| Research Article | ||
Open Vet. J.. 2024; 14(11): 2866-2876 Open Veterinary Journal, (2024), Vol. 14(11): 2866-2876 Research Article Ameliorative effect of Naja haje venom against arthritic influence of Freund’s complete adjuvant FCA in paw and ankle of male ratsNabil A. Soliman1*, Amr A. Shalaby1, Mayada R. Farag2, Shimaa Kamal1, Sara M. Abdelkarem Alashqar1, Mohamed Ahmed Ammar1 and Suzan Attia Mawed11Zoology Department, Faculty of Science, Zagazig University, Zagazig, Egypt 2Forensic Medicine and Toxicology Department, Veterinary Medicine Faculty, Zagazig University, Zagazig, Egypt *Corresponding Author: Nabil A. Soliman. Zoology Department, Faculty of Science, Zagazig University, Zagazig, Egypt. Email: nabilsoliman54 [at] yahoo.com Submitted: 05/08/2024 Accepted: 05/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
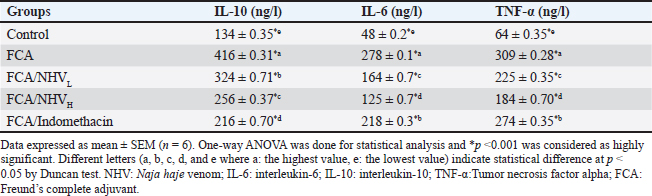
AbstractBackground: Pain and inflammation are closely associated with rheumatoid arthritis (RA), which affects the bones and joints. Aim: While there are a number of therapeutic options for arthritis, their side effects restrict their use and encourage the search for alternative, natural remedies. Methods: In male rats, we examined the anti-inflammatory and anti-arthritic properties of Naja haje venom (NHV). Thirty adult albino rats were divided into five equal groups, each consisting of six rats. The 1st group was kept as a control, while the 2nd group Freund’s complete adjuvant (FCA) received an injection of 0.05 ml of FCA for eight days to induce RA and inflammation. The 3rd group (FCA/NHVL) and the 4th group (FCA/NHVH) received an injection of FCA followed by an intraperitoneal injection of 0.042 µg/kg (low; L) and 0.085 µg/kg (high; H) of lyophilized NHV, respectively, for 14 days. The 5th group (FCA/Indomethacin) received an injection of FCA followed by an intraperitoneal injection of 0.25 mg indomethacin/kg for 14 days. Results: This study found that NHVH significantly increased the body’s total antioxidant capacity after FCA while lowering the levels of interleukins IL-10 and IL-6, as well as the activity of genes that cause inflammation, like nuclear factor kappa B and tumor necrosis factor-alpha. Furthermore, histological findings in NHVH groups showed a partial regeneration of the cartilage, bone, and synovium in the palm and ankle joint compartments. Conclusion: All of our findings suggest that NHV has anti-inflammatory and anti-arthritic qualities that may help repair the joint damaging components caused by FCA and restore cellular equilibrium. Keywords: Rheumatoid arthritis, Snake venom, Indomethacin, Freund’s complete adjuvant, Rats. IntroductionRheumatoid arthritis (RA) is a prevalent systemic inflammatory illness that is not contagious. It is characterized by inflammation of joints, pain, and destruction of cartilages and bones, in addition to synovitis, which is the primary symptom of RA. According to Zou and Hu (2019), autoimmune responses and chronic inflammation-induced synovial hyperplasia, cartilage degradation, and angiogenesis are among the pathological features of RA. Fibroblast-like synoviocytes (FLSs) are the main effector cells involved in the onset, development, and maintenance of RA, among other features of the disease (Bottini and Firestein, 2013). In addition to hyperplasia, FLSs participate in the pathogenesis of RA by reacting to secreted inflammatory mediators linked to innate immunity. These mediators include chemokines like monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 alpha, pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1 beta (IL-1β), and IL-6. Unusual high matrix concentrations of metalloproteinases, released bysynoviocytes and ultimately cause cartilage invasion and degradation, are another aggressive aspect of RA (Han et al., 2001). Furthermore, synoviocytes interact with T cells and promote the growth of inflammatory T cells, which exacerbate the inflammatory processes typical of RA (Tran et al., 2007; Bancroft et al., 2013; Boda et al., 2018). In fact, there are various therapeutics that have been used for treating RA such as non-steroidal anti-inflammatory drugs (NSAID), disease-modifying antirheumatic drugs (DMARDs), corticosteroids, and monoclonal antibodies (Kremers et al., 2004; Mamdouh Ibrahim, 2022). However, these therapeutic agents are often expensive and have undesirable side effects (Weir, 2002). Therefore, there is a need to find new agents for treating RA. Natural products are great priceless resources for biomedicine and biological therapies. Cobra venom has been utilized for treating of arthritis, inflammations, and pain in Chinese and Indian traditional medicines (Chen et al., 2015). Recently, various components of snake venoms have been utilized for drug development (Koh and Kini, 2012). Animal venoms composed of a chemical mixture of substances that have pharmacological activities, such as peptides, proteins, and enzymes with particular therapeutic actions, in addition to lipids, carbohydrates, metal ions, and other unidentified compounds (Mohamed Abd El-Aziz et al., 2019). The biological actions of venoms reported in the context of biomedical uses include their effects on disintegrating cell adhesions, the effect of L-amino acid oxidases on the promotion of apoptosis, cell dynamics, platelet functions, coagulation factors, the action of β and α neurotoxins [which belong to the three-finger toxin (TFT) family] on hypotensive effect, blockade of potassium channel, the actions of nerve growth factors, the effect of myotoxins, cardiotoxins, and TFTs, along with some other actions such as the effects on the immune cells (Cañas, Castaño-Valencia et al., 2021; Oliveira et al., 2022). Nonetheless, a small number of toxins found in venom are typically associated with toxicity (Ferraz et al., 2019). More researches are still required to identify the processes underlying the venom’s anti-inflammatory properties. A popular model in rats is Freund’s complete adjuvant (FCA)-induced arthritis. FCA injections subcutaneously or intradermally have been routinely utilized to cause inflammation in rats that mimics RA. When FCA is injected intradermally at the tail base, it causes a chronic, multi-joint, relapsing-remitting arthritis that can last for several months (Chillingworth and Donaldson, 2003). Rats are common experimental models used to investigate the disease’s etiology and assess the efficacy of anti-rheumatic medications. One of the best experimental models for examining the impacts of arthritis is adjuvant-induced arthritis, which is still often utilized in RA preclinical testing (Snekhalatha et al., 2013). The current work aims to investigate the anti-inflammatory effects of Naja haje venom (NHV) against arthritis induced by FCA in rats and compare the acquired results obtained with the widely used NSAID indomethacin, which is used to treat RA. Materials and MethodsChemicals and drugs under testA 0.25 mg/ml heat-killed Mycobacterium tuberculosis in olive oil (1:1 v/v) FCA emulsion was created by Sigma-Aldrich (Saint Louis, MO, USA). Indomethacin is purchased in injection ampoules from a nearby pharmacy under the brand name Liometacen (Zagazig, Egypt). IL-6, IL-10, and TNF-α enzyme immunoassay kits (eBioscience, San Diego, CA). The malondialdehyde marker (MDA) and total antioxidant capacity (TAC) using the enzyme-linked immunosorbent assay (ELISA) kit (Cat No. STA-360, Cell Biolabs, Inc., 7758 Arjons, Drive San Diego, CA 92126 USA). Induction of arthritis0.05 ml of this emulsion was injected into the right hind paw sub-plantarly in rats to cause RA. The same volume of saline was injected into the left hind paw (Newbould, 1963; Ghosh et al., 2016). How to prepare snake poisonCrude venom was taken from Naja haje snakes caught in Egypt’s West Delta. Before being used, snake venom in the VACSERA laboratory was milked, lyophilized, and kept in a desiccator at 4°C in the dark, then reconstituted in a saline solution. LD50 of crude venom was determined to be 0.495 mg/kg according to Abdel-Aal and Abdel-Baset (2010). Care of animals and experiment designThirty adult male Wistar albino rats, with an average body weight of 190–200 g, were purchased from The odor Bilharz Research Institute’s breeding unit in Cairo, Egypt. The rats were housed in our laboratory for 2 weeks at a temperature of 21°C ± 2°C with a 12:12 light and dark cycle. Following acclimation, the rats were randomly assigned to five equal groups, each consisting of six rats based on weight: (i) The first group, designated as the control group, was given 0.1 ml of saline daily for 21 days. (ii) The second group is the FCA group, which received an 8-day injection of 0.05 ml of (FCA) in olive oil (1:1 v/v) into the rats’ right hand paw subplantar area. (iii) The third group, FCA/NHVL, received 0.05 ml of treatment for 8 days before receiving an intraperitoneal (i.p.) injection of NHV (0.042 µg/kg; low dose) for 14 days. (iv) The fourth group, FCA/NHVH, received an intraperitoneal injection of NHV (0.085 µg/kg; high dose) after receiving 0.05 ml of FCA treatment for 8 days, for a duration of 14 days. (v) The fifth group, known as the FCA/Indomethacin group, received an injection of the standard dose of Indomethacin (0.25 mg/kg, i.p.) for 14 days after receiving 0.05 ml of FCA for 8 days. Blood and tissue collectingAt the end of the experiment, on the 22nd day, rats were given an intramuscular injection of a 2:1 ketamine-to-xylazine mixture at a dose of 1 ml/kg to induce anesthesia. Each rat in the experimental groups had its own abdominal aorta used to draw blood. Following a 15-minute centrifugation at 3,000 rpm, the resulting sera were collected and stored at −20°C until they were needed for additional experiments. After anesthesia, the skin was incised along the middle of the ankle joint until an area of 2 by 2 cm was exposed for tissue collection. The joint cavity was opened, and a surgical blade was used to carefully resect the synovial tissue surrounding the ankle. The sample of synovial tissue was washed and stored at −80°C until mRNA extraction. Rat paw and ankle joints were removed and preserved for histopathological analysis in 10% neutrally buffered formalin. Identification of serum inflammatory markersThe study utilized commercially available enzyme immunoassay kits (eBioscience, San Diego, CA) to estimate various inflammation parameters, such as IL-6, IL-10 (Chen et al., 2015), and TNF-α (Ghosh et al., 2016). The manufacturer’s instructions were strictly followed. Identification of oxidative stress markers in the serumAccording to the manufacturer’s instructions, oxidative stress can be identified by measuring lipid peroxidation using the MDA (Han et al., 2001) and by measuring TAC (Preuss et al. 1998) using the ELISA kit (Cat No. STA-360, Cell Biolabs, Inc., 7758 Arjons, Drive San Diego, CA 92126). Quantitative real-time PCR (qRT-PCR) and RNA extractionTotal RNA was isolated from the joint tissue homogenates (n=3, 1 g from each rat joint) using Trizol (Life Technologies, Carlsbad, California, USA) following the manufacturer’s instructions. A High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems TM, USA) was utilized for cDNA synthesis. SYBR Green PCR Master Mix was used in the quantitative PCR experiment (Thermo Fisher Scientific, Waltham, MA). Primers were designed using the Primer Premier 5.0 software (BIOPROCESS ONLINE, Pittsburg, CA). qRT-PCR was applied using 3 biological repeats on the MSLPCR30 Thermal Cycler system (BiobaseBiozone Co., Ltd., Guangdong, China). Genes expression differences were determined using 2-ΔΔCt method based on threshold cycle (Ct) number and GAPDH was used as the control gene. Primers of the study are shown in Table 1 as follows: Histological evaluationsRats’ paw and ankle joints were removed, and preserved in 10% buffered formalin for a whole day. For 4–5 days, the joints were decalcified in osteomol. The joints underwent additional processing, including dehydration in 50%–100% graded alcohol, xylol clearing, paraffin embedding (56°C–58°C), and block preparation. As previously noted, sections measuring 5 µm were cut using a rotary microtome (Weswox Optic, India) (Ishikawa et al., 2005; Rhodes, 2013). Stained sections were seen using a bright field microscope (Motic, Germany) to check for the presence of urolithiasis and any associated pathological alterations, such as necrosis, inflammation, proliferation, and degeneration. Motic software (Motic Images Plus 2.0 software, 217-10-24) was used to take the photos (Layton and Suvarna, 2013). Statistical analysisThe information was displayed in triplicate as mean ± standard error (SE). When comparing two-group variance, the data were statistically examined utilizing the unpaired Student’s t-test, which was followed by a one-way ANOVA. The Statistical Package for Social Sciences (SPSS, 16 ver. for Windows, Chicago, SPSS Inc) was utilized throughout this analysis. Excel and the GraphPad Prism 8 software program (GraphPad Software, La Jolla, CA) were used to create the plots and graphics. The p value was shown as (*p < 0.05; ** p < 0.01; *** p < 0.001). Ethical approvalOn February 15, 2019, the ZU-IACUC Committee, the International Animals Care and Use CommitteeatZagazig University, accepted the updated protocol and gave it the approval number ZU-IACUC/1/F/31/2019. ResultsNHV decreased the pro-inflammatory mediators that FCA had producedTable 2 demonstrated a considerable rise in pro-inflammatory cytokines, such as IL-10, IL-6, and TNf-α, following the induction of arthritis by FCA in comparison to the control group. However, injection of two distinct NHV dosages could decrease these parameters, with NHVH being the most effective dose (p < 0.001). We employed indomethacin for additional research, which demonstrated the efficacy of NHVH by lowering serum pro-inflammatory cytokines in comparison to the FCA group (p <0.001). Table 1. Genes expression differences were determined using 2-ΔΔCt method based on threshold cycle (Ct) number and GAPDH was used as the control gene.
Table 2. Effect of (NHV) and indomethacin on the serum pro-inflammatory cytokines IL-6, IL-10 andTNF-α level in FCA -treated rats.
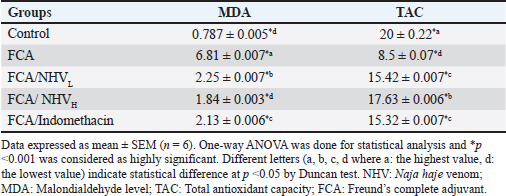
Table 3. Effect of (NHV) and indomethacin on the serum MDA and TAC levels in FCA-treated rats.
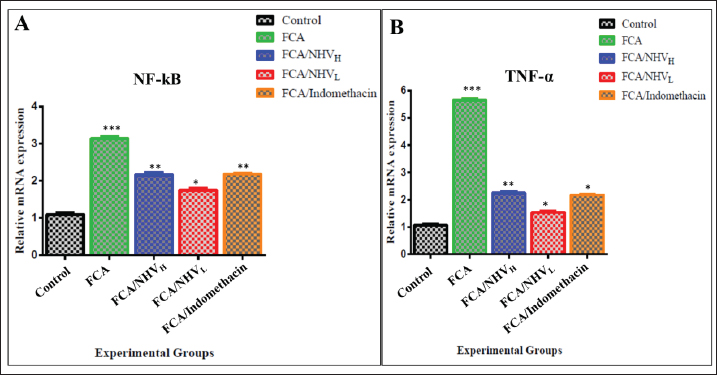
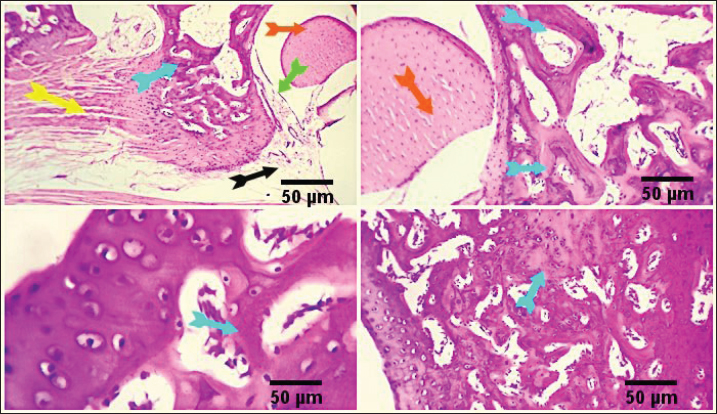
By decreasing MDA and raising TAC, NHV reduces oxidative stressThe MDA was calculated in order to assess the amount of reactive oxygen species (ROS). When compared to the FCA-induced group with arthritic symptoms, the control group displayed the lowest MDA levels. The MDA levels in the FCA/NHVL and FCA/NHVH groups showed significant (p < 0.001) lower values within the same range as the indomethacin group as compared to the FCA group. In this instance, the NHVH group outperformed the FCA arthritic group in terms of MDA levels, which were considerably lower (p < 0.001) (Table 3). On the other hand, as an indirect indicator of the production of O2 and other antioxidant species, we measured TAC. Table 2 indicates that the NHVH group had higher TAC activity than the FCA group (p < 0.001), whereas the FCA group showed the lowest value of TAC when compared to the control group. At the genetic level, NHV injection reduces the inflammatory response caused by FCAAt the genetic level, the FCA group’s nuclear factor kappa B (NF-kB) mRNA expression was higher than that of the control group, and this effect was dose-dependently eliminated following NHV injection (Fig. 1A). Similarly, the mean fold of TNF-α expression was significantly (p < 0.001) up-regulated upon FCA induction. NHV injection and indomethacin, however, significantly (p < 0.001) down-regulate TNF-α mRNA expression. Oddly, NHV outperformed indomethacin in a dose-dependent manner (Fig. 1B). NHV might reconstruct the joint elements destroyed at the histological level by FCAH&E-stained slices from the rat femur bone’s epiphyseal-joint region revealed an active covering cartilaginous cap made of dividing chondrocytes, which appeared singly or in pairs within lacunae in the control group. This cap covered a dense network of cancellous bone trabeculae that normally anastomose and contain osteocytes and bone lamellae within their lacunae. It was noted that the trabeculae’s smooth, regular, continuous endosteal surface was covered with osteoprogenitor and osteoblast cells. Large numbers of hemopoietic cells were seen in the bone marrow gaps between the trabeculae (Fig. 2). On the other hand, the FCA group showed signs of cartilage degradation, including chondrocyte necrosis and degeneration, sometimes accompanied by cystic development. Thickening of the trabeculae, osteopenia, osteoporosis, and increased inflammatory cell infiltration in the bone marrow—which occasionally infiltrates the interstitial tissue—were all evident in the underlying bone tissue. Osteoclastic cell responses were also observed. A noticeable inflammatory reaction was seen in the articular capsule, tendon, and synovial membrane (tenosynovitis). Neutrophils, macrophages, plasma cells, and lymphocytes were the main types of inflammatory cells. Aggregates of inflammatory cells were visible in the soft tissue surrounding the injured joint (Fig. 3).
Fig. 1. Expression of mRNA evaluated by qRT-PCR for inflammatory related genes indicated the up-regulation of NF-kB (A) and TNF-α (B) in FCA group that were down regulated after (NHV) injection. The results from three dependent experiments are shown as the mean ± SEM. * p < 0.05,** p < 0.01, *** p < 0.001. qRT-PCR; Real-Time Quantitative Reverse Transcription Polymerase chain reaction. mRNA; Messenger Ribonucleic acid. NF-kB: Nuclear factor kappa B; TNF-α: Tumor necrosis factor alpha; FCA: Freund’s complete adjuvant; NHV: Naja haje venom.
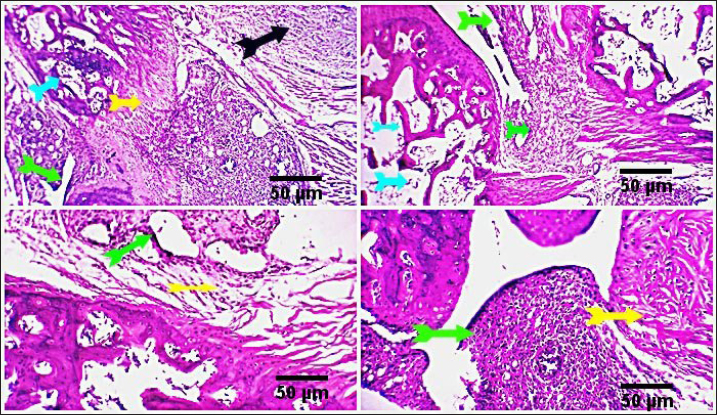
Fig. 2. Photo-micrograph from rat’s hip joint of the control group showing an active covering cartilaginous cap formed from actively dividing chondrocytes )orange arrow), cancellous bone trabeculae having bone lamellae and (light blue arrows). Smooth regular continuous endosteal surface of that trabeculae covered by osteoprogenitor and osteoblast (black arrow). Bone marrow spaces containing large number of hemopoietic cells green arrow). Scale bars 50 um. Conversely, the FCA/NHVL group displayed residual degenerative and necrotic alterations in the cartilaginous and bony tissues, accompanied by a mild to moderate inflammatory response in the soft tissue and synovial membrane (tenosynovitis). However, some of the cartilaginous-bony tissue and some synovial membranes were involved in the healing processes. The soft tissue surrounding the afflicted joints and the bone marrow both showed moderate infiltration of inflammatory cells (Fig. 4). FCA/NHVH unexpectedly revealed a moderately effective treatment efficiency. The cartilaginous cap in this instance demonstrated a regeneration process with slow ossification and remodeling, but there was also a noticeable osteoclastic reactivity, most likely to phagocytize the leftover osteonecrotic debris. In tandem with these sessions of treatment, the inflammatory responses subsided gradually, and the lesions in the synovial and soft tissues healed completely, leaving the synovial membrane looking normal (Fig. 5). Using a standard anti-arthritis drug of Indomethacin revealed marked healing effectiveness with a near complete resolving of the previously detected cartilaginous, bony, synovial membranes, and soft tissue lesions. Cartilaginous cap, cancellous bone trabeculae, intervening bone marrow, synovial membrane, tendons, capsule and other surrounding soft tissues were apparently normal (Fig. 6). DiscussionAbout 0.5%–1% of people worldwide suffer from RA, a systemic, progressive inflammatory disease marked by joint inflammation, discomfort, cartilage loss, and bone damage(Silman and Pearson, 2002). Nonsteroidal anti-inflammatory medications, corticosteroids, DMARDs, and monoclonal antibodies are being used to treat RA (Colmegna et al., 2012). Regretfully, these medications frequently come with a long list of negative side effects and are very expensive (Weir, 2002). Thus, the development of novel medications is required to treat RA. The current study’s result indicated a significant increase in the mean value of inflammatory cytokines, which may be related to T-lymphocyte and macrophage infiltration into the synovial joint. Various components of snake venom have been used for drug development in recent years (Pal et al., 2002; Koh and Kini, 2012; Chen et al., 2015). Alvarez-Flores et al. (2024) found that certain venoms can prompt macrophages to produce IL-10, indicating an anti-inflammatory effect. The levels of pro-inflammatory cytokines TNF-α, IL-6, and IL-10 were considerably decreased by cobra venom, suggesting that it may operate as a possible anti-arthritic agent by restoring the balance of cytokines. This outcome is consistent with the findings of Ghosh et al. (2016), which showed that NHV markedly decreased pro-inflammatory cytokine levels of TNF-α, IL-1β, and IL-17 (Ghosh et al., 2016). Additionally, Chen et al. (2015) confirmed that cobra toxin decreased serum levels of IL-6 and IL-10 in rats given adjuvant arthritis.
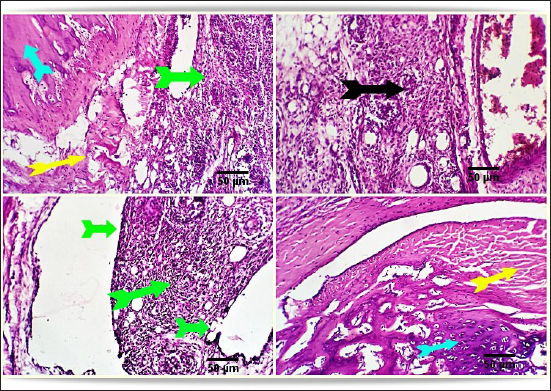
Fig. 3. Photomicrograph from rat’s hip joint of FCA group showing increased inflammatory cells infiltration (Light blue arrows). A marked inflammatory process in the synovial membrane and the adjacent articular capsule and tendon (tenosynovitis) (green and yellow arrow), aggregation of inflammatory cells (black arrow). Scale bars 50 um.
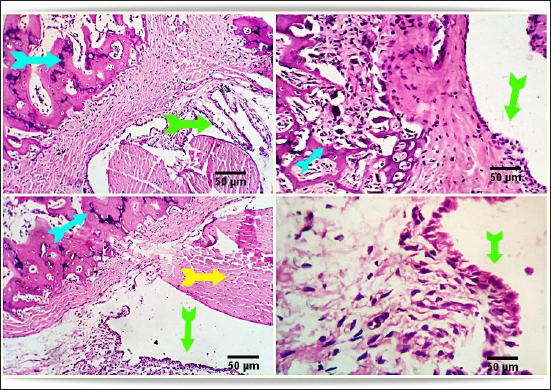
Fig. 4. Photomicrograph from rat’s hip joint of FCA/NHVLgroup showing remnants of cartilaginous and boney degenerative (light blue arrows), moderate inflammatory reaction in the synovial membrane and soft tissue (tenosynovitis) (green and yellow arrows), moderate inflammatory cells infiltration (black arrow). Scale bars 50 um.
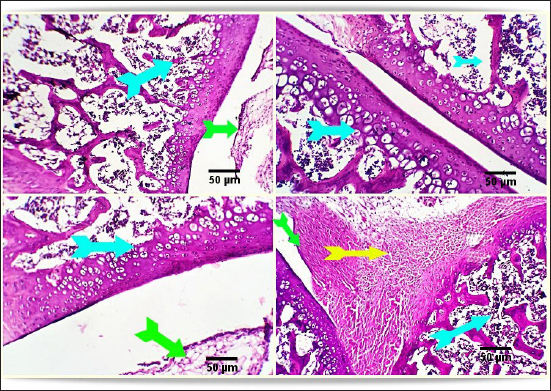
Fig. 5. Photomicrograph from rat’s hip joint of FCA/NHVH showing regenerative process with gradual ossification and remodeling process in the cartilaginous cap (light blue arrow). phagocytosis of the remaining osteonecrotic debris (yellow aarrow), synovial -soft tissue lesions are resolved with consequent complete healing and normally looking synovial membranous lining (green arrows). Scale bars 50 um.
Fig. 6. Photo-micrograph from rat’s hip joint of FCA/Indomethacin group showing apparently normal Cartilaginous cap of cancellous bone trabeculae (light blue arrows), synovial membrane (green arrows), tendons, capsule and other surrounding soft tissues (yellow arrow). Scale bars 50 um. Numerous human disorders are commonly linked to the dysregulation of gene expression, and alterations in gene expression patterns are also observed in normal tissues. This offers new targets for medication development and disease therapy (Fcollins, 2006; Li et al., 2009). The differential gene expression spectrum of synovial tissue in RA can be established, and the mechanism of the triptolide treatment effect on RA can be qualitatively and quantitatively analyzed at the gene level by examining the differences in the gene expression profile in synovial tissues of model animals with and without drug administration (Zou and Hu, 2019). Accordingly, the NF-κB family of transcription factors regulates mediators that promote inflammation (Clément, 2003). There is a strong correlation between NF-κB activation and increases in TNF-α and IL-1β mRNA levels. The TNF superfamily represents a group of cytokines that play an important role in inflammatory processes, immunity and cell proliferation, differentiation and apoptosis, and the formation of secondary lymphoid organs. This is in line with our results, which show decreases in NF-κB and TNF-α in treated groups (Ruan et al., 2013; Zou and Hu, 2019). During the acute phase of inflammation, macrophages emit TNF-α, whereas activated type 1 T helper (Th1) cells generate lymphotoxin alpha (IL-6 and TNF-α). According to Aggarwal et al. (2012) and Ruddle (2014), both have a strong proinflammatory effect and play a major role in tissue necrosis and cell apoptosis. In this case, the downregulated expression of inflammatory-related genes is suggestive of the NHV’s anti-inflammatory activity. The results showed a significant improvement in arthritis induced in the cerastes-treated group. Peroxidation damage is crucial to the development of lipopolysaccharide-induced injury. Additionally, according to Ruan et al. (2013), the TAC is a sign of O-2 and other oxidant species. ROS resulting from oxidative stress actually have a negative connection with TAC (Sharma et al., 1999; Ruan et al., 2013). In this instance, the TAC activity levels of the FCA/NHV groups were higher than those of the FCA group, suggesting that NHV has an antioxidant effect that is guaranteed by the downregulation of MDA levels. Additionally, Naja naja atra reduces reactive oxygen levels, which are created at inflammatory sites by polymorphonuclear leukocytes and macrophages through the activities of the enzymes xanthine oxidase and NADPH oxidase (Canton et al., 2021). The articular cartilage and synovium of rats treated with saline adjuvant-induced arthritis had significant hyperplasia, in contrast to normal control rats, whose articular cartilage displayed intact layers of flattened cells and smooth surfaces (Zhu et al., 2013). Additionally, Ghosh et al. (2016) showed that after receiving treatment with Bungarus fasciatus venom, the natural architecture of joints was greatly restored. The joint histological features in RA display increased degradation of the synovial membrane and decreased synovial space. Furthermore, in an inflammatory model generated by formaldehyde, it has been observed that denatured Naja naja atra venom reduces proinflammatory cytokines and attenuates paw edema (Zhu et al., 2013; Ghosh et al., 2016). Recently Metzger revealed the venoms from Elapidae and Viperidae can act as a TNF-α blocker by preventing the proliferation of FLSs, which in turn can have anti-inflammatory and analgesic effects on arthritis murine models (Metzger, 2021). Soliman, Kandeil et al. (2023) reported that groups injected with cerastesvenom showed significant improvement in arthritis. Taken together, these findings support the hypothesis that NHV can modify joint histological features back to normal following FCA. ConclusionThe current work aims to explore the anti-inflammatory and anti-oxidative properties of NHV at the biochemical, genetic, and histological levels in a model of arthritis in rats caused by FCA. In this case, FCA induced inflammation in the paw and ankle, which resulted in oxidative stress in the joint synovium tissue and histological alterations such as osteoporosis and inflammatory cell infiltration in the bone marrow. Here, the venom of Najahaje was able to repair the tissue architecture, greatly reduce joint inflammation, and boost the body’s antioxidant capacity—all of which helped to relieve the joint pain brought on by RA. Furthermore, a large dose of cobra venom produced outcomes that were almost identical to those of the commercial anti-arthritis medication indomethacin. Our combined research demonstrated the anti-inflammatory and anti-oxidative effects of denatured snake venom on RA. AcknowledgmentsNot applicable. Conflict of interestThe authors have no competing interests to declare that are relevant to the content of this article. FundingNo funds, grants, or other support was received. Authors’ contributionsThe study was conceptualized and designed by all authors, with the experiment being supervised by N.A.S., A.A.S., and the rest of the authors carried out the study’s practical components, conducted data analysis, and wrote and revised the manuscript. All authors read and appropriate the manuscript. Data availabilityAll datasets generated during this study are included in the article. ReferencesAbdel-Aal, A. and Abdel-Baset, A. 2010. Venom yield and toxicities of six Egyptian snakes with a description of a procedure for estimating the amount of venom ejected by a single snake bite. Sci. J. King Faisal Univ. Basic Appl. Sci. 11, 169–184. Aggarwal, B.B., Gupta, S.C. and Kim, J.H. 2012. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood, 119(3), 651–665. Alvarez-Flores, M.P., Batista, I.d.F.C., Boas, I.M.V., Bufalo, M.C., de Souza,J.G., Oliveira, D.S., Bonfá, G., Fernandes, C.M., Porto, R.M. and Lichtenstein, F. 2024. Snake and arthropod venoms: search for inflammatory activity in human cells involved in joint diseases. Toxicon 238, 107568. Bancroft, J.D., Layton, C. and Suvarna, S.K. 2013. Bancroft’s theory and practice of histological techniques, London, UK: Churchill Livingstone Elsevier. 49, 175. Boda, F., Banfai, K., Garai, K., Curticapean, A., Berta, L., Sipos, E. And Kvell, K. 2018. Effect of vipera ammodytes ammodytes snake venom on the human cytokine network. Toxins. 10(7), 259. Bottini, N. and Firestein, G. S.2013. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 9(1), 24–33. Cañas, C.A., Castaño-Valencia, S., Castro-Herrera, F., Cañas, F. and Tobón, G.J. 2021. Biomedical applications of snake venom: from basic science to autoimmunity and rheumatology. J. Transl. Autoimmunity 4, 100076. Canton, M., Sánchez-Rodríguez, R., Spera, I.,Viola, A. andCastegna, A. 2021. Reactive oxygen species in macrophages: sources and targets. Front. immunol. 12, 734229. Chen, C.X., Chen, J.Y., Kou, J.Q., Xu, Y.L., Wang, S.Z., Zhu, Q., Yang, L. and Qin, Z.H. 2015. Suppression of inflammation and arthritis by orally administrated cardiotoxin from Naja naja atra. Evid. Based Complement. Alternat. Med. 2015, 387094. Chillingworth, N.L. and Donaldson, L.F. 2003. Characterisation of a Freund’s complete adjuvant-induced model of chronic arthritis in mice. J. Neurosci. Methods 128(1), 45–52. Clément, M.-V. 2003.NF-κB activation: is it really redox regulated? Redox. Rep. 8(6), 323–324. Colmegna, I., Ohata, B.R. and Menard, H.A. 2012. Current understanding of rheumatoid arthritis therapy. Clin. Pharmacol. Ther. 91(4), 607–620. Fcollins, J. 2006. Gene chip analyses reveal differential genetic responses to iron deficiency in rat duodenum and jejunum. Biol. Res. 39(1), 25-37. Ferraz, C., Arrahman, A., Xie, C., Casewell, N., Lewis, R., Kool, J. and Cardoso, F. 2019. Multifunctional toxins in snake venoms and therapeutic implications: from pain to hemorrhage and necrosis. Front. Ecol. Evol. 7, 218. Ghosh, S., Saha, P.P., Dasgupta, S. and Gomes, A. 2016. Antinociceptive, anti-inflammatory and antiarthritic activities of Bungarus fasciatus venom in experimental animal models. Indian J. Exp. Biol. 54, 569–576. Han, Z., Boyle, D.L., Chang, L., Bennett, B., Karin, M., Yang, L., Manning, A.M. and Firestein, G.S. 2001.c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Investig. 108(1), 73–81. Ishikawa, T., Nishigaki, F., Miyata, S., Hirayama, Y., Minoura, K., Imanishi, J., Neya, M., Mizutani, T., Imamura, N.Y., Murai, H., Ohkubo, Y., Kagayama, A. and Mutoh, S. 2005. Prevention of progressive joint destruction in collagen-induced arthritis in rats by a novel matrix metalloproteinase inhibitor, FR255031. Br. J. Pharmacol. 144(1), 133–143. Koh, C.Y. and Kini, R.M. 2012. From snake venom toxins to therapeutics–cardiovascular examples. Toxicon. 59(4), 49–506. Kremers, H.M., Nicola, P., Crowson, C.S., O’Fallon, W.M. and Gabriel, S.E. 2004. Therapeutic strategies in rheumatoid arthritis over a 40-year period. J. Rheumatol. 31(12), 2366–2373. Layton, C. and Suvarna, K. 2013. Bancroft’s theory and practise of histological techniques, 7th ed, China. Li, Z., Wang, D., Hu, S., Wang, X., He, Y. and Zhang, Z. 2009. A gene chip study of” Jun Du Yan Bingzhi”on liver in a sepsis model of rat. Zhongguo wei Zhong Bing ji jiu yi xue=Chinese Critical Care Medicine=Zhongguo Weizhongbing Jijiuyixue. 21(1), 44–47. Metzger, M. 2021. Potential therapeutic effects of snake venom components on pain management in rheumatoid arthritis patients. University Honors Theses. Mohamed Abd El-Aziz, T., Soares, A.G. and Stockand, J.D. 2019. Snake venoms in drug discovery: valuable therapeutic tools for life saving. Toxins. 11(10), 564. Nassar, M.I. 2022. Snake venom and therapeutic potential. In Snake venom and ecology. Eds., Shah, M.M., Sharif, U., Buhari, T.R. and Imam, T.S. IntechOpen, London, UK, pp: 34. Newbould, B. 1963. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br. J. Pharmacol. 21(1), 127–136. Oliveira, A.L., Viegas, M.F., da Silva, S.L., Soares, A.M., Ramos, M.J. and Fernandes, P.A. 2022. The chemistry of snake venom and its medicinal potential. Nat. Rev. Chem. 6(7), 451–469. Pal, S.K., Gomes, A., Dasgupta, S.C. and Gomes, A. 2002. Snake venom as therapeutic agents: from toxin to drug development. Indian J. Exp. Biol. 40(12), 1353–1358. Preuss, H.G., Jarrell, S.T., Scheckenbach, R., Lieberman, S. and Anderson, R.A. 1998. Comparative effects of chromium, vanadium and gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J. Am. Coll. Nutr. 17(2), 116–123. Rhodes, A. 2013. 4—Fixation of tissues. Bancroft’s Theory and practice of histological techniques, 7th ed. Oxford, UK: Churchill Livingstone, pp: 69–93. Ruan, Y., Yao, L., Zhang, B., Zhang, S. and Guo, J. 2013. Anti-inflammatory effects of Neurotoxin-Nna, a peptide separated from the venom of Naja naja atra. BMC Complement Altern. Med. 13, 1–5. Ruddle, N.H. 2014. Lymphotoxin and TNF: how it all began—a tribute to the travelers. Cytokine Growth Factor Rev. 25(2), 83–89. Sharma, R.K., Pasqualotto, F.F., Nelson, D.R., Thomas, A.J., Jr. and Agarwal, A. 1999. The reactive oxygen species—total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum. Reprod. Open 14(11), 2801–2807. Silman, A.J. and Pearson, J.E. 2002. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 4(Suppl 3), S265–S272. Snekhalatha, U., Anburajan, M., Venkatraman, B. and Menaka, M. 2013. Evaluation of complete Freund’s adjuvant-induced arthritis in a Wistar rat model. Z. Rheumatol. 72(4), 375–382. Soliman, N.S.,Kandeil, M.A. and Khalaf, M.M. 2023. Cerastes snake venom as a promising approach in the management of complete Freund’s adjuvant-induced rheumatoid arthritis in rats: Involvement of RANKL and JAK/STAT pathway. J. Ethnopharmacol. 314, 116577. Tran, C.N., Lundy, S.K., White, P.T., Endres, J.L., Motyl, C.D., Gupta, R., Wilke, C.M., Shelden, E.A., Chung, K.C. and Urquhart, A.G. 2007. Molecular interactions between T cells and fibroblast-like synoviocytes: role of membrane tumor necrosis factor-α on cytokine-activated T cells. Am. J. Pathol. 171(5), 1588–1598. Weir, M.R. 2002. Renal effects of nonselective NSAIDs and coxibs. Cleve. Clin. J. Med. 69(Suppl 1), Si53–58. Zhu, K.-Z., Liu, Y.-L., Gu, J.-H. and Qin, Z.-H. 2013. Antinociceptive and anti-inflammatory effects of orally administrated denatured naja naja atra venom on murine rheumatoid arthritis models. Evid. Based Complement Alternat. Med. 2013, 616241. Zou, Y. and Hu, W. 2019. Investigation of gene expression profiles in a rat adjuvant arthritis model suggests an effective role of triptolide via PI3K-AKT signaling. Exp. Ther. Med. 17(5), 3999–4006. | ||
| How to Cite this Article |
| Pubmed Style Soliman NA, Shalaby AA, Farag MR, Kamal S, Alashqar SMA, Ammar MA, Mawed SA. Ameliorative effect of Naja haje venom against arthritic influence of Freund’s complete adjuvant FCA in paw and ankle of male rats. Open Vet. J.. 2024; 14(11): 2866-2876. doi:10.5455/OVJ.2024.v14.i11.15 Web Style Soliman NA, Shalaby AA, Farag MR, Kamal S, Alashqar SMA, Ammar MA, Mawed SA. Ameliorative effect of Naja haje venom against arthritic influence of Freund’s complete adjuvant FCA in paw and ankle of male rats. https://www.openveterinaryjournal.com/?mno=214271 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i11.15 AMA (American Medical Association) Style Soliman NA, Shalaby AA, Farag MR, Kamal S, Alashqar SMA, Ammar MA, Mawed SA. Ameliorative effect of Naja haje venom against arthritic influence of Freund’s complete adjuvant FCA in paw and ankle of male rats. Open Vet. J.. 2024; 14(11): 2866-2876. doi:10.5455/OVJ.2024.v14.i11.15 Vancouver/ICMJE Style Soliman NA, Shalaby AA, Farag MR, Kamal S, Alashqar SMA, Ammar MA, Mawed SA. Ameliorative effect of Naja haje venom against arthritic influence of Freund’s complete adjuvant FCA in paw and ankle of male rats. Open Vet. J.. (2024), [cited January 25, 2026]; 14(11): 2866-2876. doi:10.5455/OVJ.2024.v14.i11.15 Harvard Style Soliman, N. A., Shalaby, . A. A., Farag, . M. R., Kamal, . S., Alashqar, . S. M. A., Ammar, . M. A. & Mawed, . S. A. (2024) Ameliorative effect of Naja haje venom against arthritic influence of Freund’s complete adjuvant FCA in paw and ankle of male rats. Open Vet. J., 14 (11), 2866-2876. doi:10.5455/OVJ.2024.v14.i11.15 Turabian Style Soliman, Nabil A., Amr A. Shalaby, Mayada R. Farag, Shimaa Kamal, Sara M. Abdelkarem Alashqar, Mohamed Ahmed Ammar, and Suzan Attia Mawed. 2024. Ameliorative effect of Naja haje venom against arthritic influence of Freund’s complete adjuvant FCA in paw and ankle of male rats. Open Veterinary Journal, 14 (11), 2866-2876. doi:10.5455/OVJ.2024.v14.i11.15 Chicago Style Soliman, Nabil A., Amr A. Shalaby, Mayada R. Farag, Shimaa Kamal, Sara M. Abdelkarem Alashqar, Mohamed Ahmed Ammar, and Suzan Attia Mawed. "Ameliorative effect of Naja haje venom against arthritic influence of Freund’s complete adjuvant FCA in paw and ankle of male rats." Open Veterinary Journal 14 (2024), 2866-2876. doi:10.5455/OVJ.2024.v14.i11.15 MLA (The Modern Language Association) Style Soliman, Nabil A., Amr A. Shalaby, Mayada R. Farag, Shimaa Kamal, Sara M. Abdelkarem Alashqar, Mohamed Ahmed Ammar, and Suzan Attia Mawed. "Ameliorative effect of Naja haje venom against arthritic influence of Freund’s complete adjuvant FCA in paw and ankle of male rats." Open Veterinary Journal 14.11 (2024), 2866-2876. Print. doi:10.5455/OVJ.2024.v14.i11.15 APA (American Psychological Association) Style Soliman, N. A., Shalaby, . A. A., Farag, . M. R., Kamal, . S., Alashqar, . S. M. A., Ammar, . M. A. & Mawed, . S. A. (2024) Ameliorative effect of Naja haje venom against arthritic influence of Freund’s complete adjuvant FCA in paw and ankle of male rats. Open Veterinary Journal, 14 (11), 2866-2876. doi:10.5455/OVJ.2024.v14.i11.15 |