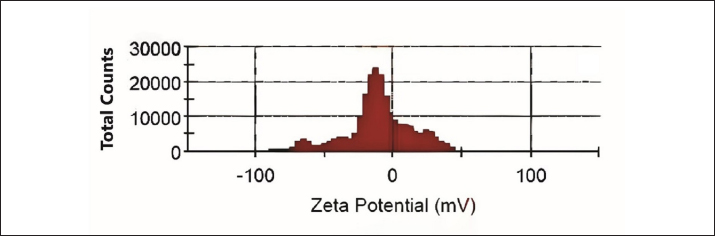
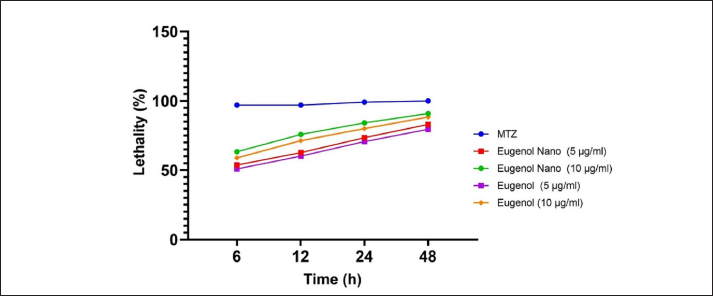
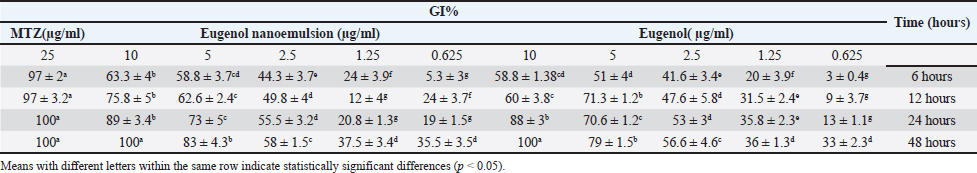
AbstractBackground: Trichomonas gallinae is a protozoan parasite responsible for canker in pigeons, a debilitating disease that causes significant economic losses. While metronidazole (MTZ) remains the primary treatment, the emergence of resistance is a growing concern. This study investigated the efficacy of eugenol and its nanoemulsion formulation against T. gallinae in both in vitro and in vivo settings. Aim: To evaluate the anti-trichomonal activity of eugenol, eugenol nanoemulsion, and MTZ against T. gallinae using in vitro and in vivo models. Methods: In vitro, T. gallinae trophozoites were exposed to varying concentrations of eugenol and eugenol nanoemulsion (0.625–10 μg/ml), as well as MTZ (25 μg/ml). Cytotoxicity was assessed using Vero cells. In vivo, 120 pigeons were experimentally infected and treated with either eugenol (10 mg/kg), eugenol nanoemulsion (10 mg/kg), MTZ (25 mg/kg), or left untreated. Treatments were administered daily for 5 days. Results: In vitro, both eugenol and its nanoemulsion at 10 μg/ml achieved 100% lethality of T. gallinae after 48 hours, while MTZ reached the same effect within 24 hours. In vivo, MTZ and eugenol (at 25 mg/kg and 10 mg/kg, respectively) resulted in 100% recovery of infected pigeons 5 days post-treatment. Notably, eugenol nanoemulsion (10 mg/kg) achieved 100% recovery within just 4 days post-treatment. Conclusion: This study highlights the potential of eugenol and its nanoemulsion as alternative treatments for T. gallinae infections in pigeons. The eugenol nanoemulsion, in particular, demonstrated promising results with faster recovery rates compared to both MTZ and eugenol, suggesting it may be especially effective against MTZ-resistant strains. Further research is warranted to explore the efficacy and safety of these agents for treating T. gallinae infections in pigeons. Keywords: Eugenol nanoemulsion, Integrative medicine, Medicinal herbs, Metronidazole, Trichomonas gallinae. IntroductionAvian trichomoniasis, caused by the protozoan parasite Trichomonas gallinae, poses a significant threat to bird populations worldwide. This parasite, belonging to the order Trichomonadida, primarily infects the digestive tract and, in some cases, the respiratory tract of various bird species (Amin et al., 2014; Rouffaer et al., 2014). The resulting lesions can range from subclinical to severe, often leading to starvation, esophageal obstruction, and ultimately death (Gerhold et al., 2008). Trichomoniasis is particularly detrimental to breeding birds, significantly increasing their mortality rates (Robinson et al., 2010; Lawson et al., 2011). In fact, it is considered a major factor contributing to the population decline of pigeons (Stockdale et al., 2015). Although nitroimidazoles, such as metronidazole (MTZ), have been the primary treatment for trichomoniasis, their effectiveness is increasingly compromised by the emergence of drug-resistant strains of T. gallinae (Dingsdag & Hunter, 2018; Gómez-Muñoz, Gómez-Molinero, Gonzalez, et al., 2022). The inappropriate prescription and prophylactic use of these drugs have exacerbated this issue. Additionally, concerns over MTZ's potential carcinogenicity (Bendesky et al., 2002) have intensified the search for safer and more effective alternatives. Given the growing challenges associated with nitroimidazole use, it is imperative to explore novel therapeutic options that can address resistance and ensure the long-term management of avian trichomoniasis. In recent years, there has been a growing interest in using plant-derived essential oils as therapeutic agents against parasites. Researchers have increasingly focused on discovering novel, naturally-derived anti-trichomonal agents (Khater et al., 2009; Khater et al., 2011; Seddiek et al., 2011; Khater, 2012, 2013; Seddiek et al., 2013). Among the promising findings, studies investigating the efficacy of Artemisia sieberi against Trichomonas parasites have shown particularly encouraging results (Youssefi et al., 2017). This expanding body of research underscores the urgent need to develop new, safe, and effective treatments for avian trichomoniasis. Exploring nature-based solutions offers significant potential for protecting bird populations from this devastating disease. Although numerous studies have investigated the antitrichomonal potential of essential oils and plant extracts, their commercial availability as therapeutic agents remains limited. This gap can be largely attributed to the inherent variability in the chemical composition of even taxonomically similar plants. Factors such as the specific plant part used, harvest time, geographical origin, and environmental conditions can significantly influence the phytochemical profile, leading to inconsistencies in the final product (Khalid et al., 2020). This variability poses a significant challenge in developing standardized and reliable plant-based treatments for trichomoniasis. One promising approach to overcome this challenge is to isolate and characterize specific bioactive compounds from plants, such as eugenol. Eugenol (C10H12O2), a phenolic compound found predominantly in cloves (Mohammadi Nejad et al., 2017), is a transparent to pale yellow liquid with a wide range of documented biological activities, including acaricidal, bactericidal, fungicidal, nematocidal, and insecticidal properties (Barboza et al., 2018; Nisar et al., 2021). However, the lipophilic nature of essential oils, including eugenol, makes them susceptible to degradation by environmental factors such as light and heat, potentially impacting their efficacy. Nanoemulsion technology offers a potential solution by encapsulating essential oils within nanoparticles, thereby protecting them from degradation, enhancing their water solubility, masking undesirable tastes, and improving bioavailability (Barradas & de Holanda e Silva, 2021). This study aimed to evaluate the efficacy of eugenol and a eugenol nanoemulsion against T. gallinae under both in vitro and in vivo conditions, comparing their performance to the current standard treatment, MTZ. Additionally, the cytotoxicity of these compounds was assessed using Vero cell lines. By exploring the potential of eugenol and its nanoemulsion formulation, this research contributes to the development of safe and effective plant-based alternatives for combating avian trichomoniasis. Material and MethodsChemicalEugenol was obtained from Sigma-Aldrich (Germany). Sorbitan monolaurate (Span 80TM) and Poly sorbitan monooleate (Tween 80TM) were purchased from Merck (Germany). MTZ was used as a standard anti-trichomonas compound. Development of nanoemulsionA mixed phase method of oil and water was applied using ultrasonic waves to achieve uniform dispersion of scattered stage particles in a nano size within the continuous stage. Therefore, poly sorbitan monooleate and sorbitan monolaurate were used as surfactants. Eugenol levels of 1,000 µl/ml, water, and a mixture of 5 wt% surfactants underwent blending. Afterward, the prepared nanoemulsion was processed using an ultrasonic processor (UP400S, Dr Haschler, Germany) operating at the ultrasonic cycle of 208 w/cm2, maximum power of 400 w, and frequency of 20 KHz, and ultrasonic time of 300 seconds. Then, dynamic light scattering (zetasizer nano series, ZEN 3,600, UK) determined the nanoemulsions droplet size distribution and average size. Measurement was done in triplicate. The obtained data were then analyzed using Zetasizer software (version 7.13). ParasitesTrichomonas gallinae was isolated from infected pigeons (8 weeks old) using the wet mount technique. Swab samples were collected from the crop of the infected birds. These swabs were gently rubbed on the surface of microscopic slides to create wet smears. Subsequently, light microscopy was employed to examine the smears for the presence of T. gallinae. For parasite culture, oral swabs were immersed in a tryptone/yeast extract/maltose medium at pH 7 supplemented with 10% fetal calf serum, followed by incubation at 37°C (Sansano-Maestre et al., 2009). The isolates were sub-cultured every 48 hours as described by Seddiek et al. (2014). In vitro analysisTo assess the sensitivity of T. gallinae to eugenol, eugenol nanoemulsion, and MTZ, sterile multi-well plates were employed for incubating T. gallinae trophozoites with varying concentrations of the treatment compounds. Each well was filled with 100 μl of culture medium containing 1 × 104 parasites. Prediluted eugenol and eugenol nanoemulsion were added to the wells to achieve concentrations of 0.625, 1.25, 2.5, 5, and 10 μg/ml. In the MTZ group, MTZ was added to the plate wells at a dose of 25 μg/ml. The control group consisted of 100 μl of culture medium with T. gallinae, without any treatment. The experiments were repeated six times. The treated plate wells were examined using a microscope at 6, 12, 24, and 48 hours. The effective dose for experimental studies was defined as the lowest concentration of the treatment compounds at which no live trophozoites of T. gallinae were observed. To distinguish between viable and non-viable trophozoites, an equal amount of trypan blue (Sigma Chemical Co.) 0.40% was supplemented to the samples. Also, 50% inhibitory concentration (IC50) was determined by GraphPad prism 9 software. The growth inhibition percentage (GI%) was calculated using the following equation: GI%=(A-B)/A × 100 where (A) represents the mean number of T. gallinae in the control group. (B) represents the mean number of T. gallinae in the treated groups (Seddiek et al., 2014). It is important to note that the term “mean number” refers to the viable trophozoites present in the respective groups. The growth inhibition percentage provides insights into the effectiveness of the tested compounds against T. gallinae, indicating either growth inhibition or lethality. In vivo analysisThe in vivo study was conducted following the laboratory animal welfare guidelines of the Pasteur Institute, Iran, as approved by the institutional animal ethics committee. A total of 120 pigeons, aged 8 weeks and initially free of T. gallinae, were selected. These pigeons were experimentally infected via oral inoculation with a 48-hour culture medium containing 4 × 104 T. gallinae trophozoites per milliliter. After 7 days of infection, confirmation of T. gallinae presence was achieved using wet mount preparations and microscopy. Subsequently, the pigeons were randomly allocated into distinct treatment groups (with 30 pigeons per group), each housed separately in individual cages. Manual administration of water and food was provided. Specifically: The eugenol group and eugenol nanoemulsion group received separate treatments with eugenol and eugenol nanoemulsion at a dose of 10 mg/kg. The MTZ group was treated with MTZ at a dose of 25 mg/kg. The control group consisted of infected pigeons that did not receive any medication. Oral treatment was administered once daily for 5 consecutive days. Parasite motility was assessed daily after completing the treatment (prior to initiating the subsequent treatment cycle) using a light microscope. Clinical effects and mortality were monitored throughout the treatment period. Cytotoxicity assayThe cytotoxicity evaluation employed the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay to investigate alterations in mitochondrial and non-mitochondrial dehydrogenase activity. Briefly, Vero cells (at a density of 5 × 103 cells/ml) were seeded onto 96-well plates and cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS) at 37°C with 5% CO2 for 24 hours. Subsequently, various concentrations (ranging from 0.625 to 10 μg/ml) of eugenol and eugenol nanoemulsion were added to the wells. Exposure periods of 12, 24, and 72 hours were selected to assess in vitro cytotoxicity. Following the incubation period, the supernatant was aspirated, and an MTT solution (0.5 mg/ml) was added to each well 30 minutes before the experiment’s conclusion. Water-insoluble dark blue formazan crystals, indicative of viable cells, formed during this process. These crystals were solubilized in dimethyl sulfoxide, and the absorbance was measured at 570 nm using a microplate reader (Biotek μQuant). Cell survival was determined by comparing the absorbance values obtained from treated cells to those of untreated cells. The 50% cytotoxic concentration (CC50) determined using GraphPad prism (version 9) software. Statistical analysisThe collected data underwent ANOVA (analysis of variance) using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). The mean values, calculated from six replicates per treatment, were presented in tables and figures, along with the standard error (SE). Tukey's test was utilized to assess the differences between means (p ≤ 0.05). Ethical approvalThe authors affirm that the current study did not involve human subjects, and all animal experiments adhered to the ARRIVE guidelines and the highest ethical standards established by internationally recognized organizations. Specifically, we ensured that the care and use of animals followed the principles outlined in the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health in the United States. Additionally, all animal-related procedures and protocols underwent review and approval by the relevant institutional animal ethics committee or review board, ensuring compliance with local regulations and guidelines. ResultsParticle size and zeta potential of eugenol nanoemulsionThe eugenol nanoemulsion was characterized by analyzing its particle size and zeta potential. Dynamic light scattering (DLS) measurements revealed a mean particle size of 97.31 nm (Fig. 1), confirming the nanoscale nature of the emulsion. Zeta potential analysis indicated a surface charge of −23.9 mV (Fig. 2), suggesting good colloidal stability. In vitro anti-trichomonal activityThe anti-trichomonal activity of eugenol and eugenol nanoemulsion against T. gallinae was evaluated in vitro. Both eugenol and its nanoemulsion formulation effectively eliminated viable T. gallinae at specific concentrations. While MTZ achieved complete eradication of trophozoites within 24 hours, eugenol and eugenol nanoemulsion required 48 hours to achieve the same outcome. Significant differences in growth inhibition percentage (GI%) were observed between the treatment groups and the control group (Table 1). Notably, the minimum inhibitory concentration (MIC) for both eugenol and eugenol nanoemulsion was determined to be 10 µl/ml. Our experimental investigation demonstrated the efficacy of these antimicrobial agents against T. gallinae. MTZ exhibited escalating lethality over time, achieving near-complete eradication of viable trophozoites after 48 hours of exposure. Both concentrations of eugenol nanoemulsion (10 nano eug and 5 nano eug) demonstrated increasing lethality, with the higher concentration being more effective. In contrast, the lethality percentages of pure eugenol (10 eug and 5 eug) were lower than their corresponding nanoemulsion formulations (Fig. 3). These findings highlight dose-dependent responses and underscore the potential of eugenol-based treatments, particularly in nanoemulsion form, for managing T. gallinae infections.
Fig. 1. Size distribution of eugenol nanoemulsion determined by DLS. The graph depicts the droplet size distribution of eugenol nanoemulsion, as measured using DLS (Zetasizer Nano Series, ZEN 3,600, UK). The average particle size within the nanoemulsion was determined to be 97.31 nm.
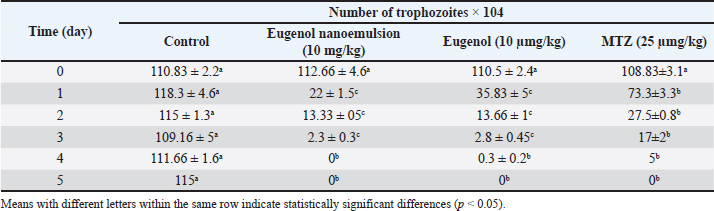
Fig. 2. Zeta potential distribution of eugenol nanoemulsion. The graph illustrates the distribution of zeta potential values for eugenol nanoemulsion, as determined using Zetasizer software (version 7.13). The peak surface charge is observed at –23.9 mV, indicating a negative surface charge for the eugenol nanoemulsion. In vivo findingsThe in vivo efficacy of eugenol, eugenol nanoemulsion, and MTZ against Trichomonas gallinae infection in pigeons was evaluated (Table 2). Initially, no significant differences in T. gallinae trophozoite counts were observed among the treatment groups. Following treatment initiation, a significant reduction in trophozoite counts was observed on day 1 in groups treated with eugenol (10 mg/kg), eugenol nanoemulsion (10 mg/kg), and MTZ (25 mg/kg) compared to the control group (p < 0.05). This reduction persisted on days 2 and 3 in both the eugenol and eugenol nanoemulsion groups (p < 0.05). Notably, by day 4, no motile T. gallinae were detected in pigeons treated with eugenol nanoemulsion. Furthermore, by day 5, motile T. gallinae were absent in both the eugenol and MTZ-treated groups. No mortalities were observed in either the treatment or control groups throughout the study period. Table 1. In vivo anti-trichomonal activity of MTZ, Eugenol and eugenol nanoemulsion against T. gallinae.
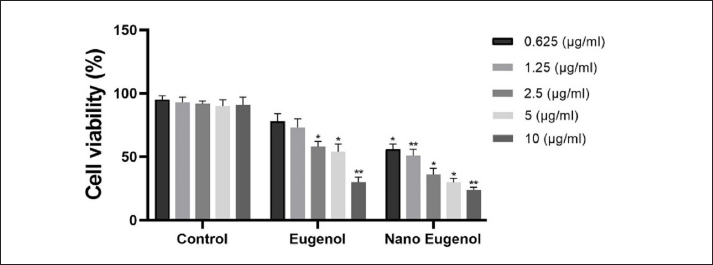
MTT assayThe cytotoxicity of eugenol and eugenol nanoemulsion on Vero cells was assessed using a range of concentrations and incubation times. At lower concentrations (0.625 and 1.25 µg/ml), both treatments exhibited minimal cytotoxicity, with cell viability comparable to the control group. However, at higher concentrations (5 and 10 µg/ml), a significant decrease in cell viability was observed for both eugenol and eugenol nanoemulsion, with eugenol demonstrating a more pronounced cytotoxic effect (Fig. 4). To further evaluate the therapeutic potential of these compounds, selectivity indices (SI=CC50/IC50) were calculated. The results indicated that eugenol nanoemulsion exhibited a higher SI compared to eugenol (Table 3), suggesting a wider therapeutic window for the nanoemulsion formulation. DiscussionNitroimidazole drugs, such as dimetridazole, MTZ, carnidazole, and ronidazole, have been widely employed for treating T. gallinae infections (Gómez-Muñoz, Gómez-Molinero, González, et al., 2022). However, prolonged use of preventive therapies based on nitroimidazoles can lead to the emergence of resistant isolates (Tabari et al., 2017; Santos et al., 2020). This challenge underscores the growing need for complementary and alternative medicine (CAM) approaches in veterinary practice. In the quest for alternative treatments, medicinal plants have gained attention due to their potential therapeutic properties (Hashemi et al., 2021). The use of CAM, including herbal remedies, is driven by a desire to enhance overall health outcomes in animals and address potential side effects of conventional treatments. Evidence suggests that certain herbal products, like Malva sylvestris, can be effective in managing specific conditions such as atopic dermatitis in pediatric patients (Meysami et al., 2021), highlighting the potential benefits of plant-derived therapies. Moreover, the antidepressant potential of essential oils from plants like Satureja khuzestanica has been demonstrated in animal models (Seyedi, Abbasi-Maleki, & Najafi, 2023), further illustrating the diverse therapeutic applications of CAM. Eugenol, a compound of plant origin, is naturally present in essential oils and extracts from various plants, including cloves, basil, and cinnamon (Graves et al., 2019; Lin et al., 2020), and represents a promising avenue for exploring novel treatments for T. gallinae infections. This in vitro study investigated and compared the antiparasitic efficacy of eugenol, eugenol nanoemulsion, and the standard drug MTZ against the avian protozoan parasite T. gallinae. Serial dilutions of eugenol and eugenol nanoemulsion (10, 5, 2.5, 1.25, and 0.62 μg/ml) were evaluated alongside MTZ and untreated controls under controlled laboratory conditions. At lower concentrations (1.25 and 0.62 μg/ml), both eugenol and its nanoemulsion formulation exhibited limited antiparasitic activity against T. gallinae trophozoites over the 48-hour observation period. In contrast, MTZ demonstrated rapid and potent efficacy, achieving 100% parasite mortality within 24 hours at these lower concentrations. However, at the higher concentration of 10 μg/ml, both eugenol and eugenol nanoemulsion achieved complete (100%) elimination of T. gallinae trophozoites, although this required a longer duration of 48 hours to match the rapid parasiticidal effect of MTZ at the same concentration. The enhanced solubility and dispersibility of eugenol in the nanoemulsion format did not significantly improve its antiparasitic activity compared to pure eugenol at the tested concentrations. This suggests that the inherent antiparasitic properties of eugenol are the primary driver of its efficacy against T. gallinae, rather than improvements in its physicochemical properties through nanoemulsion formulation (Cáceres et al., 2020). Notably, in vivo evaluation of these treatments in an infected pigeon model revealed that the eugenol nanoemulsion led to complete recovery of the infected birds after 4 days post-treatment, whereas both eugenol and MTZ groups achieved complete recovery after 5 days. This indicates the potential for the nanoemulsion format to enhance the in vivo antiparasitic efficacy of eugenol compared to the pure compound (Maurice et al., 2021).
Fig. 3. Lethality percentage in effective concentrations of eugenol and eugenol nanoemulsion, MTZ, and control groups at 6, 12, 24, and 48 hours of the treatment. Table 2. In vivo anti-trichomonal activity of MTZ, eugenol, and eugenol nanoemulsion against Trichomonas gallinae.
Eugenol, a phenylpropanoid compound prominent in clove essential oil, exhibits potent anti-parasitic activity against T. gallinae, the protozoan parasite responsible for avian trichomoniasis. Shang et al. (2021) elucidated eugenol's mechanism of action, demonstrating its inhibition of mitochondrial respiratory chain complex I activity. This inhibition occurs through binding to NADH dehydrogenase chain 2, ultimately leading to parasite mortality. This finding is consistent with previous research demonstrating the anti-trichomonal efficacy of Dennettia tripetala essential oil, which contains eugenol as a major constituent (Gbolade et al., 2009).
Fig. 4. Concentration-dependent cytotoxic effects of eugenol and eugenol nanoemulsion on vero cells. The bar graph depicts the in vitro cytotoxicity of vero cells following 48-hour exposure to varying concentrations of eugenol, eugenol nanoemulsion, and an untreated control. The asterisks (*p < 0.05, **p < 0.01) indicate the statistical significance levels of the differences between the treatment groups and the control. Table 3. Antitrichomonal activity and cytotoxic effect of eugenol and eugenol nanoemulsion on Vero cells.
The broad spectrum of biological activities attributed to clove essential oil, including its anti-parasitic, bactericidal, fungicidal, and insecticidal properties, is largely attributed to the presence of eugenol (Machado et al., 2011; El-Kady et al., 2019). Furthermore, essential oil from Artemisia sieberi, a plant rich in eugenol, has also demonstrated promising anti-trichomonal activity. Tabari et al. (2017) reported that treatment with A. sieberi essential oil resulted in faster recovery in infected pigeons compared to MTZ treatment. Notably, while MTZ achieved complete eradication of T. gallinae trophozoites at a concentration of 20 µg/ml after 24 hours of incubation, A. sieberi essential oil achieved the same outcome at a MIC of 10 µg/ml under identical conditions. Beyond clove essential oil, other natural compounds with anti-trichomonal activity have been explored. For example, Seddiek et al. (2014) reported that garlic demonstrated comparable efficacy to MTZ in suppressing T. gallinae growth both in vitro and in vivo. This study highlighted garlic's potential as a safer alternative to MTZ, considering the latter's reported side effects, including cytotoxicity, neurological dysfunction, and carcinogenicity. This study evaluated the efficacy of eugenol nanoemulsion against T. gallinae, revealing that the nanoemulsion exhibited superior efficacy compared to the control group. In vivo studies further demonstrated that eugenol nanoemulsion was more effective against T. gallinae than both pure eugenol and MTZ treatments. The enhanced efficacy of the nanoemulsion may be attributed to its improved ability to penetrate the protozoan membrane, facilitating increased interaction with cellular targets. As noted by Nair et al. (2016), nanotechnology can enhance the therapeutic effects of compounds by improving their solubility, bioavailability, and protecting the active ingredient from degradation. Therefore, the enhanced anti-trichomonal properties of eugenol nanoemulsion likely result from its increased bioavailability and stability provided by the nanoformulation (Esmaeili et al., 2016). Additionally, this study assessed the in vitro cytotoxicity of both eugenol and eugenol nanoemulsion on Vero cells, suggesting that eugenol nanoemulsion may serve as a more effective and safer therapeutic agent against T. gallinae. Our findings indicate that eugenol nanoemulsion has a broader therapeutic window compared to pure eugenol, underscoring its potential for improved efficacy with reduced side effects. While previous research has explored the antimicrobial properties of eugenol—a natural compound derived from clove oil—against various microorganisms, including T. gallinae (Machado et al., 2011; Karami et al., 2023), the cytotoxicity and therapeutic potential of eugenol have been less extensively studied. Our results are consistent with earlier findings that eugenol exhibits antimicrobial activity, as evidenced by a significant decrease in cell viability at higher concentrations. However, our study uniquely highlights the impact of eugenol nanoemulsion, a novel formulation designed to enhance therapeutic efficacy while minimizing the toxicity associated with pure eugenol. The observed higher selectivity index of eugenol nanoemulsion compared to pure eugenol suggests that the nanoemulsion formulation effectively reduces eugenol’s cytotoxic effects, potentially through alterations in its pharmacokinetic profile. This finding aligns with emerging research on the potential of nanotechnology to improve drug delivery and therapeutic outcomes (Ali et al., 2017). While this study provides valuable insights into the comparative efficacy and safety profiles of eugenol, eugenol nanoemulsion, and MTZ against T. gallinae, it is essential to acknowledge its limitations and implications for the broader field. One key limitation is the reliance on in vitro cell culture models, which may not fully recapitulate the complex in vivo interactions and pharmacokinetic properties of the tested compounds. Although the in vivo evaluation in an infected pigeon model offers more clinically relevant insights, the sample size and experimental conditions were relatively limited. Future in vivo studies with larger cohorts and a more comprehensive assessment of parameters, such as tissue distribution, metabolism, and long-term safety, are warranted to validate these findings and better understand the therapeutic potential of these compounds. The implications of this research extend beyond the immediate application in treating avian trichomoniasis. The emergence of drug-resistant T. gallinae isolates, as highlighted in the literature, underscores the urgent need for alternative therapeutic approaches to manage this persistent and challenging protozoal infection. Eugenol-based treatments, particularly eugenol nanoemulsion, hold promise as effective and safer alternatives to conventional nitroimidazole drugs, representing a significant advancement in the field of antiprotozoal drug development. Furthermore, the insights gained from this study may have broader relevance for exploring natural compounds and nanoformulations as therapeutic agents against other protozoal infections. ConclusionIn conclusion, our research sheds light on the comparative efficacy of eugenol and eugenol nanoemulsion against T. gallinae. While both forms demonstrated concentration-dependent effects, their effectiveness fell short of that observed with MTZ. Notably, a dose of 10 μg/ml of either eugenol or its nanoemulsion form resulted in complete trophozoite death after 48 hours, whereas MTZ achieved the same outcome within 24 hours. Furthermore, in an in vivo context, eugenol nanoemulsion led to faster recovery in infected pigeons compared to MTZ treatment. These findings underscore the potential of eugenol-based treatments as alternatives for managing trichomoniasis. Future studies should explore optimal dosages and mechanisms of action to enhance clinical applications. AcknowledgmentsThe authors express their gratitude to the staff of the Islamic Azad University (IAU) laboratory at Babol Branch (Iran) for their valuable cooperation during this study. Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. FundingThis research received no specific grant. Authors’ contributionsConceptualization: Mohamad Reza Youssefi; Methodology: Abdollah Khaki; Formal analysis and investigation: NadiaTaeifi Nasrabadi; Writing: original draft preparation: Abdollah khaki; Writing: review and editing: Mohamad Reza Youssefi; Funding acquisition: Abdollah khaki; Resources: NadiaTaeifi Nasrabadi; Supervision: Mohamad Reza Youssefi. Data availabilityThe data presented in this study are available on request from the corresponding author. ReferencesAli, A., Ansari, V.A., Ahmad, U., Akhtar, J. and Jahan, A. 2017. Nanoemulsion: an advanced vehicle for efficient drug delivery. Drug. Res. (Stuttg) 67(11), 617–631. Amin, A., Bilic, I., Liebhart, D. and Hess, M. 2014. Trichomonads in birds--a review. Parasitology 141(6), 733–747. Barboza, J.N., da Silva Maia Bezerra Filho, C., Silva, R.O., Medeiros, J.V.R. and de Sousa, D.P. 2018. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid. Med. Cell. Longev. 2018(1), 3957262. Barradas, T.N. and de Holanda e Silva, K.G. 2021. Nanoemulsions of essential oils to improve solubility, stability and permeability: a review. Environ. Chem. Lett. 19(2), 1153–1171. Bendesky, A., Menéndez, D. and Ostrosky-Wegman, P. 2002. Is metronidazole carcinogenic? Mutat. Res. 511(2), 133–144. Cáceres, M., Guzmán, E., Alvarez-Costa, A., Ortega, F., G. Rubio, R., Coviella, C., Santo Orihuela, P.L., Vassena, C.V. and Lucia, A. 2020. Surfactantless emulsions containing eugenol for imidacloprid solubilization: physicochemical characterization and toxicity against insecticide-resistant cimex lectularius. Molecules 25(10), 2290. Dingsdag, S.A. and Hunter, N. 2018. Metronidazole: an update on metabolism, structure-cytotoxicity and resistance mechanisms. J. Antimicrob. Chemother. 73(2), 265–279. El-Kady, A.M., Ahmad, A.A., Hassan, T.M., El-Deek, H.E.M., Fouad, S.S. and Althagfan, S.S. 2019. Eugenol, a potential schistosomicidal agent with anti-inflammatory and antifibrotic effects against Schistosoma mansoni, induced liver pathology. Infect. Drug. Resist. 12, 709–719. Esmaeili, F., Rajabnejhad, S., Partoazar, A.R., Mehr, S.E., Faridi-Majidi, R., Sahebgharani, M., Syedmoradi, L., Rajabnejhad, M.R. and Amani, A. 2016. Anti-inflammatory effects of eugenol nanoemulsion as a topical delivery system. Pharma. Dev. Tech. 21(7), 887–893. Gbolade, A.A., Arcoraci, T., D'Arrigo, M., Olorunmola, F.O., Biondi, D.M. and Ruberto, G. 2009. Essential oils of Dennettia tripetala Bak. f. stem bark and leaf. Constituents and biological activities. Planta Med. 75(09), PI32. Gerhold, R.W., Yabsley, M.J., Smith, A.J., Ostergaard, E., Mannan, W., Cann, J.D. and Fischer, J.R. 2008. Molecular characterization of the Trichomonas gallinae morphologic complex in the United States. J. Parasitol. 94(6), 1335–1341. Gómez-Muñoz, M.T., Gómez-Molinero, M.Á., Gonzalez, F., Azami-Conesa, I., Bailen, M., Garcia Piqueras, M. and Sansano-Maestre, J. 2022. Avian oropharyngeal trichomonosis: treatment, failures and alternatives, a systematic review. Microorganisms 10(11), 2297. Gómez-Muñoz, M.T., Gómez-Molinero, M.Á., González, F., Azami-Conesa, I., Bailén, M., García Piqueras, M. and Sansano-Maestre, J. 2022. Avian oropharyngeal trichomonosis: treatment, failures and alternatives, a systematic review. Microorganisms 10(11), 2297. Graves, K.J., Ghosh, A.P., Schmidt, N., Augostini, P., Secor, W.E., Schwebke, J.R., Martin, D.H., Kissinger, P.J. and Muzny, C.A. 2019. Trichomonas vaginalis virus among women with trichomoniasis and associations with demographics, clinical outcomes, and metronidazole resistance. Clin. Infect. Dis. 69(12), 2170–2176. Hashemi, N., Ommi, D., Kheyri, P., Khamesipour, F., Setzer, W.N. and Benchimol, M. 2021. A review study on the anti-trichomonas activities of medicinal plants. Int. J. Parasitol. Drugs. Drug. Resist. 15, 92–104. Karami, F., Dastan, D., Fallah, M. and Matini, M. 2023. In vitro antitrichomonal activity of Satureja khuzestanica and main essential oil components carvacrol, thymol, and eugenol. J. Infect. Dev. Ctries. 17(1), 80–85. Khalid, K.A., Essa, E.F., Ismaiel, H.M.H. and Elsayed, A.A.A. 2020. Effects of geographical locations on essential oil composition of navel orange leaves and flowers. J. Essent. Oil-Bear. Plants. 23(1), 139–148. Khater, H.F. 2012. Ecosmart biorational insecticides: alternative insect control strategies. Insecticides-advances in integrated pest management, Egypt, pp: 17–60. Khater, H.F. 2013. Bioactivity of essential oils as green biopesticides: recent global scenario. Recent Prog. Med. Plants. 37, 151–218. Khater, H.F., Hanafy, A., Abdel-Mageed, A.D., Ramadan, M.Y. and El-Madawy, R.S. 2011. Control of the myiasis-producing fly, Lucilia sericata, with Egyptian essential oils. Int. J. Dermatol. 50(2), 187–194. Khater, H.F., Ramadan, M.Y. and El-Madawy, R.S. 2009. Lousicidal, ovicidal and repellent efficacy of some essential oils against lice and flies infesting water buffaloes in Egypt. Vet. Parasit. 164(2-4), 257–266. Lawson, B., Cunningham, A.A., Chantrey, J., Hughes, L.A., John, S.K., Bunbury, N., Bell, D.J. and Tyler, K.M. 2011. A clonal strain of Trichomonas gallinae is the aetiologic agent of an emerging avian epidemic disease. Infect Genet. Evol. 11(7), 1638–1645. Lin, H.C., Chu, L.J., Huang, P.J., Cheng, W.H., Zheng, Y.H., Huang, C.Y., Hong, S.W., Chen, L.C., Lin, H.A., Wang, J.Y., Chen, R.M., Lin, W.N., Tang, P. and Huang, K.Y. 2020. Proteomic signatures of metronidazole-resistant Trichomonas vaginalis reveal novel proteins associated with drug resistance. Parasit Vectors 13(1), 274. Machado, M., Dinis, A.M., Salgueiro, L., Custódio, J.B., Cavaleiro, C. and Sousa, M.C. 2011. Anti-Giardia activity of Syzygium aromaticum essential oil and eugenol: effects on growth, viability, adherence and ultrastructure. Exp. Parasitol. 127(4), 732–739. Maurice, M.N., Huseein, E.A.M., Monib, M.E.-S.M.M., Alsharif, F.M., Namazi, N.I. and Ahmad, A.A. 2021. Evaluation of the scolicidal activities of eugenol essential oil and its nanoemulsion against protoscoleces of hydatid cysts. PLoS One 16(11), e0259290. Meysami, M., Hashempur, M.H., Kamalinejad, M. and Emtiazy, M. 2021. Efficacy of short term topical Malva Sylvestris L. Cream in pediatric patients with atopic dermatitis: a randomized double-blind placebo-controlled clinical trial. Endocr. Metab. Immune Disord. Drug Targets 21(9), 1673–1678. Mohammadi Nejad, S., Özgüneş, H. and Başaran, N. 2017. Pharmacological and toxicological properties of Eugenol. Turk. J. Pharm. Sci. 14(2), 201–206. Nair, M., Jayant, R.D., Kaushik, A. and Sagar, V. 2016. Getting into the brain: potential of nanotechnology in the management of NeuroAIDS. Adv. Drug Deliv. Rev. 103, 202–217. Nisar, M.F., Khadim, M., Rafiq, M., Chen, J., Yang, Y. and Wan, C.C. 2021. Pharmacological properties and health benefits of Eugenol: a comprehensive review. Oxid. Med. Cell. Longev. 2021(1), 2497354. Robinson, R.A., Lawson, B., Toms, M.P., Peck, K.M., Kirkwood, J.K., Chantrey, J., Clatworthy, I.R., Evans, A.D., Hughes, L.A., Hutchinson, O.C., John, S.K., Pennycott, T.W., Perkins, M.W., Rowley, P.S., Simpson, V.R., Tyler, K.M. and Cunningham, A.A. 2010. Emerging infectious disease leads to rapid population declines of common British birds. PLoS One 5(8), e12215. Rouffaer, L.O., Adriaensen, C., De Boeck, C., Claerebout, E. and Martel, A. 2014. Racing pigeons: a reservoir for nitro-imidazole-resistant Trichomonas gallinae. J. Parasitol. 100(3), 360–363. Sansano-Maestre, J., Garijo-Toledo, M.M. and Gómez-Muñoz, M.T. 2009. Prevalence and genotyping of Trichomonas gallinae in pigeons and birds of prey. Avian Pathol. 38(3), 201–207. Santos, H.M., Tsai, C.Y., Catulin, G.E.M., Trangia, K.C.G., Tayo, L.L., Liu, H.J. and Chuang, K.P. 2020. Common bacterial, viral, and parasitic diseases in pigeons (Columba livia): a review of diagnostic and treatment strategies. Vet. Microbiol. 247, 108779. Seddiek, S.A., Ali, M.M., Khater, H.F. and El-Shorbagy, M.M. 2011. Anthelmintic activity of the white wormwood, Artemisia herba-alba against Heterakis gallinarum infecting turkey poults. J. Med. Plants. Res. 5(16), 3946–3957. Seddiek, S.A., Khater, H.F., El-Shorbagy, M.M. and Ali, A.M. 2013. The acaricidal efficacy of aqueous neem extract and ivermectin against Sarcoptes scabiei var. cuniculi in experimentally infested rabbits. Parasitol. Res. 112(6), 2319–2330. Seddiek Sh, A., El-Shorbagy, M.M., Khater, H.F. and Ali, A.M. 2014. The antitrichomonal efficacy of garlic and metronidazole against Trichomonas gallinae infecting domestic pigeons. Parasitol. Res. 113(4), 1319–1329. Seyedi, S.S., Abbasi-Maleki, S. and Najafi, G. 2023. Phytochemical properties and antidepressant potential of Satureja khuzestanica Jamzad essential oil in mouse models of depression. Trad. Integr. Med. 8(4), 340–346. Shang, X.F., Dai, L.X., Yang, C.J., Guo, X., Liu, Y.Q., Miao, X.L. and Zhang, J.Y. 2021. A value-added application of eugenol as acaricidal agent: the mechanism of action and the safety evaluation. J. Adv. Res. 34, 149–158. Stockdale, J.E., Dunn, J.C., Goodman, S.J., Morris, A.J., Sheehan, D.K., Grice, P.V. and Hamer, K.C. 2015. The protozoan parasite Trichomonas gallinae causes adult and nestling mortality in a declining population of European Turtle Doves, Streptopelia turtur. Parasitology 142(3), 490–498. Tabari, M.A., Youssefi, M.R. and Moghadamnia, A.A. 2017. Antitrichomonal activity of Peganum harmala alkaloid extract against trichomoniasis in pigeon (Columba livia domestica). Br. Poult. Sci. 58(3), 236–241. Youssefi, M.R., Abouhosseini Tabari, M. and Moghadamnia, A.A. 2017. In vitro and in vivo activity of Artemisia sieberi against Trichomonas gallinae. Iran. J. Vet. Res. 18(1), 25–29. | ||
| How to Cite this Article |
| Pubmed Style Khaki A, Youssefi MR, Nasrabadi NT. Comparative efficacy of eugenol, eugenol nanoemulsion and metronidazole against Trichomonas gallinae: An experimental study. Open Vet. J.. 2024; 14(10): 2618-2627. doi:10.5455/OVJ.2024.v14.i10.11 Web Style Khaki A, Youssefi MR, Nasrabadi NT. Comparative efficacy of eugenol, eugenol nanoemulsion and metronidazole against Trichomonas gallinae: An experimental study. https://www.openveterinaryjournal.com/?mno=207864 [Access: November 22, 2025]. doi:10.5455/OVJ.2024.v14.i10.11 AMA (American Medical Association) Style Khaki A, Youssefi MR, Nasrabadi NT. Comparative efficacy of eugenol, eugenol nanoemulsion and metronidazole against Trichomonas gallinae: An experimental study. Open Vet. J.. 2024; 14(10): 2618-2627. doi:10.5455/OVJ.2024.v14.i10.11 Vancouver/ICMJE Style Khaki A, Youssefi MR, Nasrabadi NT. Comparative efficacy of eugenol, eugenol nanoemulsion and metronidazole against Trichomonas gallinae: An experimental study. Open Vet. J.. (2024), [cited November 22, 2025]; 14(10): 2618-2627. doi:10.5455/OVJ.2024.v14.i10.11 Harvard Style Khaki, A., Youssefi, . M. R. & Nasrabadi, . N. T. (2024) Comparative efficacy of eugenol, eugenol nanoemulsion and metronidazole against Trichomonas gallinae: An experimental study. Open Vet. J., 14 (10), 2618-2627. doi:10.5455/OVJ.2024.v14.i10.11 Turabian Style Khaki, Abdollah, Mohamad Reza Youssefi, and Nadia Taiefi Nasrabadi. 2024. Comparative efficacy of eugenol, eugenol nanoemulsion and metronidazole against Trichomonas gallinae: An experimental study. Open Veterinary Journal, 14 (10), 2618-2627. doi:10.5455/OVJ.2024.v14.i10.11 Chicago Style Khaki, Abdollah, Mohamad Reza Youssefi, and Nadia Taiefi Nasrabadi. "Comparative efficacy of eugenol, eugenol nanoemulsion and metronidazole against Trichomonas gallinae: An experimental study." Open Veterinary Journal 14 (2024), 2618-2627. doi:10.5455/OVJ.2024.v14.i10.11 MLA (The Modern Language Association) Style Khaki, Abdollah, Mohamad Reza Youssefi, and Nadia Taiefi Nasrabadi. "Comparative efficacy of eugenol, eugenol nanoemulsion and metronidazole against Trichomonas gallinae: An experimental study." Open Veterinary Journal 14.10 (2024), 2618-2627. Print. doi:10.5455/OVJ.2024.v14.i10.11 APA (American Psychological Association) Style Khaki, A., Youssefi, . M. R. & Nasrabadi, . N. T. (2024) Comparative efficacy of eugenol, eugenol nanoemulsion and metronidazole against Trichomonas gallinae: An experimental study. Open Veterinary Journal, 14 (10), 2618-2627. doi:10.5455/OVJ.2024.v14.i10.11 |