| Research Article | ||
Open Vet. J.. 2024; 14(11): 2848-2859 Open Veterinary Journal, (2024), Vol. 14(11): 2848-2859 Research Article Pathological, hematological, and biochemical alteration in broiler chickens infected with mycotoxin in Babylon provinceHayder Abd AL-Emier Almremdhy1*, Ghusoon Abdul Kareem Neamha1, Zina Bakir Al-Hilli2 and Rafal Jalil Awadh31Department of Pathology and Poultry Diseases, College of Veterinary Medicine, Al-Qasim Green University, Babylon 51013, Iraq 2Department of Microbiology, College of Veterinary Medicine, Al-Qasim Green University, Babylon 51013, Iraq 3Directorate of Veterinary Medicine, Babylon, Iraq *Corresponding Author: Hayder Abd AL-Emier Almremdhy. Department of Pathology and Poultry Diseases, College of Veterinary Medicine, Al-Qasim Green University, Babylon, Iraq. Email: hiederalmremdhy [at] vet.uoqasim.edu.iq Submitted: 04/08/2024 Accepted: 09/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
AbstractBackground: Mycotoxins are considered one of the most important problems and threats that face poultry producers. Aim: This study was conducted to investigate the pathological, hematological, and biochemical alterations in chickens fed on mycotoxins contamination ration. Methods: 434 feed samples were collected from poultry farms operating in Babil Governorate/Iraq, where feed samples were collected over the course of 2023, and these samples were tested by direct competitive enzyme-linked immunosorbent assay to determine the level of mycotoxins. (Aflatoxin, Ochratoxin, and Trichothecin T2) in poultry feed rations, after that, the chickens that were fed on feeds contaminated with mycotoxins were carefully examined to observe clinical signs. Then, blood samples were collected from chickens fed on feeds contaminated with mycotoxins, as well as from chickens fed on uncontaminated feeds (control group). These blood samples were divided to two parts one was put in a tube contain anticoagulant Ethylenediaminetetraacetic acid (EDTA) to examination the complete blood count, while the other part was put in a gel test tube for separating the serum which used in the biochemical tests which included the total protein and total cholesterol, uric acid and the liver enzymes [Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST)]. Then, the chickens fed on mycotoxins contaminated feed were humanly sacrificed to observe the gross lesions after that, tissue samples were collected from internal organs (liver, kidneys, bursa of Fabricius, and spleen) to examine the microscopic lesions. Results: The results showed the percentage of feed samples contaminated with mycotoxins above the limit of quantification values were 22% (94), whereas the percentage of feed samples contaminated with aflatoxin, ochratoxin, and trechothesen T2 was 12% (54), 8% (34), and 2% (6), respectively. The gross lesions were showed on the internal organs of chickens fed on mycotoxins contaminated feed paleness, enlargement and friable liver, pale, enlarged, and lobulated kidneys, hemorrhage in skeletal muscle as well as showed ascites. Microscopically, the kidneys showed necrosis in some renal tubules and glomeruli. In the liver, there was congestion of the portal vein and periportal necrosis with inflammatory cells infiltration. The results showed a significant decrease in means values of HB, PCV, RBCS, WBCS, total protein, and cholesterol while there was a significant increase in means values of AST, ALT, and Uric acid in infected chickens group compared with the healthy chicken group. Conclusion: Contamination of feed with mycotoxins is one of the most prominent challenges facing poultry producers in Babylon province. In addition, it is important to do pathological, hematological, and biochemical examinations for the diagnosis of mycotoxins in broilers. Keywords: Mycotoxins, Pathological lesions, Heamatological and Biochemical parameters, Broiler chickens, Babylon. IntroductionThe poultry farming sector in Iraq is considered one of the strongest, largest, and most innovative sectors of poultry production due to the continuous growth and development of poultry farming annually (Central Statistical Organization Iraq, 2017). In Babylon province (100 km south of Baghdad), like other provinces in Iraq, there are many obstacles that threaten this important sector, which is developing rapidly to meet the requirements of the large population increase from poultry protein (Kshash, and Oda, 2019). The diseases with various causes are the most prominent of these obstacles that threaten the future of the poultry industry due to large economic losses resulting from high mortality rates, low chick weights, low egg production, low fertility, and hatching rates, in addition to the high cost of prevention and control of these diseases (Kusi et al., 2015, Akbay and Azeez, 2016, Butler, 2016, Ume et al., 2016). Mycotoxins are considered one of the most important problems and threats faced the poultry producers due to some common effects are reduced feed intake, weight gain, feed efficiency, growth performance, immunity, and hatchability along with increased mortality, organ damage (mainly kidney and liver), carcinogenicity, teratogenicity, and decreased egg production which lead severe economic losses (Devegowda and Murthy, 2005). Mycotoxins are secondary metabolite products of fungi that grow in various poultry feed raw materials when the appropriate conditions are provided for them, such as humidity, temperature, under aerobic conditions. There are about 200 species of fungi that can produce more than 500 types of mycotoxins. Mycotoxins produce a variety of diseases, collectively called “mycotoxicoses,” directly or in combination with other primary stressors such as pathogens (Raju and Devegowda, 2000). It was found that Aspergillus, Fusarium, and Penicillium are the most abundant genera that produce pathogenic mycotoxins such as ochratoxin (OTA), aflatoxin (AF), T-2 toxin, fumonisin, zearalenone, and deoxynivalenol in food and feed that can significantly impact the health and productivity of poultry species (Pitt and Hocking, 2009; Patial et al., 2018; Adeyeye, 2020). Acute cases marked by clear clinical indications and post-mortem lesions brought on by ingesting high quantities of mycotoxins may result in mortality as well as a significant reduction in poultry productivity. But most of the time, mycotoxicosis is a chronic condition brought on by low-level consumption of fungal metabolites, which causes observable performance declines as well as nonspecific alterations such as immunosuppression and subcutaneous bleeding in broilers (D’mello et al., 1999). In the field, mycotoxicosis is suggested by subpar performance in the absence of a clear pathogenic, environmental, or management component, or by a nutritional deficit. Due to the density of poultry farming in Babylon province, which is located in central Iraq, and the lack of studies dealing with the study of mycotoxins and their impact on the health and productivity of chickens, this study therefore aimed to study contamination with mycotoxins in poultry feed and their impact on the health, performance and immunity of poultry in Babylon province. Materials and MethodsThis study was conducted in the Pathology and Poultry Diseases Department, at the College of Veterinary Medicine, Al-Qasim Green University, in cooperation with the Veterinary Teaching Hospital in Babylon province, where this study extended from January to December of 2023. 434 food samples were collected from poultry farms operating in Babil province during the year 2023, and the number of collected samples have been distributed over the months of the year (Table 1) for the purpose of examining them and detecting whether this feed had been loaded with mycotoxins (AF, OTA, and trechothesenT2) through a competitive direct enzyme-linked immunosorbent assay testing and using Veratox® AF, OTA and T-2/HT-2. The assay identifies the T-2 toxin and the T-2 and HT-2 toxins together (NEOGEN company kits, USA) as explained below. limit of quantification (LOQ) for the three toxins were T2=100ppb, Ochra=5ppb, and Afla=20ppb. The examination was conducted according to the manufacturer’s instructions. After that, around of 500–1,000 broiler chickens (Rose 308) were aged 3–6 weeks from both sex, were examined in each months for observing clinical signs of mycotoxins on chickens that were fed with mycotoxins-contaminated feed. Then, blood samples were collected from chickens fed on feeds contaminated with mycotoxins, as well as from chickens fed on uncontaminated feeds (control group). These blood samples were divided into two parts one put in a tube containing EDTA anticoagulant to conduct some hematological analysis which was performed in the laboratory of a veterinary teaching hospital in Babylon by using Auto hematology Analyzer according to (Coles, 1986), where the other part was put in a gel test tube for separating the serum which used in the biochemical tests which included the total protein and total cholesterol (TC), uric acid and the liver enzyme (ALT, AST) were determined with an apparatus SMT 100V / Seamaly 20 photometer using the original Seamaly Diagnostics kits, according to the manufacturer instructions which briefly described 200 μl was injected in specific disc then the disc inserted in apparatus (SMT 100V) then the result was read after 12 minutes. The chickens fed on mycotoxin-contaminated feed were humanly sacrificed for the purpose of conducting gross lesions examination and collecting samples of the affected tissues to conduct a histopathological examination of tissue samples were collected from internal organs including bursa of Fabricius, spleen, liver, kidneys, and heart. The organs were fixed in 10% neutral buffered formalin and processed in paraffin embedding according to (Bancroft and Gamble, 2008). The histopathological sections (3–5 μm) were stained with Haematoxylin and Eosin and exanimated under the light microscope. Statistical analysisThe experiment data were analyzed with a completely randomized design, and the differences between the parameters means were tested by analysis of variance, and the L.S.D. test tested the significant differences at a 0.05 probability level. The ready-made statistical program SAS, was used and the association between mycotoxin infection rate and each of the animals (was detected using the chi-square test, Probability values of p < 0.05 were considered statistically significant using the Statistical Analysis System program 9.6th ed. (SAS). Table 1. Shows the types of mycotoxins identified in feed samples by the ELIZA test in each month during the study period.
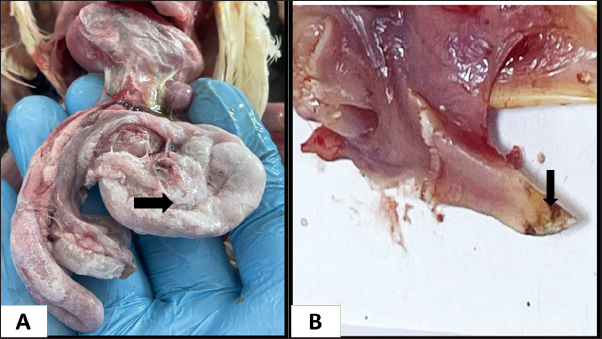
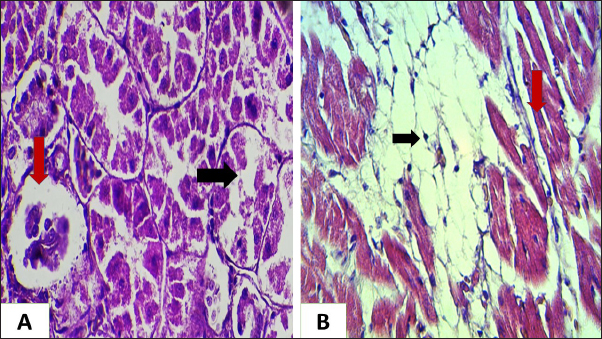
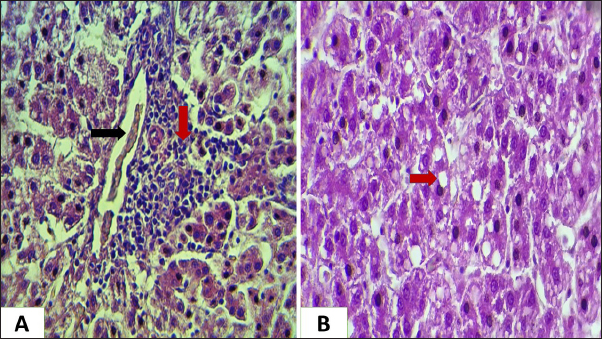
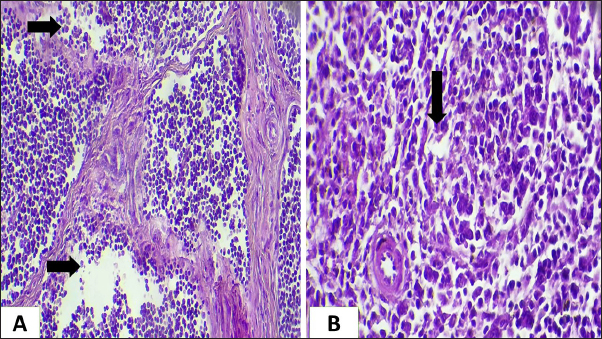
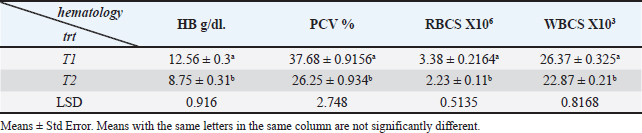
Ethical approvalNot needed for this study. ResultsIn this investigation had been collected 434 food samples from poultry farms operating in Babil province during the year 2023 for examining the mycotoxins (AF, ochera toxin, and trechothesen T2) by ELIZA. The results showed the percentage of numbers of feed samples contaminated with myotoxins above the level of LOQ were 22% (94) while the percentage of numbers of feed samples contaminated with myotoxins under the level of LOQ were 78 % (340). On the other hand, the percentage of numbers of feed samples contaminated AF, OTA, and Trechothesen T2) were (12(54), 8(34), and 2(6)) %, respectively, also the results appeared highest percentage of numbers of feed sample contaminated with myotoxins were recorded in months January and November, respectively, were recorded 14 positive food samples in each month, while as did not record any positive food sample in months July and August, respectively, as shown in Table 1. In the current study, the clinical signs showed in mycotoxins loaded chickens’ group were lethargy, loss of appetite, decreased weight, average growth, opisthotonus, diarrhea, the passage of undigested feed particles, arched back, neck muscle spasms, and then death with the legs extended backward. These signs were observed in birds from 2 weeks of age and older. Post-mortem lesions of the sacrificed mycotoxicated chickens showed change in muscle color with severe petechial and ecchymosis hemorrhage (Fig. 1A) and pectoral muscle (Fig. 1B), pale, enlargement and friable liver (Fig. 2A), pale, enlarged and lobulated kidneys (Fig. 2B) as well as showed ascites in some chickens (Fig. 3A), also, showed erosion in the gizzard (Fig. 3B), while some chickens revealed accumulation of urate in visceral organs (Fig. 4A) also, some birds showed ulceration in the tongue (Fig. 4B). The result of the histopathological examination of chicken tissues infected with mycotoxicosis were sever changes in different organs. In the kidneys were showed necrosis in some renal tubules and glomeruli (Fig. 5A), In the heart was showed atrophy of myocardial muscles due to infiltration of inflammatory edema (Fig. 5B). The histopathological changes in liver was congestion of the portal vein and periportal necrosis with inflammatory cells infiltration (mainly lymphocyte and macrophage) (Fig. 6A). Also showed fatty like change in the hepatocyte (Fig. 6B). The bursa of Fabricius was showed depletion of the lymphoid follicles due to lymphocytic necrosis with (Fig. 7A) and spleen showed depletion in the white bulb (Fig. 7B). Where the results showed a significant decrease in means values of HB, PCV, RBCS, and WBCS in the infected chickens group were recorded (8.75 g/dl, 26.25%, 2.23 × 106, and 22.87 × 103), respectively, in compared with means values which recorded in the healthy chicken group (12.56 g/dl, 37.68%, 3.38 × 106, and 26.37 × 103), respectively, as shown in Table 2.
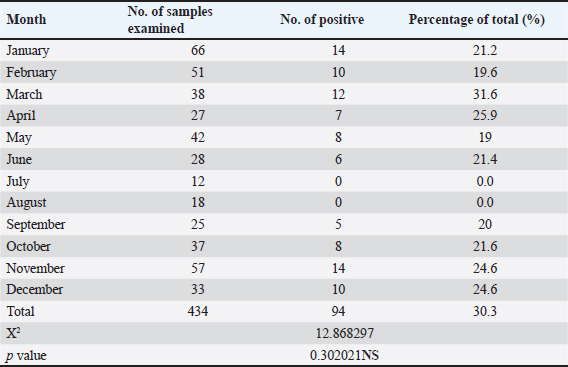
Fig. 1. Gross picture for muscles of chickens infected with mycotoxicosis reveal change in muscles color with severe petechial and ecchymosis hemorrhage A-in thigh muscle, B-pectoral muscle.
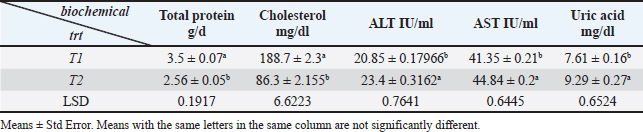
Fig. 2. Gross picture of chicken infected with mycotoxins (A): liver appears pale, enlarged, and brittle with petechial hemorrhage. (B) chicken kidneys appear pale, enlarged pale, enlarged, and hemorrhagic. Table 2 illustrates the results, which showed a significant increase in the means of AST, ALT, and uric acid in the infected chicken group recorded 44.48 IU/ml, 23.4 IU/ml, and 9.29 mg/dl, respectively, in comparison to the means values recorded in the healthy chicken group (41.35 IU/ml, 20.95 IU/ml, and 7.61 mg/dl). The results also showed a significant decrease in the means values of total protein and cholesterol in the infected chicken group (2.56 g/dl and 86.3 mg/dl, respectively) in comparison with the means values recorded in the healthy chicken group (3.5 g/dl, 188.7 mg/dl, respectively) (Table 3). DiscussionMycotoxins are known to be a prevalent secondary fungal metabolite contaminant in feed stuffs produced by mold and to be a substantial global issue with significant economic impact (Pappas et al., 2016). The Food and Agriculture Organization has indicated that about 1 billion metric tons of feed is destroyed annually throughout the world due to contamination with mycotoxins. This organization has estimated that 25% of agricultural crops that are used in the manufacture of feed and food products around the world are contaminated with mycotoxins.
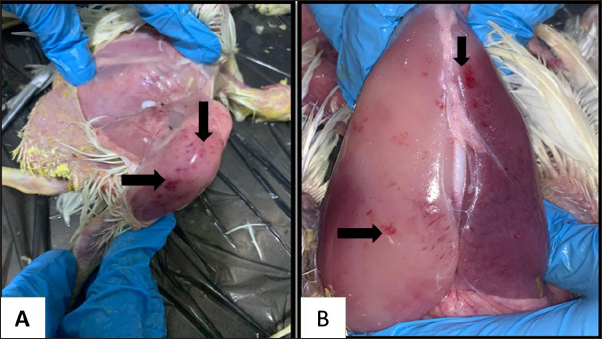
Fig. 3. Gross picture of chicken infected with mycotoxins(A): abdominal cavity reveals severe ascites. (B): gizzard of chickens showing sever erosion.
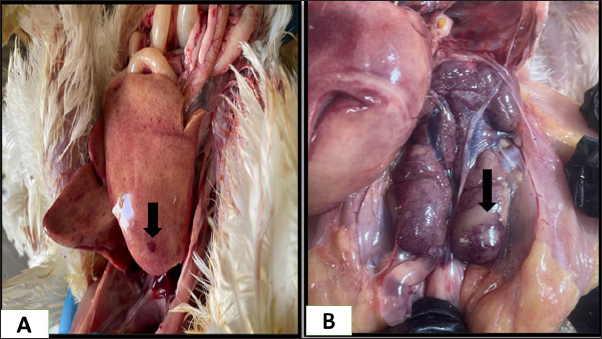
Fig. 4. Gross picture of the chicken infected with mycotoxin (A) visceral organs showing accumulation of urate crystals. (B) chicken tongue of showing severs ulceration. There have been reports of mycotoxins in poultry feed all over the world, which pose a serious risk to the wellbeing and output of chickens (Morrison et al., 2017; Ma et al., 2018). The problem of mycotoxicosis in Iraqi conditions, is one of the common problems that occur in chicken fields due to the lack of modern technology used in drying yellow corn, especially when harvesting the fall corn, which coincides with the period of rainfall and high humidity, and this exposes it to the growth of molds that secrete their toxins on yellow corn, when such grains are introduced into the feed, it will cause mycotoxicosis (Ibrahim and Al-Jubouri, 1986).
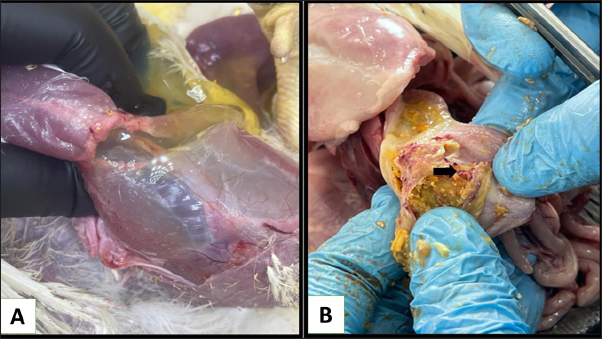
Fig. 5. Histopathological section of chicken infected with mycotoxin (A): kidney showing sloughing in the epithelia lining the renal tubules due to necrosis (black arrow) and collapse of glomeruli (red arrow) (B): chicken heart showing atrophy of myocardial muscles (red arrow) due to infiltration of inflammatory edema (black arrow) (H &E, stains, 400x).
Fig. 6. Histopathological section for chicken liver infected with mycotoxin (A): showing congestion of the portal vein (black arrow), periportal necrosis with inflammatory cells infiltration (red arrow). (B): showing fatty-like change or vacuolation of hepatocyte (red arrow) (H &E, stains, 200x). The results of this investigation referred to that 22% of the food samples were contaminated with mycotoxins in different types (OTA, trichothecene T-2 toxin) where this percentage consisted of 12%, 8%, and 2% for AF, ochera toxin, and trechothesen T2, respectively. These results are nearly similar with results obtained by Hegazy et al. (2022) who was detected positive mycotoxin in feed sample (26%), representing (15.4%) AF and (11.5%) OTA.
Fig. 7. Histopathological section of chicken infected with mycotoxin (A): bursa of Fabricius showing depletion of the lymphoid follicles due to lymphocytic necrosis with (black arrow). (H&E, stains, 200x). (B): spleen showing depletion in the white bulb (black arrow). (H&E, stains, 400x). Table 2. Explain the mean ± st. error of HB, PCV, RBCS, and WBCS in Mycotoxicosis chickens group and healthy chickens group.
Table 3. Explain the mean ± st. error of total protein, TC, AST, ALT, and uric acid in mycotoxicosis chickens group and healthy chickens group.
Through the results achieved in this study, we note that the percentage of contamination of poultry feed with AF and OTA toxins occupies the largest proportions, and this is consistent with Williams et al. (2004); Perrone et al. (2007). According to Khatoon and Abidin (2018), among the agriculturally significant metabolites, aflatoxins, ochratoxins, and mycotoxins are the most detrimental to the chicken business. These metabolites can be found in a variety of raw materials derived from plant and animal sources that are used in the production of animal feed. On the other hand, the results of this study indicate an increase in the incidence of contamination of poultry feed with mycotoxins in the months that fall within the fall and winter seasons, and a significant decrease in the summer season. The reason for this may be due to the availability of appropriate conditions for the growth of fungi, such as humidity and temperatures appropriate for the growth of fungi and the lack of drying of the grains that Install the poultry feed well after harvesting. The reason may be poor storage of the raw materials used in the manufacture of poultry feed, or poor storage of feed after manufacturing, which leads to the growth of fungi and thus the production of their metabolites, which are mycotoxins. This interpretation is consistent with Dutta and Das (2001) who pointed to that Aspergillus flavus, Aspergillus parasiticus, and Penicillium are produce their toxins in feed whenever they encounter a favorable environment (temperature, humidity, CO2, and O2). Also, in a study conducted by Streit et al. (2012) observed that there are several factors influenced in fungus growth and mycotoxins formation in the field, during storage and transit these factors include environmental factors such as grains, season, drought, and harvest time. Chickens are exposed to multiple mycotoxins due to ingestion of mycotoxin-contaminated rations, which lead to appear clinical signs and pathological lesions. These clinical signs and pathological lesions are influenced by many factors such as poultry species, mycotoxin type, dose consumed, and duration of exposure. Accordingly, naturally contaminated broiler diets with mycotoxins at permissible levels (T2=100ppb, Ochra=5ppb, and Afla=20ppb), chickens may exhibit signs and lesions of mycotoxicosis (Rodrigues and Naehrer, 2012; El Nabarawy et al., 2020). Since mycotoxins can have a negative impact on an animal’s health and productivity, their presence in feed is extremely undesired (Antonissen et al. 2014; Kipper et al. 2020). Mycotoxin exposure need not result in death; instead, the primary adverse effect on chickens is a decrease in food intake, which might impair development performance by reducing nutrient uptake and metabolism (Abd-Elhak et al., 2018; Wang and Hogan 2019). The clinical signs shown on infected chickens in this study similar to clinical signs were recorded by Gholami, et al. (2016); Kraidi et al. (2019); and Hegazy et al. (2022) who observed depression, ruffled feathers, diarrhea, loss of appetite, poor body performance, incoordination, vitamin deficiency like symptoms and high mortality rate on chickens infected with mycotoxins. Mycotoxins in chickens lead to prominent pathological changes that can be used as an indicator for clinical diagnosis. The most important of these changes is a change in the size of the internal organs, such as an increase in the size of the liver, spleen, and kidneys, and a decrease in the size of the bursa and thymus gland. As well as changes in the color and texture of the organs. The liver, for example, becomes a distinctive yellow color and is friable, with noticeable fatty infiltration. The macroscopic lesions seen in this investigation were consistent with those reported by Kumar and Balachandran (2009), who detailed the liver’s pale or yellowish coloring, as well as the enlargement and distention of the gall bladder, caused by AF B1 in chickens. The kidneys were enlarged, pale in color, and showed signs of congestion and intermittent petechial hemorrhages. In contrast, Mohiuddin et al. 1992, and Kumar et al. (2004) reported gross lesions caused by OTA A, which is more of a nephrotoxin than a hepatotoxin and is a potent and severe nephrotoxic in broiler chickens infected by enlarged and congested kidneys, reduced the size of lymphatic organs like the spleen and bursa, and increased size of the liver. The results of histopathological lesions in the liver were observed in this study were similar with results of previous studies Ortatali et al. (2005); Kumar and Balachandran (2009); Bakeer et al. (2013); and Kraidi et al. (2019) who noted hepatocyte deterioration, fatty alterations, and vacuolar degeneration under a microscope. They also noted the infiltration of various lymphocytic cells, including mononuclear cells and heterophils, as well as bile duct congestion and hyperplasia. Although OTA A is a nephrotoxin rather than a hepatoxin, it produces lesions in liver tissue as described by Kumar et al. (2004); Elaroussi et al. (2008); and Abidin et al. (2011) who demonstrated mononuclear cell infiltration in the parenchyma of liver tissue where the outer layer of the liver becomes thickened. The portal areas appear congested and the sinusoidal spaces are congested and swollen. In later stages of poisoning (due to continued exposure), the hepatic cords deteriorate and necrosis of individual cells can be observed. Vacuolar atrophy has been reported. OTA A causes proliferation of the bile duct epithelium with its degeneration. As a result of the oxidative stress caused by aflatoxins, which can lead to lipid peroxidation and oxidative damage to DNA, Solcan et al. (2008) and Bakeer et al. (2013) linked the degenerative and necrotic changes seen in the liver tissues of chickens infected with aflatoxicosis to the damage of essential cellular macromolecules (lipids, DNA, and proteins). Also, the microscopic lesions of kidneys in chickens infected with mycotoxin in this study were concurred with results obtained by many study Kumar et al. (2004); Ortatali et al. (2005); Carmen et al. (2008); Kumar and Balachandran (2009); Abidin (2010); Bakeer et al. (2013) and Kraidi et al. (2019) who revealed the OTA-caused tiny kidney lesions An infection is defined as the following: a swollen and deteriorated epithelium of proximal convoluted tubules; pyknotic nuclei in the cytoplasm of epithelial cells; degeneration in the epithelium of convoluted tubules and Malphighi corpuscles; and a rise in the size of epithelial cells. Moreover, glomerular infiltration, enlarged glomerular gaps, and necrosis of tubular epithelial cells have been observed. Mycotoxins produce follicular atrophy and a decrease in the amount of lymphocytic mass in the medulla of the Bursa of Fabricius. Vacuolar degeneration is also evident in the cortex and medulla follicles (Kumar et al., 2004; Khatoon, 2010). Nearly, the same lesions have been seen in this study. In the present study, the microscopic lesions in the spleen of mycotoxiosis chicken showed depletion in the white bulb, while as Bakeer et al. (2013) described the lesions in the spleen of chickens fed Mycotoxin, after 2 weeks, the red pulp showed significant erythrocyte proliferation while the white pulp showed lymphoid depletion. Based on the results of the hematological study, a significant difference was seen between the groups of healthy control hens and the mycotoxin-infected birds. The investigation’s hematological examination results are in line with those of earlier research (Ortatatli et al., 2005; Moregaonkar, 2007; Rathod et al., 2017), which found that chickens fed mycotoxin-contaminated feed—particularly AF B—even at low concentrations experienced a decline in average hemoglobin concentration values as well as packed cell volumes and red blood cell counts, indicating that the birds were anemic. In addition to their toxic effect on the process of hematopoiesis in the bone marrow, which results in a decrease in the production of red blood cells, white blood cells, hemoglobin concentration, and packed cell volumes, mycotoxins are also thought to be responsible for anemia in chickens infected with them (Sakhare et al., 2007, Prameela et al., 2011; Anjorin and Cyriacus, 2014; Abena et al., 2015; Hussain et al. 2016; Khan et al. 2017). This study also found that mycotoxin-loaded chickens had a decrease in white blood cells. These results are consistent with those of Rathod et al. (2017) and Hegazy et al. (2022) who found that broiler chickens fed AF-contaminated feed at varying concentrations may experience leukopenia. The chickens in the mycotoxin-infected group and the healthy group, which served as control groups, showed a substantial difference in the biochemical examination results. The lower serum total protein concentration in the mycotoxin-infected chickens group in the present study may be due to the toxic effects of mycotoxins on protein synthesis through their inhibition of mRNA transcription or amino acid transport leading to inhibition of protein synthesis (Kubena et al, 1993). The results of Goda et al. (2008), Zhao et al. (2010); Rathod et al. (2017); Naseem et al. (2018); and Hegazy et al. (2022) are consistent with the findings of the current study. Also, the finding of the present study referred to decreased cholesterol concentration in mycotoxin infected chickens group in compared with the healthy chickens group that might be attributed to disturbances in fat mobilization, metabolism, and its utilization in the body resulting in hepatic damage. This result had been concurred with results obtained by Abou-Zeid et al. (2015) and Rathod et al. (2017). The results of serum enzyme activity (ALT and AST) examined in the current study indicate that serum enzyme activity increased in the group of chickens infected with mycotoxins compared to the healthy chicken group, and this may indicate the presence of damage to liver tissue. As the activity of these enzymes in the blood reflects the activity of vital organs in the body, and the increased level of ALT and AST is linked to damage to liver cells, which are considered the main target organ for AF B1, as indicated by (Hassan et al. 2012). The results of the current study are consistent with the results reached by Zaho et al. (2010); Khan et al. (2017); Rathod et al. (2017); and Naseem et al. (2018). The results of this study reported a high concentration of uric acid in mycotoxins infected chickens group as compared to the healthy chickens group indicating severe kidney damage. The increase in serum uric acid concentration might be due to severe injury to renal tubules that leads to impaired renal excretory function during mycotoxicosis especially OTA intoxication (Abidin et al., 2011). Similar findings were also observed by Mohamed and Mohamed (2009); Magnoli et al., (2011); Bhatti et al. (2016); Rathod et al. (2017); and Naseem et al. (2018). ConclusionBased on the results of this study, we conclude that contamination of feed with mycotoxins is one of the most prominent challenges facing poultry producers in Babil Governorate. The feed loaded with mycotoxins used in feeding chickens leads to health problems in chickens and poor productivity, thus causing serious economic losses to poultry producers. Pathological, hematological, and biochemical changes are considered important initial indicators for diagnosing cases of fungal poisoning in birds. Common occurrence arose from the multi-mycotoxin effect, which means synergistic or antagonistic effects can occur. This study recommends creating a comprehensive database on the extent of contamination of poultry feed with mycotoxins. This will help find ways to reduce the burden of mycotoxins on the health of birds. AcknowledgmentsThe researchers would like to thank the veterinary hospital in Babylon for providing the samples and data. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research received no external funding. Authors’ contributionsConceptualization and design: HA and GA; Practical work: HA, GH, and ZA; formal analysis and interpretation of data: HA, ZA; writing-original draft preparation: HA and GH; All authors revised and approved the final manuscript for publication. Data availabilityAll data supporting the findings of this study are available within the manuscript and no additional data sources are required. ReferencesAbd El-Hack, M.E., Samak, D.H., Noreldin, A.E., El-Naggar, K. and Abdo, M. 2018. Probiotics and plant-derived compounds as ecofriendlyagents to inhibit microbial toxins in poultry feed: a comprehensive review. Environ Sci Pollut Res. Nov;25(32):31971–31986. Abeena, B., George, A.J., Mohammed Shejir, R., Nair, N.D. and C.B. Manomohan. 2015. Haematobiochemical changes of layer chicken in experimental Aflatoxicosis. Indian Vet. J. 92(10):84–86. Abidin, Z. 2010. Ameliorative effects of L-carnitine and vitamin E upon toxico-pathological alterations induced by ochratoxicosis in white Leg-horn cockerels. M. Phil Thesis, Department of Pathology, Faisalabad, Pakistan: University of Agriculture Faisalabad. Abidin, Z., Khatoon, A. and Numan, M. 2011. Mycotoxins in broilers: pathological alterations induced by aflatoxins and ochratoxins, diagnosis and determination, treatment and control of mycotoxicosis. Worlds Poult Sci J.Vol. 67, 485–496. Abou-Zeid, A.E., El-Damarawy, S.Z. and El-Rayes, T.K. 2015. Biochemical, immunological and pathological studies on broiler fed AflatoxinB1 b1 contaminated diet treated biologically by lactobacillus acidophilus or saccharomyces cerevisiae. Egyptian J. Nutrition and Feeds. 18(2), 409–420. Adeyeye, S.A.O., 2020. Aflatoxigenic fungi and mycotoxins in food: a review. Crit Rev Food Sci Nutr. 60, 709–721. Akbay, C. and Azeez, J. 2016. Factors affecting on mortality rate in the broiler chicken production farms in Erbil, Iraq. Pakistan Journal of Food Science, 26(3), 119–128. Anjorin, S.T. and Cyriacus, C.O. 2014. Haematological effect of Aspergillus species metabolites on broiler chicks. American J. of Research Communication, 2(1), 172–184. Antonissen, G., Van Immerseel, F., Pasmans, F., Ducatelle, R., Haesebrouck, F., Timbermont, L., Verlinden, M., Janssens, G.P.J., Eeckhaut, V. and Eeckhout, M. 2014. The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens induced necrotic enteritis in broiler chickens. PLoS One. Sep 30;9(9), e108775. Bakeer, A.M., Farid, A.S. and AbdElKarim, M.F. 2013. The hepatotoxic and nephrotoxic effects of mycotoxin in broiler chickens. (BVMJ), 25(1), 29–45. Bancroft, J.D. and Gamble, M. 2008. Theory and practice of histology techniques, London, UK: Churchill Livingstone Elsevier, pp. 83–134. Batikh, M.M., El-Nabarawy, A.M., Shakal, M.A.S., Hegazy, A.H.M. and Morsy, E.A. 2021. The effect of mycotoxins in naturally contaminated diet on the pathogenicity of Escherichia coli in broiler chickens. World Vet. J. 11(4), 745–757. Bhatti, S.A., Khan, M.Z., Saleemi, M.K., Muhammad Saqib, M.S. 2016. Aflatoxicosis and ochratoxicosis in broiler chicks and their amelioration with locally available bentonite clay. Pak Vet J. 36, 68–72. Butler, J. 2016. Prospects and challenges of poultry farming in the Wa municipality of the upper west region of Ghana. Afr. J. Poult. Farming 4(1), 103–112. Carmen, S., Coman, I., Solcan, G., Miron, L. and Oprean, O.Z. 2008. Histological and ultrastructural lesions of the kidney in experimental ochratoxicosis of broiler chickens. Medicine. J Vet Med Educ. 65, 91–95 Central Statistical Organization Iraq. 2017. Report of poultry for 2016. Baghdad, Iraq: Central Statistical Organization Iraq. Coles, E.H. 1986. Veterinary Clinical Pathology, 4th Edition, Philadelphia, PA: W.B.Saunders Company, pp. 17–19. Devegowda. G. and Murthy, D.E. 2005. Diaz mycotoxins: their effects in poultry and some practical solutions the mycotoxin blue book, Nottingham, UK: Nottingham University Press, pp. 25–56. D’Mello, J.P.F., Placinta, C.M. and Macdonald, A.M.C. 1999. Fusarium mycotoxins: a review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 80(3–4), 183–205. Dutta, T.K. and Das, P.I. 2001. Isolation of aflatoxigenic strains of Aspergillus and detection of aflatoxin B1 from feeds in India. Mycopathologia 151, 29–33. Elaroussi, M.A., Mohamed, P.R., Elgendy, M.S., El Blarkouky, E.M., Abdou, A.M. and Hatab, M.H. 2008. Ochratoxicosis in broiler chickens: functional and histological changes in targetorgans. Int. J. Poult. Sci. 5, 414–422. El Nabarawy, A.M., Ismael, E., Shaaban, K.A., El Basuni, S.S., Batikh, M.M. and Shakal, M. (2020). Mycotoxins contamination levels in broiler Feeds and aflatoxin residues in broiler Tissues. J. World Poult. Res. 10, 133–144. Gholami, M, Rangsaz, N. and Azizi, S. 2016. Evaluation turmeric (Curcum longa) effect on biochemical and pathological parameters of liver and kidney in chicken aflatoxicosis. Pharm. Biol. 54(5), 780–787. Gowda, N.K., Ledoux, D.R., Rottinghaus, G.E., Bermudez, A.J. and Chen, Y.C. 2008. Efficacy of turmeric (Curcuma longa), containing a known level of curcumin and a hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of aflatoxin in broiler chicks. Poult. Sci. 87, 1125–1130. Ibrahim, I.K. and Al-Jubouri, K.M.T. 1998. Mycotoxins, their effects and dangers. First edition, Higher Education and Scientific Research Press, Abaa Center for Agricultural Research. Hassan, Z.U., Khan, M.Z., Khan, A., Javed, I., Sadique, U. and Khatoon, A. 2012. Ochratoxicosis in White Leghorn breeder hens: production and breeding performance. Pak. Vet. J. 32, 557–561. Hegazy, A., A El Sisi, M., K El Bendari, E., M Abd-allah, E. and MN Toliba, H. 2022.Problems of some mycotoxins in broiler farms in Egypt. KVMJ, 20(1), 6–11. Hussain Z., Khan M.Z., Saleemi M.K., Khan, A. and Rafique, S. 2016. Clinicopathological effects of prolonged intoxication of aflatoxin B1 in broiler chicken. Pak. Vet. J. 36, 477–481. Khan A., Aalim M.M., Khan M.Z., Saleemi, M.K., He, C., Naseem, M.N. and Khatoon, A. 2017. Does distillery yeast sludge ameliorate moldy feed toxic effects in White Leghorn hens? Toxin. Rev. 36, 1–8. Khatoon, A. 2010. Effects of ochratoxin A feeding to chicks upon immune system and their amelioration by silymarin and vitamin E. M-Phil Thesis, Department of Pathology, University of Agriculture Faisalabad, Faisalabad, Pakistan. Khatoon, A. and Abidin, Z, 2018. An extensive review of experimental ochratoxicosis in poultry: I. Growth and production parameters along with histopathological alterations. World Poult. Sci. J. 74, 627–646. Kipper, M., Andretta, I., Ribeiro, A.M.L., Pires, P.G.D., Franceschina C.S., Cardinal K.M., Moraes P.D. and Schroeder B. 2020. Assessingthe implications of mycotoxins on productive efficiency of broilers and growing pigs. Sci. Agric. 77(3), e20180236. Kraidi, Q.A., Abbas, S.S. and Chayan, M.A. 2019. Study of pathological changes caused by mycotoxins in broilers in Al-Qurna City, Basra, Iraq. Plant Arch. 19(2), 2579–2584. Kshash, B. and Oda, H. (2019) Constraints facing poultry producers in Iraq. J. Agri. Exten. 23(2), 90–100. Kubena, L.F., Harvey, R.B., Phillips, T.D. and Clement, B.A. 1993. Effect of hydrated sodium calcium aluminosilicates on aflatoxicosis in broiler chicks. Poult. Sci. 72, 651–657. Kumar, A., Jindal, N., Shukla, C.L., Asrani R.K., Ledoux D.R. and Rottinghaus G.E. 2004. Pathological changes in broiler chickens fed ochratoxin A and inoculated with Escherichia coli. Avian Pathol. 33(4), 413–417. Kumar, R. and Balachandran, C. 2009. Histopathological changes in broiler chickens fed AflatoxinB1 and cyclopiazonic acid. Vet. Arhiv. 79, 31–40. Kusi, L., Asabre, P., Kosi, I. and Nyarku, K. 2015. The challenges and prospects of poultry farmers: the case of Dormaa ahenkro municipal area. SH&SS, 2(4), 214–224. Ma, R, Zhang, L, Liu, M, Su, Y.T., Xie, W.M., Zhang, N.Y., Dai, J.F., Wang, Y., Rajput, S.A., Qi, D.S. and Karrow, N.A. 2018. Individual and combined occurrence of mycotoxins in feed ingredients and complete feeds in China. Toxins 10, 113. Magnoli, A.P., Monge, M.P., Miazzo, R.D., Cavaglieri, L.R., Magnoli, C.E., Merkis, C.I., Cristofolini, A.L., Dalcero, A.M. and Chiacchiera, S.M. 2011. Effect of low levels of AflatoxinB1 B1 on performance, biochemical parameters, and AflatoxinB1 B1 in broiler liver tissues in the presence of monensin and sodium bentonite. Poult. Sci. 90, 48–58. Mohamed A.H. and Mohamed H.M. 2009. Haemato-biochemical and pathological studies on aflatoxicosis and treatment of broiler chicks in Egypt. Vet. Ital. 45(2), 323–337. Mohiuddin, S.M., Reddy, M.V. and Ahmed, S.R. 1992 Studies on ochratoxicosis in broiler chicks Indian Vet. J. 69, 1011–1014. Moregaonkar, S.D. 2007. Efficacy of a commercial mycotoxin binder in preventing aflatoxic and ochratoxic pathology in broiler birds, I. J. V.Pathol. 31(1), 79. Morrison,, D.M., Ledoux D.R.., Chester, L.F. and Samuels, C.A. 2017. A limited survey of aflatoxins in poultry feed and feed ingredients in Guyana. Vet. Sci. 4, 60. Naseem M.N., Saleemi M.K., Abbas R.Z., Khan A, Khatoon A., Gul S.T., Imran M., Sindhu, Z.U.D. and Sultan A. 2018. Hematological and serum biochemical effects of aflatoxin B1intoxication in broilers experimentally infected with fowl adenovirus-4 (FAdV-4). Pak Vet. J. 38(2), 209–213. Ortatatli, M., Oguz, H., Hatipogl, F. and Karaman, M. 2005. Evaluation of pathological changes in broilers during chronic aflatoxinb1 (50 and 100 ppb) and clinoptilolite exposure. Res Vet Sci. 78, 61–68. Pappas, A. Tsiplakou, D., Tsitsigiannis, M., Georgiadou, M., Iliadi, K. and Zervas, G. 2016. The role of bentonite binders in single or concomitant mycotoxin contamincation of chicken diets. Br Poult Sci. 57(4), 551–558. Perrone, G., Susca, A., Gozzi, G., Ehrlich, K., Varga, J., Frisvad, J.C., Meijers, M., Noonim, P., Mahakarnchanakuls, W. and Samson, R.A. 2007. Biodiversity of Aspergillus species in some important agricultural products. Stud. Mycol. 59, 53–66. Pitt J. and Hocking A. 2009. Fungi and food spoilage, 3rd edition, Berlin, Germany: Springer. Prameela R.M., Nissar A.N., Eswara P.P. and Sri Latha, C. 2011.Hematological and Biochemical changes of stunting syndrome in Broiler chicken. Vet. World 4(3), 124–125. Raju, M.V. and Devegowda, G. 2000. Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T- 2 toxin). Br. Poult. Sci. 41, 640. Rathod, P., Gangadhar, K., Gangane, G. and Bhojane, N.2017. Effect of aflatoxin on haematological and biochemical alteration in broilers. Int. J. Sci. Environ. Tech. 6(1), 824–831 Rodrigues I. and Naehrer K. 2012. Prevalence of mycotoxins in feedstuffs and feed surveyed worldwide in 2009 and 2010. Phytopathol. Mediterr. 175–192. Sakhare P..S, Harne S.D. and Kalorey D.R. 2007. Effect of toxiroak polyherbal feed supplement during induced aflatoxicosis, ochratoxicosis and combined mycotoxicoses in broilers. Vet. Arhiv. 77, 129–146. SAS. 2018. Statistical Analysis System, User’s Guide. Statistical. Version 9.6th ed. Cary, NC: SAS. Inst. Inc. Streit, E., Schatzmayr, G., Tassis, P., Tzika, E., Marin, D., Taranu, I., Tabuc, C., Nicolau, A., Aprodu, I., Puel, O. and Oswald, I.P. 2012. Current situation of mycotoxin contamination and co-occurrence in animal feed-focus on Europe. Toxins, 4, 788–809. Solcan, C., Coman, I. and Solcan, G.H. 2008. Histological lesions of the liver in chicken’sochratoxicosis. Lucrari Stiinłifice Med. Vet. 8, 898–903. Ume I., Jiwuba, C., Obi, I. and Elisha, D. 2016. Economics of broiler production among rural women in Ahiazu Mbaise L.G.A of Imo State, Nigeria. ARJA, 1(2), 1–8. Patial, V., Asrani, R.K. and Thakur, M. 2018. Food-Borne Mycotoxicoses: Pathologies and Public Health Impact. Foodborne Diseases, 1, 239–274. Wang, A.H. and Hogan, N.S. 2019. Performance effects of feed-borne Fusarium mycotoxins on broiler chickens: Influences of timing and duration of exposure. Anim. Nutr. 5(1), 32–40. Williams, J.H., Phillips, T.D., Jolly, P.E., Stile, J.K., Jolly, C.M. and Aggarwal, D. 2004. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences and interaction. Am. J. Clin. Nutr. 80, 1106–1122. Zhao, J, Shirley, R.B., Dibner, J.D., Uraizee, F., Officer, M., Kitchell, M., Vazquez-Anon, M. and Knight, C.D.2010. Comparison of hydrated sodium calcium aluminosilicate and yeast cell wall on counteracting aflatoxicosis in broiler chicks. Poult. Sci. 89, 214–56. | ||
| How to Cite this Article |
| Pubmed Style Almremdhy HAA, Neamha GAK, Al-hilli ZB, Awadh RJ. Pathological, hematological and biochemical alteration in broiler chickens infected with mycotoxin in Babylon province. Open Vet. J.. 2024; 14(11): 2848-2859. doi:10.5455/OVJ.2024.v14.i11.13 Web Style Almremdhy HAA, Neamha GAK, Al-hilli ZB, Awadh RJ. Pathological, hematological and biochemical alteration in broiler chickens infected with mycotoxin in Babylon province. https://www.openveterinaryjournal.com/?mno=214120 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.13 AMA (American Medical Association) Style Almremdhy HAA, Neamha GAK, Al-hilli ZB, Awadh RJ. Pathological, hematological and biochemical alteration in broiler chickens infected with mycotoxin in Babylon province. Open Vet. J.. 2024; 14(11): 2848-2859. doi:10.5455/OVJ.2024.v14.i11.13 Vancouver/ICMJE Style Almremdhy HAA, Neamha GAK, Al-hilli ZB, Awadh RJ. Pathological, hematological and biochemical alteration in broiler chickens infected with mycotoxin in Babylon province. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 2848-2859. doi:10.5455/OVJ.2024.v14.i11.13 Harvard Style Almremdhy, H. A. A., Neamha, . G. A. K., Al-hilli, . Z. B. & Awadh, . R. J. (2024) Pathological, hematological and biochemical alteration in broiler chickens infected with mycotoxin in Babylon province. Open Vet. J., 14 (11), 2848-2859. doi:10.5455/OVJ.2024.v14.i11.13 Turabian Style Almremdhy, Hayder Abd Al-emier, Ghusoon Abdul Kareem Neamha, Zina Bakir Al-hilli, and Rafal Jalil Awadh. 2024. Pathological, hematological and biochemical alteration in broiler chickens infected with mycotoxin in Babylon province. Open Veterinary Journal, 14 (11), 2848-2859. doi:10.5455/OVJ.2024.v14.i11.13 Chicago Style Almremdhy, Hayder Abd Al-emier, Ghusoon Abdul Kareem Neamha, Zina Bakir Al-hilli, and Rafal Jalil Awadh. "Pathological, hematological and biochemical alteration in broiler chickens infected with mycotoxin in Babylon province." Open Veterinary Journal 14 (2024), 2848-2859. doi:10.5455/OVJ.2024.v14.i11.13 MLA (The Modern Language Association) Style Almremdhy, Hayder Abd Al-emier, Ghusoon Abdul Kareem Neamha, Zina Bakir Al-hilli, and Rafal Jalil Awadh. "Pathological, hematological and biochemical alteration in broiler chickens infected with mycotoxin in Babylon province." Open Veterinary Journal 14.11 (2024), 2848-2859. Print. doi:10.5455/OVJ.2024.v14.i11.13 APA (American Psychological Association) Style Almremdhy, H. A. A., Neamha, . G. A. K., Al-hilli, . Z. B. & Awadh, . R. J. (2024) Pathological, hematological and biochemical alteration in broiler chickens infected with mycotoxin in Babylon province. Open Veterinary Journal, 14 (11), 2848-2859. doi:10.5455/OVJ.2024.v14.i11.13 |