| Research Article | ||
Open Vet. J.. 2025; 15(5): 2230-2237 Open Veterinary Journal, (2025), Vol. 15(5): 2230-2237 Research Article Assessment of the environmental transmission dynamics of Escherichia coli-producing ESBL in wet market broiler chickens: A high-burden food safety concernKuntaman Kuntaman1*, Masfufatun Masfufatun2, Freshinta Jellia Wibisono3, Rosantia Sarassari4, Wahyu Setyorini5, Putu Oky Ari Tania6 and Toshiro Shirakawa71Department of Medical Microbiology, Faculty of Medicine, Universitas Wijaya Kusuma Surabaya, Surabaya, Indonesia 2Department of Biochemistry, Faculty of Medicine, Universitas Wijaya Kusuma Surabaya, Surabaya, Indonesia 3Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Wijaya Kusuma Surabaya, Surabaya, Indonesia 4Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Cibinong, Indonesia 5Institute of Tropical Disease, Universitas Airlangga, Surabaya, Indonesia 6Department of Biomedical and Biomoleculer Research, Faculty of Medicine, Universitas Wijaya Kusuma Surabaya, Surabaya, Indonesia 7Department of Organ Therapeutics, Faculty of Medicine, Kobe University, Kobe, Japan *Corresponding Author: Kuntaman Kuntaman. Department of Medical Microbiology, Faculty of Medicine, Universitas Wijaya Kusuma Surabaya, Indonesia. Email: kuntaman [at] uwks.ac.id Submitted: 05/03/2025 Revised: 20/04/2025 Accepted: 26/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
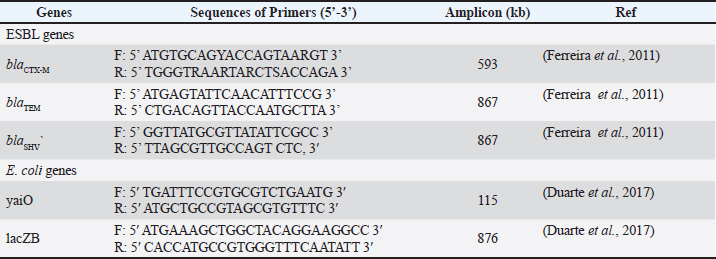
ABSTRACTBackground: Foodborne pathogens, particularly antibiotic-resistant strains, pose a significant threat to public health globally. Objective: To explore the emergence and spread of extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli in the food chain, focusing on broiler chickens in wet markets. Materials and Methods: Specimens were inoculated into TBX medium supplemented with cefotaxime 4 ug/ml for selective isolation of ESBL-producing E. coli. Phenotypic and genotypic characterization of the isolates was performed, including species detection using PCR for E. coli identification with specific primers targeting the gene and a double diffusion synergy test for ESBL identification. Results: A total of 262/305 (86%) samples were confirmed positive for ESBL-producing E. coli from broiler chickens and free-range chickens. blaCTX-M were mostly identified among ESBL-producing E. coli from broiler chickens (91%) or free-range chicken (100%), followed by blaTEM 20% and 23%, respectively. There were no identified blaSHV genes. Among 50 ESBL producers, E. coli (31; 17.4% from broiler chickens and 19; 22.6% from free-range chicken) were identified with a co-incidence of blaCTX-M and blaTEM. Conclusion: There is an increased prevalence of ESBL-producing E. coli in environmentally contaminated foods. Hygiene and antibiotic use regulations for poultry farms should be improved. ESBL genes among chickens are mainly blaCTX-M (94%), followed by blaTEM (21%), which has a lower incidence; no blaSHV was detected. Keywords: Antimicrobial resistance, Chicken, Escherichia coli, ESBL, Food chain, Infectious disease. IntroductionFoodborne pathogens, particularly antibiotic-resistant strains, pose significant threats to public health globally. One pressing concern is the emergence and dissemination of extended-spectrum beta-lactamase (ESBL)-producing E. coli in the food chain, with broiler chickens in wet markets being a key reservoir (Tellez et al., 2012; Eger et al., 2022). The wet market environment, characterized by poor hygiene and cross-contamination, facilitates the amplification and transmission of multidrug-resistant bacteria between poultry, vendors, and consumers. Broiler chicken production in the United States, the largest producer and second-largest exporter of broiler chicken meat in the world, is increasingly moving toward antibiotic-free programs, which has led to an increase in morbidity and mortality within broiler chicken farms due to pathogens like E. coli and Clostridium perfringens (Fancher et al., 2020). The dissemination of antibiotic resistance genes, such as those encoding ESBLs and plasmid-mediated AmpC beta-lactamases (pAmpC), poses a substantial threat in hospital effluents and urban rivers as a potential route for the spread of ESBL-producing bacteria and their respective genes into the natural environment, further exacerbating this public health crisis (Walia et al., 2016; Song et al., 2020). Table 1. Primer sets for detection of Escherichia coli and ESBL genes in broiler and free-range chickens.
Addressing this complex issue requires a multifaceted approach, including the development of novel vaccines, prebiotics, probiotics, and improved housing management strategies. It is important to mitigate the impact of these pathogens on broiler chicken health and productivity while also implementing robust surveillance and control measures to limit the environmental transmission of antibiotic-resistant bacteria in wet markets and beyond. The spread of ESBL-producing bacteria from broiler chickens in wet markets to humans is a significant food safety concern that requires urgent attention and action from public health authorities, the poultry industry, and the scientific community. The prevalence of ESBL-producing microorganisms in hospital effluents and urban rivers further complicates the issue, highlighting the need for a comprehensive One Health approach to address these complex challenges (Gay et al., 2023; Primeau et al., 2023). This study aimed to elucidate the environmental transmission dynamics of ESBL-producing E. coli in the wet market broiler chickens and free-range chicken ecosystems and explore potential interventions to mitigate this critical food safety concern. Materials and MethodsThis study was conducted in the Surabaya wet market in East Java, Indonesia, a region predicted to have a high prevalence of ESBL-producing E. coli in broiler chickens. A total of 200 broiler chickens and 105 free-range chicken samples were collected from four different wet market stalls over 4 months, from March 18th, 2024 to June 4th, 2024. Samples were obtained from the cecum of chickens. The specimens were inoculated into TBX medium supplemented with cefotaxime (CTX) 4 ug/ml for selective isolation of ESBL-producing E. coli (Freire et al., 2023). Phenotypic and genotypic characterization of the isolates was performed, including species detection using PCR for E. coli identification with specific gene targeting primers (Zimoń et al., 2024) and a DDST (Double diffusion synergy test) for ESBL identification (Kim et al., 2021). We used four antibiotics for the DDST test, CTX, CAZ, Ceftriaxone (CRO), and Amoxycillin-Clavulanic acid (AMC), as in our previous study (Aydin et al., 2024). Escherichia coli was identified using a PCR-based method, as previously described (Ferreira et al., 2011). Two pairs of primers were used (Table 1). ESBL gene detection (blaCTX-M, blaTEM, and blaSHV) was performed by PCR as previously described (Ma et al., 2005; Rahman et al., 2012). The primer set is shown in Table 1. The data were analyzed using Chi-Square to compare the incidence of ESBL-E coli between broiler chickens and free-range chickens, whereas the trend of the dynamic incidence of ESBL-E. coli in the two groups of samples was analyzed using the Phi score. Ethical approvalSamples were collected from the chicken seller and butcher with the consent of those who agreed to participate and met the criteria of the Research Ethics Committee of Universitas Wijaya Kusuma Surabaya (approval number 95/SLE/FK/UWKS/2024). ResultsSample collectionBetween March 18th and June 4th 2024, the location and number of wet markets in Surabaya were identified, and the region was then plotted to collect random samples. Fifteen markets were selected to represent all the chicken-selling and slaughtering areas in Surabaya. As a precaution to guarantee that the samples were sterile and free of contamination, sampling was always performed at dawn (04.00–05.00 am). All the chicken sellers and butchers of both broiler and free-range chickens were supplied from traditional farming by chicken farm companies in East and Central Java. The slaughtering process at all sampling locations used standard procedures involving groundwater (dig-water) in every process. The slaughtering was done on the floor without a mat. Table 2. Prevalence and association between ESBL-producing E coli among broiler chickens, free-range chickens, and the Surabaya Area, distributor’s location p-value.
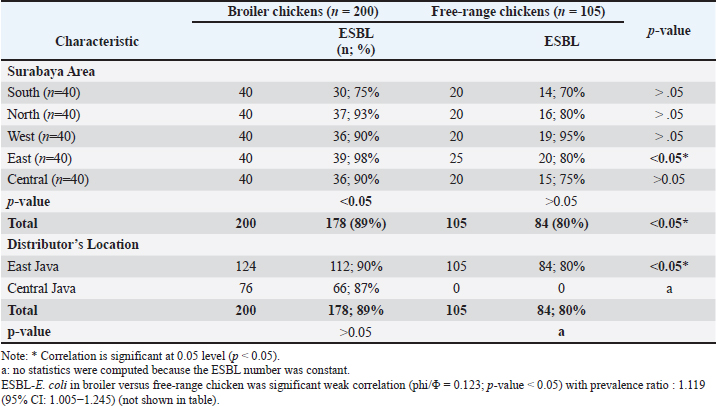
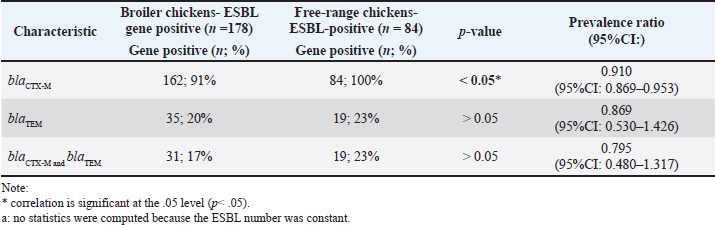
Prevalence of ESBL-producing E. coli from chicken cecum in SurabayaA total of 305 samples were isolated and collected from broiler and free-range chickens in 5 areas of wet markets in Surabaya, East Java, Indonesia. The sampling locations were chicken sellers and butchers who purchased chicken from distributors in East Java and Central Java. The isolates were identified as E. coli using conventional biochemical methods and were confirmed using PCR. ESBL detection was confirmed by the DDST using four antibiotics, CTX, CAZ, CRO, and Amoxicilin + Clavularic acid (AMC), as in our previous study (Aydin et al., 2024). ESBL prevalence varies between locations. A total of 262 out of 305 (86%) of the samples were confirmed positive for ESBL-producing E. coli from broiler chickens and free-range chickens (Table 2). blaCTX-M was the most commonly identified ESBL-producing E. coli from broiler chickens (91%) or free-range chickens (100%), then blaTEM 20% and 23%, respectively. There were no identified blaSHV genes. Fifty ESB-producing E. coli (31; 17.4% from broiler chickens and 19; 22.6% from free-range chickens) were identified co-existence with both blaCTX-M and blaTEM. Five different areas represent regions in Surabaya. The southern area is represented by Dukuh Kupang and Simo Rukun, and the northern area is represented by Pabean and Kapas Krampung. The western and eastern areas were Darmo Permai, Asem Rowo, and Bulak, while Keputran, Pucang Sewu, and Gubeng represented the city center. The prevalence of ESBL-E coli was weakly associated (phi/Φ=0.123; p value < 0.05) with prevalence ratio: 1.119 (95% CI: 1.005−1.245) between broiler versus free-range chickens. Table 3. Distribution of ESBL genes in ESBL-producing E. coli chickens
The distribution of ESBL-producing E. coli between broiler and free-range chickens was significantly different in total samples (p < 0.05; Table 2) and specifically in the East Surabaya area (p < 0.05; Table 2). There were no significant differences in the prevalence of ESBL-producing E. coli in broiler chickens between distributors from East Java and Central Java provinces (p > 0.05; Table 2). The highest prevalence of ESBL-producing E. coli was in the east area of Surabaya (90.7%), and the lowest in the southern area (73.3%). Among 178 (89%) of 200 broiler chickens and 84 (80%) of 105 free-range chickens were inhabited by ESBL-producing E. coli. It was significantly different (p < 0.05; Table 2). The prevalence of ESBL genes was mostly blaCTX-M 162 (91%) among 178 ESBL-E coli isolates from broiler chickens and all 84 (100%) isolates from free-range chickens, p < 0.05. The blaTEM was the next gene discovered, with 35 (20%) in broiler chickens and 19 (23%) in free-range chickens (p> .05). There were no identified blaSHV genes (Table 3). DiscussionPersistence of Escherichia coli ESBL in the food chainOur study showed that approximately 86% of broiler and free-range chickens sold in wet markets in Surabaya carried ESBL-producing E. coli. The presence of antibiotic-resistant bacteria, particularly ESBL-producing E. coli, in the food chain poses significant public health concerns. Numerous studies have reported the detection of ESBL-producing organisms in various food sources, including fruits, vegetables, and animal products such as chicken meat (Shrestha et al., 2017; Bushen et al., 2021). This highlights the potential for these resistant bacteria to be transmitted to humans by consuming contaminated food, for example, when handling raw meat or cross-contamination in the kitchen. The extensive and indiscriminate use of antibiotics in animal farming, particularly in low- and middle-income countries, is a significant contributing factor to the emergence and spread of drug-resistant bacteria. The contamination of chicken meat with ESBL-producing E. coli in wet markets is a particularly worrying issue, as these retail establishments often lack proper hygiene and food safety practices, potentially facilitating the cross-contamination of pathogens between different food sources (Shrestha et al., 2017; Bushen et al., 2021; Junaid et al., 2021). A study conducted in India found that Gram-negative bacteria, including E. coli, Citrobacter spp., and Salmonella spp., were isolated from chicken meat samples, with a significant proportion exhibiting multidrug resistance and ESBL production (Shrestha et al., 2017). Similarly, a study in China reported that over 96.7% of E. coli isolates from sick chickens were resistant to at least one of the tested antimicrobial agents, and 87.2% were multidrug-resistant (Chen et al., 2016). Investigating the spread of ESBL E. coli from chicken farms to the food chainThe rapid emergence and dissemination of ESBL-producing E. coli has become a global public health concern. Poultry farms have been identified as a significant reservoir of these antibiotic-resistant bacteria, and the extensive use of antimicrobials in chicken production contributes to the selection and proliferation of ESBL-producing strains (Chong et al., 2018). These resistant bacteria can then potentially spread to humans through the food chain, posing a significant threat to public health. The Agriculture Ministry’s Decree 14/Permentan/PK 350/5/2017 prohibits antimicrobial agents as animal feed additives, but compliance with this decree remains uncertain. Parawidnyaningsih et al., in their research on food sold on the roadside, especially food without proper coverings, found that 20-70% of food groups were colonized by ESBL E. coli (Parawidnyaningsih et al., 2023). The higher prevalence of ESBL E coli in chickens, higher than 80% of samples, is a source of environmental ESBL-producing bacterial transmission. Several studies have highlighted the prevalence of ESBL-producing E. coli in chicken farms and their ability to contaminate various food products. The extensive use of antibiotics in chicken farming has been a significant driver of this phenomenon, leading to the evolution and dissemination of multidrug-resistant strains (Bushen et al., 2021). Some studies have found ESBL-producing organisms in fruits, vegetables, and other food items, indicating that contamination can occur during the production, processing, or distribution stages (Bushen et al., 2021). Therefore, hygiene practices in markets and the regulation of antibiotic use in poultry farming need to be improved. The impact of ESBL-producing bacteria on clinical practice is also a growing concern. These strains are often resistant to a wide range of antibiotics, including commonly used cephalosporins, limiting treatment options and leading to increased morbidity and mortality in infected individuals (Chong et al., 2018; Stanley et al., 2018). Moreover, the genetic diversity and multidrug resistance patterns of ESBL-producing E. coli isolates underscore the complex epidemiology and the need for comprehensive surveillance and control measures.
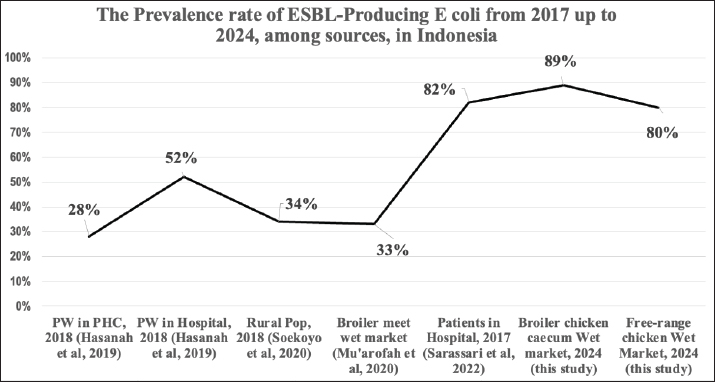
Fig. 1. The prevalence trend of ESBL-producing E. coli in studies from 2017 to 2024. Note: PW=Pregnant women, rectal swab; PHC=Primary Health care Center; Pop=Population; The Y-axis is the prevalence (%) of ESBL-producing E. coli; X-axis is the sample source and year of publication. Our study showed that the pattern of ESBL genes among E. coli isolates from broiler chickens and free-range chickens sold in Surabaya was mostly blaCTX-M (91% and 100%), respectively, whereas blaTEM had a lower burden, 20% in broiler chickens and 23% in free-range chickens. These figures show a close spread of ESBL E. coli among them. One exciting result concerns the pattern of ESBL E. coli found between distributors in two areas of broiler chicken distribution: East Java and Central Java; there were no significant differences in the prevalence of ESBL E. coli between these two areas. This means that the East and Central Java centers have similar protocols for managing chicken farming, mainly a culture of antibiotic usage. This topic needs to be explored further in the future. The environmental circulation of ESBL-producing bacteria in our AMR surveillance study groups, especially in inpatient studies, showed that the carrier rate of ESBL-producing E. coli in the gut flora was high and is increasing. The prevalence rate of ESBL-producing E. coli among pregnant women in primary health care centers in 2018 was 28%, and that among hospitalized pregnant women in Dr. Soetomo Hospital, Surabaya, was 51% (Hasanah et al., 2019). In 2017, the study in post-neonatal aged primary health center patients had a prevalence rate of 37% (Happy et al., 2020), while a rural population in 2018 had a prevalence rate of 34% (Soekoyo et al., 2020). Broiler chicken meat sold in the wet market in 2018 had a prevalence rate of 33% (Mu’arofah et al., 2020), whereas our current study in 2024 of broiler chickens and free-range chickens cecum found an 89% and 80% prevalence rate, respectively. Although the specimens were not collected serially, the prevalence of ESBL-producing E. coli in the community is fast increasing (Fig. 1). According to recent findings from several countries, there is a growing trend of E. coli contamination in chicken meat and visceral organs. Multiple factors affect the recovery rate of E. coli, including isolation techniques, sample source, host-related factors (chicken breeds, health status, and/or vaccination), feed, water, husbandry conditions, ambient temperature, litter management, and miscellaneous environmental factors, including trade between regions (Chen at al., 2016; Junaid et al., 2021). According to Puspandari et al (Sunarno et al., 2023), ESBL-producing E. coli was detected in 100 samples from pregnant women, with a prevalence of approximately 40%. This result is quite high considering that pregnant women constitute a vulnerable group in the community. Isolates from humans and broiler chickens in Ghana were closely related, as evidenced by the genetic links of ESBL-E. coli between human and poultry populations (Falgenhauer et al., 2019). A study in Egypt identified similarities in the phenotypes and genotypes of ESBL-producing E. coli found in poultry and human samples, indicating the possibility of zoonotic transmission. The contamination of ESBL-E. coli in broiler and free-range chickens samples was 178 (89%) and 84 (80%), respectively, a surprisingly high result. This outcome was comparable to an analysis of 99 out of 115 broiler chicken samples (86.1%) positive for E. coli resistant to beta-lactam antibiotics in East Java Province (Faridah et al., 2023). The present study identified 162 samples (81%) with blaCTX-M genes and 35 (17.5%) positive for blaTEM genes in broiler chickens, whereas 84 (80%) samples were detected for blaCTX-M and 19 (18%) for blaTEM genes in free-range chickens. However, none of the broiler chicken and free-range chicken samples were detected to have the blaSHV gene. blaCTX-M was the predominant gene in this study, similar to research in Israel, Spain, New York, the United Kingdom, and South Africa, which showed that the blaCTX-M gene was the most frequently detected gene in ESBL-E. coli samples (Bajpai et al., 2017). Globally, CTX-M enzymes are becoming a severe public health concern linked to epidemics worldwide. An antibiotic resistance study in Syria identified K. pneumoniae and E. coli as the two most common ESBL producers (Al-Subol and Youssef, 2015). The high prevalence of ESBL-E. coli could be attributed to the widespread empirical use of antibiotic medications due to their affordability and ease of administration as oral antibiotics. ConclusionThere is an increasing prevalence of ESBL-producing E. coli in environmentally contaminated foods. The monitoring of food chains, which is a major issue in public health, should be given a higher priority, especially for chicken, which is the main food consumed by most countries worldwide. Our study (2024) showed that over 80% of the chicken sold in the wet market in Surabaya, East Java, Indonesia is contaminated with ESBL-producing E. coli and multiple drug-resistant bacteria. This was a follow-up to the 2017–2018 study. Broiler chickens were more contaminated than free-range chickens. The ESBL genes identified from the chickens were mainly blaCTX-M (94%), followed by blaTEM (21%); no blaSHV was detected. Antibiotic usage in chicken feed would appear to be the likely source driving this phenomenon. Our study suggests that hygiene practices and antibiotic regulations for poultry farming should be improved. AcknowledgmentsThe authors thank the Institute of Tropical Disease, Universitas Airlangga, for supporting this research. The authors also thank Nadila, Sucia, and all the technicians for their essential support. FundingThis study was supported by Riset dan Inovasi untuk Indonesia Maju (RIIM) Batch 4 of the National Research and Innovation Agency (BRIN) and the Indonesia Endowment Fund for Education Agency (LPDP); Contract: 157/IV/KS/11/2023 & 265/MoA/LPPM/UWKS/XI/2023. Authors contributionsFor research articles with several authors, a short paragraph describing their individual contributions is required. The following statements should be used: “Conseptualization, K.K. and M.M.; software, P.O.A.; validation, K.K., M.M.; formal analysis, K.K.; investigation, K.K., M.M.; resources, F.J.W., W.S., P.O.A.; data curation, R.S., K.K.; writing-original draft preparation, K.K.; writing-review and editing, K.K., M.M., T.S.; visualization, K.K., M.M.; supervision, K.K.; project administration, M.M., P.O.A., W.S; funding acquisition, K.K. All authors have read and agreed to the published version of the manuscript. Conflicts of interestThe authors declare no conflict of interest. ReferencesAl-Subol, I. and Youssef, N. 2015. Prevalence of CTX-M, TEM, and SHV Beta-Lactamases in clinical isolates of Escherichia coli and Klebsiella pneumoniae isolated from Aleppo university hospitals, Aleppo, Syria. Arch. Clin. Infect. Dis. 10, 1–6 Arizandy, R.Y., Utomo, B. and Kuntaman, K. 2020. Detection of Extended Spectrum Β-Lactamase (ESBL) gene patterns of enterobacteriaceae in broiler chicken meat sold in traditional markets in the east Surabaya. SJIK 9(1), 12–19. Aydin, A. Suleymanoglu, A.A., Abdramanov, A., Paulsen, P. and Dumem, E. 2024. Detection of Extended-Spectrum ß-Lactamase-producing Escherichia coli with biofilm formation from chicken meat in Istanbul. Foods. 13(7), 1–14 Bajpai, T., Pandey, M., Varma, M. and Bhatambere, G.S. 2017. Prevalence of TEM, SHV, and CTX-M Beta-Lactamase genes in urinary isolates from a tertiary care hospital. Avicenna J. Med. 7(1), 12–16 Bushen, A., Tekalign, E. and Abayneh, M. 2021. Drug- and multidrug-resistance patterns of enterobacteriaceae isolated from the droppings of healthy chickens on a poultry farm in southwest Ethiopia. Infect. Drug Resist. 14, 2051–2058. Chen, C.J., Chen, C.C. and Ding, S.J. 2016. Effectiveness of hypochlorous acid to reduce the biofilms on titanium alloy surfaces in vitro. Int. J. Mol. Sci. 17(7), 1–12 Chong, Y., Shimoda, S. and Shimono, N. 2018. Current epidemiology, genetic evolution, and clinical impact of Extended-Spectrum β-Lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. 61, 185–188. Eger, E., Domke, M., Heiden, S.E., Padits, M., Balau, V., Huxdorf, C., Zimmermann, D., Homeier-Bachmann, T. and Schaufler, K. 2022. Highly virulent and multidrug-resistant Escherichia coli Sequence type 58 from a sausage in Germany. Antibiotics 11(8), 1–14 Falgenhauer, L., Imirzalioglu, C., Oppong, K., Akenten, C.W., Hogan B, Krumkamp, R., Poppert, S., Levermann, V., Schwengers, O., Sarpong, N., Owusu-Dabo, E., May, J. and Eibach, D. 2019. Detection and characterization of ESBL-Producing Escherichia coli from humans and poultry in Ghana. Front. Microbiol. 10, 1–8. Fancher, C.A., Zhang, L., Kiess, A.S, Adhikari, P.A., Dinh, T.T.N. and Sukumaran, A.T. 2020. Avian pathogenic and: challenges in no antibiotics ever broiler chicken production and potential solutions. Microorganisms 8(10), 1–27. Faridah, H.D., Wibisono, F.M., Wibisono, F.J., Nisa, N., Fatimah, F., Effendi, M.H., Ugbo, E.N., Khairullah, A.R., Kurniawan, S.C. and Silaen, O.S.M. 2023. Prevalence of the blaCTX-M and blaTEM genes among extended-spectrum beta lactamase-producing Escherichia coli isolated from broiler chickens in Indonesia. J. Vet. Res. 67(2), 179–186. Ferreira, C.M., Ferreira, W.A., Almeida, N.C., Naveca, F.G. and Barbosa, Md. 2021. E Extended-spectrum beta-lactamase-producing bacteria isolated from hematologic patients in Manaus, State of Amazonas, Brazil. Braz. J. Microbiol. 42(3), 1076–1084. Freire, S., Grilo, T., Rodrigues, B., Oliveir, R., Esteves, C., Marques, A., Poirel, L. and Aires-de-Sousa, M. 2023. ESBL- and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae among bivalves from Portuguese shellfish production areas. Microorganisms 11(415), 1–11. Gay, N., Rabenandrasana, M.A.N., Panandiniaina, H.P., Rakotoninidrina, M.F., Ramahatafandry, I,T., Enouf, V., Roger, F., Collard, J.M., Cardinale, E., Rieux, A. and Loire, E. 2023. One health compartment analysis of ESBL-producing Escherichia coli reveals multiple transmission events in a rural area of Madagascar. J. Antimicrob. Chemother. 78(8), 1848–1858. Happy, T.A., Setyarini, W., Ranuh, I.G.M.R.G. and Kuntaman, K. 2020. Prevalence ESBL producing Escherichia coli among children in Indonesia. Indian J. Public Health Res. Dev. 11(05), 792–797. Hasanah, M., Setyarini, W., Parathon, H. and Kuntaman, K. 2019. The Prevalence of Extended-Spectrum Beta-Lactamase (ESBL) producing gut flora among pregnant Women’s peripartum in community and hospital, Indonesia. Indian J. Public Health Res. Dev. 10(12). 1839–1844. Junaid, K., Ejaz, H., Asim, I., Younas, S., Yasmeen, H., Abdalla, A.E., Abosalif, K.O.A. Alameen, A.A.M., Ahmad, N., Bukhari, S.N.A. and Rehman, A. 2021. Heavy metal tolerance trends in extended-spectrum β-lactamase encoding strains recovered from food samples. Int. J. Environ. Res. Public Health. 18(9), 1–12. Kim, H., Kim, Y.A., Seo, Y.H. and Lee, K. 2021. Prevalence and molecular epidemiology of Extended-Spectrum-β-Lactamase (ESBL)-producing Escherichia coli from multiple sectors of poultry industry in Korea. Antibiotics 10(9), 1–7. Ma, L, Chang, F.Y., Fung, C.P., Chen, T.L., Lin, J.C., Lu, P.L., Huang, L.Y., Chang, J.C. and Siu, L.K. 2005. Variety of TEM-, SHV-, and CTX-M-type beta-lactamases present in recent clinical isolates of Escherichia coli, Klebsiella pneumoniae, and Enterobacter cloacae from Taiwan. Microb. Drug Resist. 11(1), 31–39. Madaruaga-Venegas, F., Fernandez-Soto, R., Duarte, L.F., Suarez, N., Delgadillo, D., Jara, J.A., Fernandez-Ramires, R., Urzua, B. and Molina-Berrios, A. 2017. Characterization of a novel antibiofilm effect of Nitric Oxide-releasing Aspirin (NCX-4040) on Candida albicans isolates from denture stomatitis patients. PLoS One. 12(5), 1–15. Parawidnyaningsih, P.T.A., Masfufatun., Listyawati, A.F., Kuntaman, K. and Sudibya, A. 2023. Detection of Enterobacteriaceae lactose fermenter bacteria roducing Extended Spectrum Beta-Lactamase (ESBL) in food samples at Surabaya. Bioma 12(2), 38–45. Primeau, C.A., Bharat, A., Janecko, N., Carson, C.A., Mulvey, M., Reid-Smit R., McEwen, S., McWhirter, J.E. and Parmley, E.J. 2023. Integrated surveillance of Extended-Spectrum Beta-Lactamase (ESBL)-producing Salmonella and Escherichia coli from humans and animal species aised for Huhman consumption in Canada from 2012 to 2017. Epidemiol. Infect. 151(14), 1–11. Puspandari, N., Sunarno, S., Febrianti, T., Febriyana, D., Saraswati, R.D., Rooslamiati, I., Amaloa, N., Nursofiah, S., Hartoyo, Y., Herna, H., Mursinah, M., Muna, F., Aini, N., Risniati, Y., Dhewantara, P.W., Allamanda, P., Wicaksana, D.N.,Sukoco, R.,Efadeswarni, Nelwan, W.J. and Matheu, J. 2021. Extended Spectrum Beta-Lactamase-producing Escherichia coli surveillance in the human, food chain, and environment sectors: a tricycle project (pilot) in Indonesia. One Health. 13, 1–8. Rahman, N.M.W., Lutfor, A.B., Jhora, S.T., Yasmin, M. and Haq, J.A. 2012. Detection of the CTX-M gene in Extended Spectrum Beta Lactamase (ESBL) producing Escherichia coli and Klebsiella species of different hospitals. Bangladesh J. Med. Microbiol. 4(2), 28–31. Shrestha, A., Bajracharya, A.M., Subedi, H., Turha, R.S.., Kafle, S., Sharma, S., Neupane, S. and Chaudhary, D.K. 2017. Multi-drug resistance and extended spectrum beta actamase producing gram negative bacteria from chicken meat in Bharatpur Metropolitan, Nepal. BMC Res. Notes. 10(1), 1–5. Soekoyo, A.R., Sulistiawati, S., Setyorini, W. and Kuntaman, K. 2020. Epidemiological patterns and risk factor of ESBL (Extended Spectrum Β-Lactamase) producing enterobacteriaceae in the gut bacterial flora of dairy cows and people surrounding in rural area, Indonesia. IJTID 8(3), 144–151. Song, H.J., Moon, D.C., Mechesso, A.F., Kang, H,Y., Kim, M.H., Choi, J.H., Kim, S.J., Yoon, S.S. and Lim, S.K. 2020. Resistance profiling and molecular characterization of extended-spectrum/ plasmid-mediated Ampc β-Lactamase-producing Escherichia coli isolated from healthy broiler chicken in South Korea. Microorganisms 8(9), 1–17. Stanley, I.J., Kajumbula, H., Bazira, J., Kansiime, C., Rwego, I.B. and Asiimwe, B.B. 2018. Multidrug resistance among Escherichia coli and Klebsiella pneumoniae carried in the gut of out-patients from pastoralist communities of Kasese district, Uganda. PLoS One 13(7), 1–12. Sunarno, S., Puspandari, N., Fitriana, F., Nikmah, U.A., Idrus, H.H. and Panjaitan, N.S.D. 2023. Extended-Spectrum Beta Lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae in Indonesia and southeast Asian countries: GLASS Data 2018. AIMS Microbiol. 9, 218–227 Tellez, G., Pixley, C., Wolfenden, R.E., Layton, S.L. and Hargis, B.M. 2012. Probiotic/ direct fed microbials for Salmonella control in poultry. Food Res. Int. 45(2), 628–633. Walia, S., Murleedharm, C., band, J., Kanwar, M. and Kumar, A. 2016. Quantitation of antibiotic resistance genes pollution in hospital waste water effluent and Urban Clinton River Water, Michigan, USA. Curr. Med. Res. Pract. 6(4), 149–151. Zimoń, B., Psujek, M., Matczak, J., Guziński, A., Wójcik, E. and Dastych, J. 2024. Novel multiplex-PCR test for Escherichia coli detection. Microbiol. Spectr. 12, 1–16. | ||
| How to Cite this Article |
| Pubmed Style Kuntaman K, Masfufatun M, Wibisono FJ, Sarassari R, Setyarini W, Tania POA, Shirakawa T. Assessment of the environmental transmission dynamics of Escherichia coli-producing ESBL in wet market broiler chickens: A high-burden food safety concern. Open Vet. J.. 2025; 15(5): 2230-2237. doi:10.5455/OVJ.2025.v15.i5.40 Web Style Kuntaman K, Masfufatun M, Wibisono FJ, Sarassari R, Setyarini W, Tania POA, Shirakawa T. Assessment of the environmental transmission dynamics of Escherichia coli-producing ESBL in wet market broiler chickens: A high-burden food safety concern. https://www.openveterinaryjournal.com/?mno=245867 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.40 AMA (American Medical Association) Style Kuntaman K, Masfufatun M, Wibisono FJ, Sarassari R, Setyarini W, Tania POA, Shirakawa T. Assessment of the environmental transmission dynamics of Escherichia coli-producing ESBL in wet market broiler chickens: A high-burden food safety concern. Open Vet. J.. 2025; 15(5): 2230-2237. doi:10.5455/OVJ.2025.v15.i5.40 Vancouver/ICMJE Style Kuntaman K, Masfufatun M, Wibisono FJ, Sarassari R, Setyarini W, Tania POA, Shirakawa T. Assessment of the environmental transmission dynamics of Escherichia coli-producing ESBL in wet market broiler chickens: A high-burden food safety concern. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2230-2237. doi:10.5455/OVJ.2025.v15.i5.40 Harvard Style Kuntaman, K., Masfufatun, . M., Wibisono, . F. J., Sarassari, . R., Setyarini, . W., Tania, . P. O. A. & Shirakawa, . T. (2025) Assessment of the environmental transmission dynamics of Escherichia coli-producing ESBL in wet market broiler chickens: A high-burden food safety concern. Open Vet. J., 15 (5), 2230-2237. doi:10.5455/OVJ.2025.v15.i5.40 Turabian Style Kuntaman, Kuntaman, Masfufatun Masfufatun, Freshinta Jellia Wibisono, Rosantia Sarassari, Wahyu Setyarini, Putu Oky Ari Tania, and Toshiro Shirakawa. 2025. Assessment of the environmental transmission dynamics of Escherichia coli-producing ESBL in wet market broiler chickens: A high-burden food safety concern. Open Veterinary Journal, 15 (5), 2230-2237. doi:10.5455/OVJ.2025.v15.i5.40 Chicago Style Kuntaman, Kuntaman, Masfufatun Masfufatun, Freshinta Jellia Wibisono, Rosantia Sarassari, Wahyu Setyarini, Putu Oky Ari Tania, and Toshiro Shirakawa. "Assessment of the environmental transmission dynamics of Escherichia coli-producing ESBL in wet market broiler chickens: A high-burden food safety concern." Open Veterinary Journal 15 (2025), 2230-2237. doi:10.5455/OVJ.2025.v15.i5.40 MLA (The Modern Language Association) Style Kuntaman, Kuntaman, Masfufatun Masfufatun, Freshinta Jellia Wibisono, Rosantia Sarassari, Wahyu Setyarini, Putu Oky Ari Tania, and Toshiro Shirakawa. "Assessment of the environmental transmission dynamics of Escherichia coli-producing ESBL in wet market broiler chickens: A high-burden food safety concern." Open Veterinary Journal 15.5 (2025), 2230-2237. Print. doi:10.5455/OVJ.2025.v15.i5.40 APA (American Psychological Association) Style Kuntaman, K., Masfufatun, . M., Wibisono, . F. J., Sarassari, . R., Setyarini, . W., Tania, . P. O. A. & Shirakawa, . T. (2025) Assessment of the environmental transmission dynamics of Escherichia coli-producing ESBL in wet market broiler chickens: A high-burden food safety concern. Open Veterinary Journal, 15 (5), 2230-2237. doi:10.5455/OVJ.2025.v15.i5.40 |