| Review Article | ||
Open Vet. J.. 2022; 12(2): 242-249 Open Veterinary Journal, (2022), Vol. 12(2): 242–249 Review Article Changes in the gut microbiome and colic in horses: Are they causes or consequences?Felipe Lara1, Rodrigo Castro2 and Pamela Thomson3*1Unidad de Medicina y Cirugía Equina, Hospital Clínico Veterinario, Escuela de Medicina Veterinaria, Facultad de Ciencias de la Vida, Universidad Andrés Bello, Santiago, Chile 2Escuela de Medicina Veterinaria, Facultad de Recursos Naturales y Medicina Veterinaria, Universidad Santo Tomás, Chile 3Laboratorio Microbiología Clínica y Microbioma, Escuela de Medicina Veterinaria, Facultad de Ciencias de la Vida, Universidad Andrés Bello, Santiago, Chile *Corresponding Author: Pamela Thomson. Laboratorio Microbiología Clínica y Microbioma, Escuela de Medicina Veterinaria, Facultad de Ciencias de la Vida, Universidad Andrés Bello, Santiago, Chile. Email: pamela.thomson [at] unab.cl Submitted: 06/02/2022 Accepted: 22/03/2022 Published: 06/04/2022 © 2022 Open Veterinary Journal
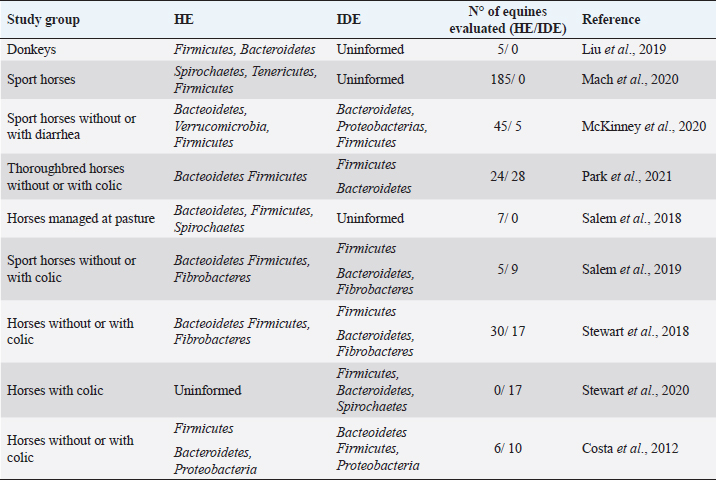
AbstractThe gut microbiome is a compound for millions of microorganisms that coexist in an organized way and contribute to the fermentation of different types of indigestible fibers by the small intestine. Some techniques, such as the massive sequencing of the 16S ribosomal RNA gene, have made it possible to obtain information about the abundance and functionality of the microorganisms that compose the equine gut microbiome and the interaction with their environment. Recent studies have identified the change in the composition of the intestinal microbiome during and after a colic episode, although is not clear if it is a cause or a consequence. The objective of this review was to elucidate whether there is a direct relationship between the changes that occur in the gut microbiome and colic in the equine. A systematized search in Embase, Web of Science, and PubMed was realized. Although there is good evidence that horses with colic have a change in their gut microbiome, it is not fully understood whether these changes are causes or effects. It is necessary to delve into this topic, considering studying larger population sizes. In addition, it would be of great value to previously know the normal intestinal microbiome of a group of healthy horses, which in the future could develop an episode of colic, to compare the before and after in the same individual. Keywords: Colic, Equine, Gut, Microbiome. IntroductionIn equine veterinary medicine, colic is the general term to refer to visceral abdominal pain and acute abdominal disease (Worku et al., 2017; Marshall and Blikslager, 2019). This pain is considered a symptom that may be reflecting various disorders of different magnitudes and severity at the digestive level, such as a simple obstruction, strangulating obstruction, or severe inflammatory processes, among others (Cook and Hassel, 2014; Marshall and Blikslager, 2019; Bowden et al., 2020). Despite the treatments and surgical interventions to address these episodes, there is a high morbidity and mortality associated with economic losses for the owners (Tinker et al., 1997; Proudman et al., 2002; Cook and Hassel, 2014; Tannahill et al., 2019). Therefore, identifying and understanding the variables that act as predisposing or determining factors have been key to suggesting management practices that reduce the prevalence of colic (Scantlebury et al., 2015; Blikslager, 2019; Kaufman et al., 2020; Vitale et al., 2020). In a 2019 report, these risk factors were evaluated and classified into three large areas: horse-related factors, management-related factors, and environment-related factors. It was concluded that the most common risk factors are related to diet, either due to changes in the concentrate or hay consumed by the animal and/or due to a decrease in water intake (Mehdi and Mohammad., 2006; Curtis et al., 2019). The equine gut microbiome is a complex and diverse ecosystem composed primarily of bacteria, which cohabit with viruses, archaea, and fungi (Rodriguez et al., 2015; Arroyo et al., 2018; Nishida et al., 2018). As in other mammals, the intestinal microbiome of horses generates a complex and symbiotic environment, which in addition to its role in the digestion of food, fulfills metabolic functions of protection against pathogens and stimulation of the immune system, directly or indirectly affecting different physiological processes of the host (Icaza-Chavez, 2013; Shirazi-Beechey, 2008; Barko et al., 2018). Modifications in the composition of the microbiome, known as dysbiosis (Blackmore et al., 2013; Venable et al., 2016; Salem et al., 2019), have been related to diets high in concentrate, poor quality forage, confinement, stress, fasting, and age, among other causes. Interestingly, in recent years, some authors have associated alterations in the gut microbiome of horses with colic, since changes in the abundance of certain bacterial groups that have an important role in maintaining a healthy gastrointestinal tract have been observed (Costa and Weese, 2018; Blikslager, 2019; Salem et al., 2018, 2019). The objective of this review was to elucidate whether there is a direct relationship between the changes that occur in the intestinal microbiome and colic in equines. Gut microbiomeThe equine gastrointestinal tract is made up of a complex consortium of microorganisms called the microbiome, which plays an important role in both health and disease. It is estimated that there are about 1015 microorganisms that compose it, with about 160 described species (Stewart et al., 2018, 2019). The equine gut microbiome colonization process begins at birth, where the neonates acquire the first microorganisms occurring through the birth canal, for the direct contact with the mother and later for the contact with the environment (Barko et al., 2018). In humans and horses, it has been shown that the assembly and establishment of the gut microbiome are essential for proper growth and development (Mueller et al., 2015; Matsuki et al., 2016). The first colonizers are facultative anaerobic bacteria, such as Enterobacteriales, Enterococcus, and Streptococcus; these bacteria will consume the oxygen present in the intestinal lumen, creating an anaerobic environment that will allow the growth of strict anaerobes, such as Bacteroides and Clostridium (Dougal et al., 2014; Lindenberg et al., 2019). The equine gut microbiome is totally different in the first months of life from that existing or established in adulthood, which related to the intake of colostrum, milk, coprophagia, and/or the incorporation of fiber in the diet (Costa and Weese, 2018). In a study conducted in foals and their mothers, a marked stabilization of the gut microbiome was observed around day 50 postpartum, which was associated with the gradual introduction of the adult diet well before weaning. The early microbiome was characterized by bacteria previously identified in the milk and birth canal, while from day 50 there was a significant increase in certain anaerobic species capable of fermenting fiber (Lindenberg et al., 2019). Apparently, the diversity of species decreases with age, determined by a study concluding that horses between 19 and 28 years have lower bacterial richness compared to younger horses (Dougal et al., 2014). Venable et al. (2016) support the idea that the aging process in the horse generates a reduction in microbial diversity. Another study conducted on fecal samples found that the group made up of old horses had a higher relative abundance of the Proteobacteria phylum compared to the control group (Morrison et al., 2018). The use of culture-independent identification methods, such as the massive sequencing of 16S ribosomal RNA gene, allowed obtaining information about the abundance and functionality of the microorganisms that compose the equine gut microbiome and the interaction with their environment (Costa and Weese, 2012). Most of these studies have been carried out from stool samples, being representative of the microbiome present in the large intestine, mainly in the caecum (Venable et al., 2016; Salem et al., 2019). In healthy adult horses, the microbial profiles that have been found are mostly represented by bacteria from the phyla Firmicutes (44%) and Bacteroidetes (38%), followed by Spirochaetes (2.5%), Fibrobacter (2.0%), Proteobacteria (0.8%), and Tenericutes (0.5%) (Stewart et al., 2019). On the contrary, other studies indicate that the most abundant phylum in healthy horses is Bacteroidetes, followed by Firmicutes, Verrucomicrobia, and methanogenic archeas (Costa and Weese, 2018; Kauter et al., 2019; Stewart et al., 2019). However, some studies have concluded that bacteria belonging to the phylum Firmicutes outnumber Bacteroidetes in a ratio of 4:1, in contrast to current data reporting that this ratio is 1: 1. Indeed, these differences may be influenced by factors such as geographic location, race, diet, intestinal segment, methodological differences in the DNA extraction technique, and even the sequencing platform used (Costa et al., 2012; Dougal et al., 2013; Ericsson et al., 2016; Kauter et al., 2019) (Table 1). Additionally, some reports indicate that the microbiome is diverse and multifunctional throughout the gastrointestinal tract, which is related to the physiology of each segment. In general, the richness and diversity are much greater in the large intestine than in the stomach and small intestine. At the edge level, the Proteobacteria is dominant in the ileum, while Firmicutes and Bacteroides are abundant in the large intestine (Dougal et al., 2013; Abreu and Taga, 2016; Liu et al., 2019). One of the main activities of the gut microbiome is to contribute to the fermentation of various types of indigestible fibers by the small intestine. Horses, as nonruminant herbivores, depend largely on the colon and the caecum to carry out fermentation processes where fibrolytic bacteria, mainly anaerobic, are capable of depolymerizing pectin, starch, cellulose, and hemicellulose to their respective monosaccharides through the Embden Meyerhoff pathway (Jassmin and Andrews, 2009). This activity results in the release of short-chain fatty acids (SCFA), such as butyrate, propionate, and acetate (Tazoe et al., 2008; Carpuso, 2016; Cerqueira et al., 2020). Butyrate has been shown to affect the growth and differentiation of colonocytes and has a positive role in reducing inflammation at the intestinal level (Zimmerman et al., 2012; Louis et al., 2014; Chung et al., 2017, Thomson et al., 2018; Chen et al., 2020). In addition, it has a direct impact on the development and activity of the immune system, considering that the intestinal epithelium represents a stable barrier between the lymphoid tissue and the microbiome itself (Janssen and Kersten, 2015; Tamburini et al., 2016; Thaiss et al., 2016). It is important to understand that microbial interactions are key to shaping the composition of the gut microbiome, where competition for nutrients is commonly observed (Rakoff-Nahoum et al., 2014; Abreu and Taga, 2016; Sung et al., 2017). These interactions are dependent on the chemical nature of dietary compounds and could influence health, according to the different SCFA profiles that each individual manifests (Medina et al., 2017; Adamberg et al., 2018; Thomson et al., 2018). The diet is the main factor that contributes to gut microbiome modification in horses (Willing et al., 2009; Daly et al., 2012; Steelman et al., 2012; Destrez et al., 2015, 2019; Hansen et al., 2015; Harlow et al., 2015; Venable et al., 2017; Barko et al., 2018). Table 1. Relative abundance of the main intestinal bacterial phyla reported for healthy equines (HE) and intestinal disease equines (IDE).
Alterations of gut microbiomeIn human and animal patients, it has been observed that losses of microbiome homeostasis, or dysbiosis, are associated with some diseases, which are explained by the imbalance and loss of some bacterial species (Núñez et al., 2021). An example of this is inflammatory bowel disease, which contains Crohn’s disease and ulcerative colitis, in addition to metabolic syndrome and irritable bowel disease and chronic relapsing Clostridium difficile infection (Icaza-Chavez, 2013; Zhou et al., 2017; Costa and Wesse, 2018; Barko et al., 2018). One of the strongest links between irritable bowel syndrome (IBS) and intestinal microbiome was indicated in a study where fecal matter was transplanted from human subjects with IBS to healthy mice, observing that the mice showed changes associated with the disease, such as impaired intestinal motility, increased permeability of the intestine, and visceral hypersensitivity (Crouzet et al., 2013). Currently, there is little research evaluating the relationship between the intestinal microbiome and colic in horses; one of them was carried out by Venable et al. (2013), where they analyzed fecal samples obtained from thoroughbred horses during the colic episode, and compared them with those obtained 30 and 90 days later; they reported an increase in the relative abundance of Clostridium phytofermentans and Bacteroides sp. in all samples obtained during colic, compared to samples collected at 30 and 90 days after the episode. Other studies mention that colicky horses have a significantly higher percentage of Clostridioides difficile compared to healthy horses (Niwa et al., 2013; Schoster et al., 2019). In this regard, Nomura et al. (2020) relate the administration of antimicrobials in hospitalized horses with diarrhea caused by C. difficile, where a clear increase in this species has been observed. Undoubtedly, among all the external factors that generate changes in the gut microbiome, the administration of antibiotics could have the most serious consequences (Jalanka-Tuovinen et al., 2011) since it favors the rapid proliferation of pathogenic bacteria (Britton and Young, 2014; Theriot et al., 2016). In equine clinical practice, the oral administration of antibiotics can induce dysbiosis, which often results in diarrhea and colitis (Barr et al., 2012; Diab et al., 2013; Liepman, 2015). Indirectly, the administration of the fecal transplantation from a healthy donor horse to a patient with diarrhea has made it possible to generate a causal relationship between the composition of the microbiome and disease (McKinney et al., 2020, 2021) situation that has been reported in human patients (Coignard et al., 2006; Kachrimanidou and Tsintarakis 2020; Núñez et al., 2021; Thomson et al., 2021). It has been shown that the diversity of species decreases in horses with colic, showing significant differences in 46 of 304 identified operational taxonomic units. Likewise, they point out that the relative abundance of bacteria such as Prevotella, Clostridia, and Lachnospiraceae is reduced while Christenellaceae, Streptococcus, and Sphaerochaeta increase in horses with colic compared to elective cases (Stewart et al., 2019). The decrease in the relative abundance of members of the family Lachnospiraceae producers of butyrate bacteria has been previously mentioned (Costa et al., 2012; Weese et al., 2015). Interestingly, Stewart et al. (2019) found an association between Firmicutes: Proteobacteria ratio and the appearance of colic with an odds ratio of 0.95, indicating that the higher this proportion, the lower the probability of developing a colic episode, information you can use to predict and prevent these types of events. However, other study could not find this relationship and found an overgrowth of lactic acid-producing bacteria, such as Lachnospiraceae and Lactobacillaceae, and a decrease of methanogenic bacteria (Park et al., 2021). Weese et al. (2015) found in postpartum mare that developed colic a higher relative abundance in Proteobacteria 1–76 days (mean 17.5) before the onset of the colic episode (Table 1). However, neither significant differences in community membership, structure, diversity, or evenness were found. Likewise, several studies carried out in humans support the idea of considering the Proteobacteria phylum as a marker of the alteration of the intestinal microbiome, and an increase in the relative abundance of this group of microorganisms associated with various gastrointestinal diseases has been observed (Van Nood et al., 2013; Cammarota et al., 2017; Chamorro et al., 2021). Epidemiological studies have established an increased risk of colic associated with sudden changes in diet, which result from an alteration of fermentation patterns and a metabolic disorder within 4–6 days after a dietary change (Shirazi-Beechey, 2008; Salem et al., 2018; Stewart et al., 2019). Some authors explain that when horses are exposed to the sudden incorporation of high levels of soluble carbohydrates in the diet, the normal enzymatic degradation in the small intestine is overloaded and large amounts of starch pass to the large intestine, where the microbial fermentation occurs (Beuvink and Spolestra, 1992; Hansen et al., 2015; Warzecha et al., 2017). As a result of this fermentation, an accumulation of lactic acid and gas occurs by bacteria such as Lactobacillus and Streptococcus (Milinovich, 2006, 2010; Hansen et al., 2015), causing a rapid drop in the pH of the hindgut and consequently a loss in fibrolytic bacteria. Using culture-independent techniques, it was possible to confirm a lower abundance of the Lachnospiraceae, Ruminococcaceae and Fibrobacter families (Dougal et al., 2013; Hansen et al., 2015; Julliand and Grimm, 2017; Warzecha et al., 2017). It is also known that grass-fed horses have a higher relative abundance of bacteria belonging to the Clostridiaceae, Eubacterium, and Spirochataceae families in the hindgut (Daly et al., 2001; Daly and Shirazi-Beechey, 2003). On the other hand, those fed grain show a decrease in the relative abundance of Fibrobacter (Blackmore et al., 2013; Hansen et al., 2015). In addition, pregnant mares are at greater risk to have colic because of changes in their feeding management during the peripartum period that produce significant alterations in the pH of the colon, which can cause variations in the hindgut microbiota, leading to a significant increase in the phylum Proteobacteria (Salem et al., 2019). ConclusionDifferent factors can alter the composition of the intestinal microbiome in equines, modifying the relative abundance of the main bacterial phyla. The bacterial community composition in horses with colic is different from healthy horses, characterized by a less diverse population and changes in the relative abundance of some phyla and bacteria species. In healthy animals, a predominance of the phyla Firmicutes and Bacteroidetes has been identified, while in horses with colic these phyla decrease and Proteobacteria increases. However, more studies are required to establish a causal relationship between these alterations and the presentation of colic in horses. AcknowledgmentsThe authors would like to thank the ANID PAI Project # 77190079. Conflicts of interestThe authors declare that they have no conflicts of interest. Authors’ contributionsFelipe Lara: conception of the study, wrote the first draft of the manuscript, and critically revised the manuscript. Rodrigo Castro: wrote the first draft of the manuscript and critically revised the manuscript. Pamela Thomson: conception and design of the study, wrote the first draft of the manuscript, critically revised the manuscript, and funding acquisition. ReferencesAbreu, N.A. and Taga, M.E. 2016. Decoding molecular interactions in microbial communities. FEMS Microbiol. Rev. 40, 648–663. Adamberg, K., Kolk, K., Jaagura, M., Vilu, R. and Adamberg, S. 2018. The composition and metabolism of faecal microbiota is specifically modulated by different dietary polysaccharides and mucin: an isothermal microcalorimetry study. Benef. Microbes. 9, 21–34. Arroyo, L.G., Gomez, D.E. and Martins, C. 2018. Equine duodenitis-proximal jejunitis: a review. Can. Vet. J. 59, 510–517. Barko, P.C., McMicheal, M.A., Swanson, K.S. and Williams, D.A. 2018. The gastrointestinal microbiome: a review. J. Vet. Intern. Med. 32, 9–25. Barr, B.S., Waldridge, B.M., Reed, S.M., Clark, C., Belgrave, R., Donecker, J.M. and Weigel, D.J. 2012. Antimicrobial-associated diarrhoea in three equine referral practices. Equine. Vet. J. 45, 154–158. Beuvink, J.M.W. and Spoelestra, S.F. 1992. Interactions between substrate, fermentation end-products, buffering systems and gas production upon fermentation of different carbohydrates by mixed rumen microorganisms in vitro. Appl. Microbiol. Biotechnol. 37, 505–509. Blackmore, T., Dugdale, A., Argo, C., Curtis, G., Pinloche, E., Harris, P., Worgan, H., Girdwood, S., Dougal, K., Newbold, C. and McEwan, N. 2013. Strong stability and host specific bacterial community in faeces of ponies. PLoS One 8, e75079. Blikslager, A.T. 2019. Colic prevention to avoid colic Surgery: a surgeon’s perspective. J. Equine Vet. Sci. 76, 1–5. Bowden, A., England, G.C.W., Brennan, M.L., Mair, T.S., Furness, W.A., Freeman, S.L. and Burford, J.H. 2020. Indicators of ‘critical’ outcomes in 941 horses seen ‘out-of-hours’ for colic. Vetrec. 187, 105881. Britton, R.A., Young VB. 2014. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology 146, 1547–1553. Cammarota G, Ianiro, G., Tilg, H., Rajilić-Stojanović, M., Kump, P., Satokari, R., Sokol, H., Arkkila, P., Pintus, C., Hart, A., Segal, J., Aloi, M., Masucci, L., Molinaro, A., Scaldaferri, F., Gasbarrini, G., Lopez-Sanroman, A., Link, A., De Groot, P., De Vos, W., Högenauer, C., Malfertheiner, P., Mattila, E., Milosavljević, T., Nieuwdorp, M., Sanguinetti, M., Simren, M. and Gasbarrini, A. 2017. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 66, 569–580. Carpuso, L. 2016. The intestinal microbiota. Recenti. Prog. Med. 107, 257–266. Cerqueira, F.M., Photenhauer, A.L., Pollet, R.M., Brown, H.A. and Koropatkin, N.M. 2020. Starch Digestion by Gut Bacteria: Crowdsourcing for Carbs. Trends Microbiol. 28, 95–108. Chamorro, N., Montero, D.A., Gallardo, P., Farfán, M., Contreras, M., De la Fuente, M., Dubois, K., Hermoso, M.A., Quera, R., Pizarro-Guajardo, M., Paredes-Sabja, D., Ginard, D., Rosselló-Móra, R. and Vidal, R. 2021. Landscapes and bacterial signatures of mucosa-associated intestinal microbiota in Chilean and Spanish patients with inflammatory bowel disease. Microb. Cell. 8, 223–238. Chen, D., Jin, D., Huang, S., Wu, J., Xu, M., Liu, T., Dong, W., Liu, X., Wang, S., Zhong, W., Liu, Y., Jiang, R., Piao, M., Wang, B. and Cao, H. 2020. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 469, 456–467. Chung, W.S., Meijerink, M., Zeuner, B., Holck, J., Louis, P., Meyer, A.S., Wells, J.M., Flint, H.J., and Duncan, S.H. 2017. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol. Ecol. 93(11); doi: 10.1093/femsec/fix127. Coignard, B., Barbut, F., Blanckaert, K., Thiolet, J.M., Poujol, I., Carbonne, A., Petit, J.C. and Desenclos, J.C.2006. Emergence of Clostridium difficile toxinotype III, PCR-ribotype 027-associated disease, France, 2006. Euro. Surveill. 11, E060914.1. Cook, V. and Hassel, D. 2014. Evaluation of the colic in horses: decision for referral. Vet. Clin. North Am. Equine Pract. 30, 383–398. Costa, M.C., Arroyo, L.G., Allen–Vercoe, E., Stampeli, H.R., Kim, P.T., Sturgeon, A. and Weese, J.S. 2012. Comparison of the fecal microbiota of healthy horses and horses with colitis by High throughput sequencing of the v3-v5 region of the 16S rRNA gene. PLoS One. 7, e41484. Costa, M.C. and Weese, J.S. 2012. The equine intestinal microbiome. Anim. Health Res. Rev. 13, 121–128. Costa, M.C. and Weese, J.S. 2018. Understanding the intestinal microbiome in health and disease. Vet. Clin. North Am. Equine Pract. 34, 1–12. Crouzet, L., Gaultier, E., Del’Homme, C., Cartier, C., Delmas, E., Dapoigny, M., Fioramonti, J. and Bernalier-Donadilleet, A. 2013. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol. Motil. 25, e272–e282. Curtis, L., Burford, J.H., England, G.C. and Freeman, S.L. 2019. Risk factors for acute abdominal pain (colic) in the adult horse: a scoping review of risk factors, and a systematic review of the effect of management-related changes. PLoS One 14, e0219307. Daly, K., Stewart, C.S., Flint. H.J. and Shirazi–Beechey, S.P. 2001. Bacterial diversity within the equine large intestine as revealed by molecular analysis of cloned 16S rRNA genes. FEMS Microbiol. Ecol. 38, 141–151. Daly, K. and Shirazi-Beechey, S.P. 2003. Design and evaluation of groupspecific oligonucleotide probes for quantitative analysis of intestinal ecosystems: their application to assessment of equine colonic microflora. FEMS Microbiol. Ecol. 44, 243–252. Daly, K., Proudman, C.J., Duncan, S.H., Flint HJ, Dyer J. and Shirazi-Beechey, S.P. 2012. Alterations in microbiota and fermentation products in equine large intestine in response to dietary variation and intestinal disease. Br. J. Nutr. 107, 989–995. Destrez A, Grimm P, Cézilly F, Julliand V. 2015. Changes of the hindgut microbiota due to high–starch diet can associate with behavioral stress response in horses. Physiol Behav 149, 159–164. Destrez, A., Grimm, P. and Julliand, V. 2019. Dietary-induced modulation of the hindgut microbiota is related to behavioral responses during stressful events in horses. Physiol. Behav. 202, 94–100. Diab, S.S., Songer, G. and Uzal, F.A. 2013. Clostridium difficile infection in horses: a review. Vet. Microbiol. 167, 42–49. Dougal, K., De la Fuente, G., Harris, P.A., Girdwood, S.E., Pinloche, E. and Newbold, C. 2013. Identification of a core Bacterial community within the large intestine of the horse. PLoS One 8, e77660. Dougal, K., De la Fuente, G., Harris, P.A., Girdwood, S.E., Pinloche, E., Geor, R.J., Nielsen, B.D., Schott, H.C., Elzinga, S. and Newbold, J. 2014. Characterisation of the faecal bacterial community in adult and elderly horses fed a high fibre, high oil or high starch diet using 454 pyrosequencing. PLoS One 9, e87424. Ericsson, A.C., Johnson, P.J., Lopes, M.A., Perry, S.C. and Lanter, H.R. 2016. A microbiological map of the Healthy equine gastrointestinal tract. PLoS One 11, e0166523. Hansen, N.C.K., Avershina, E., Mydland, L.T., Næsset, J.A., Austbø, D., Moen, B., Måge, I. and Rudi, K. 2015. High nutrient availability reduces the diversity and stability of the equine caecal microbiota. Microb. Ecol. Health Dis. 26, 27216. Harlow, B.E., Donley, T.M., Lawrence, L.M. and Flythe, M.D. 2015. Efect of starch source (corn, oats or wheat) and concentration on fermentation by equine faecal microbiota in vitro. J. Appl. Microbiol. 119, 1234–1244. Icaza-Chávez, M.E. 2013. Microbiota intestinal en la salud y la enfermedad. Revista de Gastroenterología de México 78, 240–248. Jalanka – Tuovinen, J., Salonen, A., Nikkila, J., Immonen, O., Kekkonen, R., Lahti, L., Palva, A. and Willem, M. 2011. Intestinal microbiota in Healthy adults: Temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS One 6, e23035. Janssen, A.W. and Kersten, S. 2015. The role of the gut microbiota in metabolic health. FASEB J. 29, 3111–3123. Jassmin, R.A.M. and Andrews, F.M. 2009. The Bacterial Community of the horse gastrointestinal tract and its relation to fermentative acidosis, laminitis, colic, and stomach ulcers. Vet. Clin. North Am. Equine Pract. 25, 199–215. Julliand, V. and Grimm, P. 2017. The impact of diet on the hindgut microbiome. J. Equine Vet. Sci. 52, 23–28. Kachrimanidou, M. and Tsintarakis, E. 2020. Insights into the Role of Human Gut Microbiota in Clostridioides difficile Infection. Microorganisms 8, 200. Kaufman, J.M., Nekouei, O., Doyle, A.J. and Biermann, N.M. 2020. Clinical findings, diagnoses, and outcomes of horses presented for colic to a referral hospital in Atlantic Canada (2000–2015). Can. Vet. J. 61, 281–288. Kauter, A., Epping, L., Semmler, T., Antao, E., Kannapin, D., Stoeckle, S.D., Gehlen, H., Lübke-Becker, A., Günther, S., Wieler, L.H., Walther, B. 2019. The gut microbiome of horses: current research on equine enteral microbiota and future perspectives. Anim. Microbiome. 1, 14. Liepman, R. 2015. Alterations in the fecal microbiome of Healthy horses in response to antibiotic treatment. Thesis, Ohio State University, Columbus, OH. Lindenberg, F., Krych, L., Kot, W., Fielden, J., Frøkiær, H., Van Galen, G., Nielsen, D.S., Hansen, A.K.2019. Development of the equine gut microbiota. Sci. Rep. 9, 14427. Liu, G., Bou, G., Su, S., Xing, J., Qu, H., Zhang, X., Wang, X., Zhao, Y. and Dugarjaviin, M. 2019. Microbial diversity within the digestive tract contents of Dezhou donkeys. PLoS One. 14, e0226186. Louis, P., Hold, G.L. and Flint, H.J. 2014. The gut microbiota, bacterial metabolites, and colorectal cancer. Nat. Rev. Microbiol. 12, 661–672. Mach, N., Ruet, A., Clark, A., Bars-Cortina, D., Ramayo-Caldas, Y., Crisci, E., Pennarun, S., Dhorne-Pollet, S., Foury, A., Moisan, M. and Lansade, L. 2020. Priming for welfare: gut microbiota is associated with equitation conditions and behavior in horse athletes. Sci Rep. 10, 8311 Marshall, J. and Blikslager, A. 2019. Colic: Diagnosis, surgical decision, preoperative management, and surgical Approaches to the abdomen. In Equine Surgery, Eds., Auer, J.A., Stick, J,A., Kummerle, J.M. and Prange, T. St. Louis, MO: Saunders Elsevier, pp: 521–528. Matsuki, T., Yahagi, K., Mori, H., Matsumoto, H., Hara, T. Tajima, S., Ogawa, E., Kodama, H., Yamamoto, K., Yamada, T., Matsumoto, S. and Kurokawa, K. 2016. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat. Commun. 7, 11939. McKinney, C.A., Oliveira, B.C.M., Bedenice, D., Paradis, M.R. and Mazan, M., 2020. The fecal microbiota of healthy donor horses and geriatric recipients undergoing fecal microbial transplantation for the treatment of diarrhea. PLoS One 15, e0230148. McKinney, C.A., Bedenice, D., Pacheco, A.P., Oliveira, B.C.M., Paradis, M.R., Mazan, M. and Widmer, G. 2021. Assessment of clinical and microbiota responses to fecal microbial transplantation in adult horses with diarrhea. PLoS One. 16, e0244381. Medina, D., Pinto, F., Ovalle, A., Thomson, P. and Garrido, D. 2017. Prebiotics Mediate Microbial Interactions in a Consortium of the Infant Gut Microbiome. Int. J. Mol. Sci. 18, 2095. Mehdi, S. and Mohammad, V. 2006. A farm – based prospective study of equine colic incidence and associated risk factors. J. Equine Vet. Sci. 26, 171–174. Milinovich, G.J., Trott, D.J., Burrell, P.C., Van Eps, A.W., Thoefner, M.B. Blackall, L.L., Al Jassim, R.A.M., Morton, J.M. and Pollitt, C.C. 2006. Changes in equine hindgut bacterial populations during oligofructose-induced laminitis. Environ. Microbiol. 8, 885–898. Milinovich, G.J., Klieve, A.V., Pollitt, C.C. and Trott, D.J. 2010. Microbial events in the hindgut during carbohydrate - induced equine laminitis. Vet. Clin. North Am. Equine Pract. 26, 79–94. Morrison, P.K., Newbold, C.J., Jones, E., Worgan, H.J., Grove-White, D.H., Dugdale, A.H., Barfoot, C., Harris, P.A. and McG Argo, C. 2018. The equine gastrointestinal microbiome: Impacts of age and obesity. Front. Microbiol. 9, e3017. Mueller, N.T., Bakacs, E., Combellick, J., Grigoryan, Z. and Dominguez-Bello, M.G. 2015. The infant microbiome development: mom matters. Trends Mol. Med. 21, 109–117. Nishida, A., Inoue, R., Inatomi, O., Bamba, S., Naito, Y. and Andoh, A. 2018. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 11, 1-10. Niwa, H., Kato, H., Hobo, S., Kinoshita, Y., Ueno, T., Katayama, Y., Hariu, K., Oku, K., Senoh, M., Kuroda, T. and Nakai, K. 2013. Postoperative Clostridium difficile infection with PCR ribotype 078 strain identified at necropsy in five Thoroughbred racehorses. Vet. Rec. 173, 607. Nomura, M., Kuroda, T., Tamura, N., Muranaka, M. and Niwa, H. 2020. Mortality, clinical findings, predisposing factors and treatment of Clostridioides difficile colitis in Japanese thoroughbred racehorses. Vet. Rec. 187, e14. Núñez, F.P., Quera, R., Bay, C. and Thomson, P. 2021. Fecal microbiota transplant, its usefulness beyond Clostridioides difficile in gastrointestinal diseases. Gastroenterol. Hepatol. S0210, 5705. Park, T., Cheong, H., Yoon, J., Kim, A., Yun, Y. and Unno, T. 2021. Comparison of the fecal microbiota of horses with intestinal disease and their healthy counterparts. Vet. Sci. 8, 113. Proudman, C.J., Smith, J.E., Edwards, G.B. and French, N.P. 2002. Long-term survival of equine surgical colic cases. Part 1: Patterns of mortality and morbidity. Equine Vet. J. 34, 432–437. Rakoff-Nahoum, S., Coyne, M.J. and Comstock, L.E. 2014. An ecological network of polysaccharide utilization among human intestinal symbionts. Curr. Biol. 24, 40–49. Rodríguez, J.M., Murphy, K., Stanton, C., Ross, R.P., Kober, O.I., Juge, N., Avershina, E., Rudi, K., Narbad, A., Jenmalm, M.C., Marchesi, J.R. and Collado, M.C. 2015. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 26, 26050. Salem, S.E., Maddox, T.W., Berg, A., Antczak, P., Ketley, J.M., Williams, N.J. and Archer, D.C. 2018. Variation in faecal microbiota in a group of horses managed at pasture over a 12-month period. Sci. Rep. 8, e8510. Salem, S., Hough, R., Probert, C., Maddox, T., Antczak, P., Ketley, J.M., Williams, N.J., Stoneham, S.J. and Archer, D.C. 2019. A longitudinal study of the faecal microbiome and metabolome of periparturient mares. Peer. J. 7, e6687. Scantlebury, C.E., Archer, D.C., Proudman, C.J. and Pinchbeck, G.L. 2015. Management and horse-level risk factors for recurrent colic in the UK general equine practice population. Equine Vet. J. 47, 202-206. Schoster, A., Kunz, T., Lauper, M., Graubner, C., Schmitt, S. and Weese, J.S. 2019. Prevalence of Clostridium difficile and Clostridium perfringens in Swiss horses with and without gastrointestinal disease and microbiota composition in relation to Clostridium difficile shedding. Vet. Microbiol. 239, 108433. Shirazi-Beechey, S.P. 2008. Molecular insights into dietary induced colic in the horse. Equine Vet. J. 40, 414–421. Steelman, S.M., Chowdhary, B.P., Dowd, S., Suchodolski, J. and Janecka, J. 2012. Pyrosequencing of 16S rRNA genes in fecal samples reveals high diversity of hindgut microflora in horses and potential links to chronic laminitis. BMC Vet. Res. 8, e231. Stewart, H.L., Pitta, D., Indugu, N., Vecchiarelli, B., Engiles, J.B., Southwood, L.L. 2018. Characterization of the fecal microbiota of healthy horses. Am. J. Vet. Res. 79, 811–819 Stewart, H.L., Southwood, L.L., Indugu, N., Vecchiarelli, B., Engiles, J.B. Pitta, D. 2019. Differences in the equine fecal microbiota between horses presenting to a tertiary referral hospital for colic compared to an elective surgical procedure. Equine Vet. J. 51, 336–342. Sung, J., Kim, S., Cabatbat, J.J.T., Jang, S., Jin, Y.S., Jung, G.Y., Chia, N. and Kim, P. 2017. Global metabolic interaction network of the human gut microbiota for context-specific communityscale analysis. Nat. Commun. 8, 15393. Tamburini, S., Shen, N., Wu, H.C. and Clemente, J.C. 2016. The microbiome in early life: implications for health outcomes. Nat. Med. 22, 713–722. Tannahill, V.J., Cardwell, J.M. and Witte, T.H. 2019. Colic in the British military working horse population: a retrospective analysis. Vet. Rec. 184, 24. Tazoe, H., Otomo, Y., Kaji, I., Tanaka, R., Karaki, S.I. and Kuwahara, A. 2008. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J. Physiol. Pharmacol. 59, 251–262. Thaiss, C.A., Zmora, N., Levy, M., Elinav, E. 2016. The microbiome and innate immunity. Nature 6, 65–74. Theriot, C.M, Bowman, A.A. and Young, V.B. 2016. Antibiotic-induced alterations of the gut microbiota alter secondary bile acid production and allow for Clostridium difficile spore germination and outgrowth in the large intestine. mSphere 1, e00045–15. Thomson, P., Medina, D.A., Ortúzar, V., Gotteland, M. and Garrido, D. 2018. Anti - inflammatory effect of microbial consortia during the utilization of dietary polysaccharides. Food Res. Int. 109, 14–23. Thomson, P., Núñez, P., Quera, R. and Bay, C. 2021. Gastrointestinal microbiome, what is behind faecal microbiota transplantation? New Microbes New Infect. 42, 100898. Tinker, M.K., White, N.A., Lessard, P., Thatcher, C.D., Pelzer, K.D., Davis, B. and Carmel, D.K. 1997. Prospective study of equine colic incidence and mortality. Equine Vet. J. 29, 448–453. Van Nood, E., Vrieze, A., Nieuwdrop, M., Fuentes, S. Zoetenda, E.G., De Vos, W.M., Visser, C.E., Kuijper, E.J., Bartelsman, J.F., Tijssen, J.G., Speelman, P., Dijkgraaf, M., Keller, J.J. 2013. Duodenal Infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415. Venable, E.B., Kerley, M.S. and Raub, R. 2013. Assessment of equine fecal microbial profiles during and after a colic episode using pyrosequencing. J. Equine. Vet. Sci. 33, 347–348. Venable, E.B., Bland, S.D., McPherson, J.L. and Francis, J. 2016. Role of the gut microbiota in equine health and disease. Animal Frontiers 6, 43–49. Venable, E., Fenton, K., Braner, V., Reddington, C., Halpin, M., Heitz, S.A., Francis, J.M., Gulson, N.A., Goyer, C.L., Bland, S.D., Cross, T.L., Holscher, H.D. and Swanson, K.S. 2017. Effects of Feeding management on the equine cecal microbiota. J. Equine Vet. Sci. 49, 113–121. Vitale, V., Viu, J., Armengou, L., Ríos, J., Cunilleras, J.E. 2020. Prognostic value of measuring heart rate variability at the time of hospital admission in horses with colic. Am. J. Vet. Res. 81, 147–152. Warzecha, C.M., Coverdale, J.A., Janecka, J.E., Leatherwood, J.L., Pinchak, W.E., Wickersham, T.A., McCann, J.C. 2017. Influence of short-term dietary starch inclusion on the equine cecal microbiome. J. Anim. Sci. 95, 5077–5090. Weese, J.S., Holcombe, S.J., Embertson, R.M., Kurtz, K.A., Roessner, H.A., Jalali, M. and Wismer, S.E. 2015. Changes in the faecal microbiota of mares precede the development of post-partum colic. Equine Vet. J. 47, 641–649. Willing, B., Vörös, A., Roos, S., Jones, C., Jansson, A. and Lindberg, J.E. 2009. Changes in faecal bacteria associated with concentrate and forage-only diets fed to horses in training. Equine Vet. J. 41, 908–914. Worku, Y., Wondimagegn, W., Aklilu, N., Assefa, Z., Gizachew, A. 2017. Equine colic: clinical epidemiology and associated risk factors in and around Debre Zeit. Trop. Anim. Health Prod. 49, 959–965. Zhou, M., He, J., Shen, Y., Zhang, C., Wang, J. and Chen, Y. 2017. New frontiers in genetics, Gut Microbiota, and immunity: a Rosetta stone for the pathogenesis of inflammatory bowel disease. BioMed. Res. Int. 2017, 8201672. Zimmerman, M.A., Singh, N., Martin, P.M., Thangaraju, M., Ganapathy, V., Waller, J.L., Shi, H., Robertson, K.D., Munn, D.H., Liu, K. 2012. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1405–G1415. | ||
| How to Cite this Article |
| Pubmed Style Lara F, Castro R, Thomson P. Changes in the gut microbiome and colic in horses, are they cause or consequence?. Open Vet. J.. 2022; 12(2): 242-249. doi:10.5455/OVJ.2022.v12.i2.12 Web Style Lara F, Castro R, Thomson P. Changes in the gut microbiome and colic in horses, are they cause or consequence?. https://www.openveterinaryjournal.com/?mno=28765 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i2.12 AMA (American Medical Association) Style Lara F, Castro R, Thomson P. Changes in the gut microbiome and colic in horses, are they cause or consequence?. Open Vet. J.. 2022; 12(2): 242-249. doi:10.5455/OVJ.2022.v12.i2.12 Vancouver/ICMJE Style Lara F, Castro R, Thomson P. Changes in the gut microbiome and colic in horses, are they cause or consequence?. Open Vet. J.. (2022), [cited January 25, 2026]; 12(2): 242-249. doi:10.5455/OVJ.2022.v12.i2.12 Harvard Style Lara, F., Castro, . R. & Thomson, . P. (2022) Changes in the gut microbiome and colic in horses, are they cause or consequence?. Open Vet. J., 12 (2), 242-249. doi:10.5455/OVJ.2022.v12.i2.12 Turabian Style Lara, Felipe, Rodrigo Castro, and Pamela Thomson. 2022. Changes in the gut microbiome and colic in horses, are they cause or consequence?. Open Veterinary Journal, 12 (2), 242-249. doi:10.5455/OVJ.2022.v12.i2.12 Chicago Style Lara, Felipe, Rodrigo Castro, and Pamela Thomson. "Changes in the gut microbiome and colic in horses, are they cause or consequence?." Open Veterinary Journal 12 (2022), 242-249. doi:10.5455/OVJ.2022.v12.i2.12 MLA (The Modern Language Association) Style Lara, Felipe, Rodrigo Castro, and Pamela Thomson. "Changes in the gut microbiome and colic in horses, are they cause or consequence?." Open Veterinary Journal 12.2 (2022), 242-249. Print. doi:10.5455/OVJ.2022.v12.i2.12 APA (American Psychological Association) Style Lara, F., Castro, . R. & Thomson, . P. (2022) Changes in the gut microbiome and colic in horses, are they cause or consequence?. Open Veterinary Journal, 12 (2), 242-249. doi:10.5455/OVJ.2022.v12.i2.12 |