| Case Report | ||
Open Vet. J.. 2022; 12(2): 303-307 Open Veterinary Journal, (2022), Vol. 12(2): 303–307 Case Report A new Montanide™ Seppic IMS1313-adjuvanted autogenous vaccine as a useful emergency tool to resolve a Salmonella enterica subsp. enterica serovar abortus equi abortion outbreak in maresMarica Stazi1†, Martina Pellegrini1†, Elisa Rampacci2, Monica Sforna2, Fabrizio Passamonti2, Antonella Di Paolo1* and Giulio Severi11Istituto Zooprofilattico Sperimentale dell’Umbria e delle Marche “Togo Rosati”, Perugia, Italy 2Department of Veterinary Medicine, University of Perugia, Perugia, Italy #Both authors contributed equally to this work. *Corresponding Author: Antonella Di Paolo. Istituto Zooprofilattico Sperimentale dell’Umbria e delle Marche “Togo Rosati”, Perugia, Italy. Email: a.dipaolo [at] izsum.it Submitted: 09/12/2021 Accepted: 07/04/2022 Published: 23/04/2022 © 2022 Open Veterinary Journal
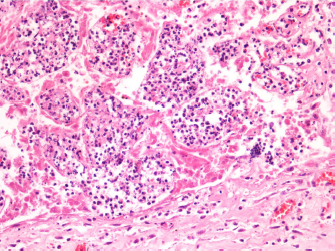
AbstractBackground: In Italy, an autogenous registered vaccine, adjuvanted with aluminum hydroxide, can be administrated to contrast Salmonella enterica subsp. enterica serovar abortus equi infection, coupled to a specific antimicrobial treatment. Case Description: Here, we report the case of an abortion outbreak by Salmonella abortus equi in Central Italy where mares were vaccinated but immediately developed a strong local reaction, maybe due to the adjuvant. Promptly, another autogenous vaccine, substituting the aluminum hydroxide with a new generation adjuvant (Montanide™ Seppic IMS1313), was produced and administrated. The new formulated vaccine did not cause any adverse outcome and conferred high protection titers against the infection. To the best of our knowledge, this is the first reported case of immunization by a vaccine adjuvanted with Montanide™ Seppic IMS1313 in horses. Conclusion: This approach may be used as a preventive strategy for further outbreaks in association with the application of recommended biosafety principles. Keywords: Salmonella abortus equi, Abortion, Autogenous vaccine, Montanide™. IntroductionSalmonella is a genus of ubiquitous Gram-negative, rod-shaped, facultative anaerobic, intracellular bacteria, which are capable of infecting many species of warm-blooded animals and humans (Marenzoni et al., 2012; Chandra and Kaur, 2018; Jajere, 2019). Salmonella spp. belongs to the family Enterobacteriaceae and its taxonomy is nowadays based on genetic analysis. The genus includes two species, Salmonella bongori and Salmonella enterica, which have more than 2,600 antigenically distinct serovars and many of these are capable of causing infections named salmonellosis (Maxie, 2016; Grandolfo et al., 2018; Jajere, 2019). Salmonella enterica subsp. enterica serovar abortus equi (Salmonella abortus equi), also indicated as Salmonella abortus or Salmonella abortivoequina or Salmonella abortus equi, is a host-restricted bacterium recognized as an etiological agent of equine abortion in mares and pneumonia, enteritis, and polyarthritis in foal (Llorente et al., 2016; Bustos et al., 2016; Chandra and Kaur, 2018; Grandolfo et al., 2018). The abortion occurs in the latter half of the gestation period with acute placentitis and retention of the placenta (Chandra and Kaur, 2018), usually without premonitory signs, except for mares with clinically manifested forms. Cases of abortion in equids associated with Salmonella abortus equi have been reported in Asia and Africa and sporadically in Europe, United States, and Argentina (Bustos et al., 2016; Llorente et al., 2016; Grandolfo et al., 2018). Recently, an abortion outbreak in donkeys occurred in China (Wang et al., 2019). Salmonella is also a public health concern and it is responsible for economic losses due to infertility, fetal loss, and foal mortality in equine farming (Chandra and Kaur, 2018). Vaccine administration and good hygiene practices are key factors to preventing the infection spread. Here, we describe an outbreak of Salmonella abortus equi-induced abortion in a horse stable located in Central Italy, for which an emergency immunization intervention was applied using a new formulation of autogenous vaccine, after the onset of anaphylactic reaction to the standard vaccine. Case DetailsDuring winter 2020, an abortion outbreak occurred in a horse stable located in Umbria region (Central Italy) that hosted nine pregnant mares. Anamnestic data reported an earlier outbreak of abortion cases caused by Salmonella abortus equi in donkeys housed in a boarding farm. In total, four mares aborted at 5–6 months of pregnancy without any clinical signs. All animals were negative for Equid alphaherpesvirus 1 (EHV-1) and Equid alphaherpesvirus 4 (EHV-4). The aborted fetuses and their placentas were delivered to the Department of Veterinary Medicine of Perugia for necropsy and laboratory investigations. Necropsy did not reveal evident gross lesions of the fetuses. Specimens of liver, spleen, lungs, and placenta were fixed in 10% neutral buffered formalin for routine histopathological examination. Samples were embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin and periodic acid–Schiff to exclude fungal infection. Histopathological examination of hepatic, splenic, and pulmonary samples revealed only parenchymal congestion; placental samples were characterized by diffused congestion of mucosa and submucosal layers with multifocal hemorrhages in the submucosa. Placental villi showed multifocal areas of necrosis associated with neutrophilic infiltration (Fig. 1). Lungs, liver, spleen, placenta, and stomach content were collected for bacteriological investigations. One gram of each organ was first pre-enriched in 9 ml of buffered peptone water (Liofilchem, Roseto degli Abruzzi, TE, Italy) and incubated at 37°C for 20 hours. One hundred microliters of the suspension was transferred in 10 ml of Rappaport-Vassiliadis Salmonella Enrichment Broth (Liofilchem, Roseto degli Abruzzi, TE, Italy) and incubated at 42°C. After 24 hours incubation, 10 µl of broth culture was seeded on the surface of Chromogenic Salmonella Agar and Hektoen Enteric Agar (Liofilchem, Roseto degli Abruzzi, TE, Italy). The plates were incubated at 37°C for 24 hours. All incubations were carried out in aerobic conditions. To exclude the growth of bacteria other than Salmonella spp., the samples were also cultured on 5% defibrinated sheep blood agar, MacConkey, Sabouraud, and Mannitol Salt agar (Liofilchem, Roseto degli Abruzzi, TE, Italy) at 37°C for 3 days in a standard air incubator. For the anaerobic culture, 5% defibrinated sheep blood agar plates were incubated at 37°C for 3 days in an anaerobic chamber. Identification of grown bacteria was based on colony characteristics, Gram staining, and biochemical characteristics evaluated through commercially available API identification systems (BioMérieux, Marcy-l’Étoile, France). Liver, placenta, and stomach content grew a species of Salmonella initially identified as Salmonella spp. No other bacterium was isolated. Serotyping investigations confirmed the detection of Salmonella abortus equi from the abortions, according to the technique reported in the EN ISO 6579-1:2020.
Fig. 1. Histological section of the placenta. Placental villi are congested and multifocally necrotic with neutrophilic infiltration. Foci of hemorrhages are present in the submucosa. (H&E, 100×). An antimicrobial susceptibility test was carried out by disk diffusion on cation-adjusted Mueller Hinton Broth (CAMHA). Briefly, after colony growth and identification, a bacterial suspension was prepared and adjusted to McFarland 0.5 and it was swabbed onto CAMHA. Antibiotic disks were dispensed to the agar surface and the plate was incubated at 37°C for 18–20 hours. The bacterium was susceptible in vitro to amikacin, gentamicin, cefazolin, enrofloxacin, marbofloxacin, and trimethoprim/sulfamethoxazole and intermediate to ampicillin and ceftiofur. Once antimicrobial susceptibility results became available, the horses were first treated with gentamicin at a dosage of 6.6 mg/kg PO q24h and then, circa 45 days later, with an autogenous vaccine, custom-made at Istituto Zooprofilattico Sperimentale dell’Umbria e delle Marche “Togo Rosati” (IZSUM). The institute was authorized by the Italian Ministry of Health by legislative decree (17th March 1994, n. 287) to produce emergency medical tools. Following the standard production process, an autogenous vaccine was produced and adjuvanted with aluminum hydroxide. All culture media for the vaccine production were made at the pharmaceutical unit, Culture Media Laboratory. Briefly, the bacterial strain isolated in the stable was submitted for vaccine batch production after serotyping and characterization. The production process started with the culture of the characterized strain: one, isolated and pure colony of Salmonella abortus equi was spread by sliding on a tryptic soy agar (TSA) and incubated in a humid chamber for 24 hours at 37°C. After incubation and purity control by Gram staining, a TSA block containing a pure colony was transferred into a Tryptone soy broth (TSB) flask and left to incubate for further 24 hours at 37°C under agitation. After a second purity check, fresh TSB medium in the ratio 1/10 (v/v) was added to the obtained matrix, for the amplification of the strain. Once the requested vaccine batch volume was reached, a third purity control was carried out before the chemical inactivation and concentration step. Briefly, a formaldehyde solution 0.05% (v/v) was added to the amplified matrix; subsequently, it was scalarly diluted (10-fold) in three tubes containing TSB and incubated at 37°C for 48 hours, then adjusted to a final concentration of 1.5 × 109 CFU/ml based on the McFarland scale. The inactivated culture was then adjuvanted with aluminum hydroxide (20% v/v). The filled vaccine was checked for neutral pH (6.8–7.4) and safety in five BalbC mice (in vivo residual pathogenicity test) by administering subcutaneously a dose 10 times higher (0.5 ml) than that usually adopted, as stated in the legislative decree (17th March 1994, n. 287, Chapter III). Animals were housed in the animal facility of IZSUM and all welfare and care practices were applied according to the Italian legislative decree n. 26 of 4th March 2014, application of 2010/63/UE Directive (2010/63/UE Directive on the protection of animals used for scientific purposes). Mice were monitored for 72 hours after the injection; particularly, abnormal/local reactions, temperature rising, and global wellness were carefully evaluated by the designed veterinarian and personnel. For the sterility requirement testing before release, an aliquot from the batch was delivered to the microbiology unit. The sample was inoculated in selective media for aerobes, anaerobes, and fungi for 15 days at different temperatures, as described in the X edition of European Pharmacopeia (European Pharmacopeia, 2019). After confirmation of vaccine sterility and safety, the aluminum hydroxide-adjuvanted formulation was administered subcutaneously (5 ml) in the neck region to pregnant mares and to those that had an abortion. After 72 hours from the administration of the vaccine adjuvanted with aluminum hydroxide, a transient local tumefaction and neck stiffness were observed in mares. Additionally, the animals developed fever (39°C) and lack of appetite for 2 days. A small granuloma had persisted until this time. Therefore, for the alternative inoculation and the subsequent booster dose, it was decided to produce a new vaccine batch by performing the above-described procedure using 20% v/v of Montanide™ Seppic IMS1313, a ready to dilute adjuvant consisting of liquid particles (10–500 nm) dispersed in an aqueous phase containing an immunostimulating compound. The in vivo residual pathogenicity test was carried out in five mice, as detailed above. Twenty-one days after the immunization with the vaccine adjuvanted with aluminum hydroxide, 5 ml dose of the Montanide™-adjuvanted vaccine was initially inoculated to one mare for a safety test. Only after 72 hours, when the absence of any kind of immediate or delayed systemic and/or locally reactions was verified, it was administered to eight remaining mares. Moreover, a recall dose after 6 months was administrated to all mares housed in the stall—still pregnant and not pregnant. The vaccine adjuvanted with Montanide™ Seppic IMS1313 did not cause any local response and/or adverse reaction after the first and second administration. One week after the recall vaccination with Montanide™ Seppic IMS1313, the five mares treated during pregnancy and their foals were tested for Salmonella serological test at a private laboratory. All animals showed high titers (>1:320; cutoff sample/positive >1:40). After 1 year from the last immunization treatment, abortion cases were not observed. Figure 2 shows all the phases described above. DiscussionGenerally, salmonellosis occurs as an asymptomatic infection in horses, and infected animals play an important epidemiological role in disease transmission. When these carriers become stressed, they can show diarrhea, by which the bacterium is eliminated and spread in the environment. Clinically infected horses can have acute or hyperacute forms characterized also by septicemia, enterocolitis, and fever for a period of 1–2 weeks, followed by recovery or death. When bacteremia ensues, Salmonella must be able to survive and replicate in macrophages to disseminate to different anatomical sites, particularly liver, lungs, joints, meninges, or placenta, and fetus (Maxie, 2016; Chandra and Kaur, 2018). In this scenario, when an outbreak of Salmonella occurs, veterinarians apply a standard procedure, combining custom-made vaccination (when a commercial vaccine is not available) and antimicrobial treatment. After diagnosing Salmonella abortus equi as the etiological agent of the abortion outbreak, an autogenous vaccine and gentamicin at a dosage of 6.6 mg/kg PO q24h were administrated to mares. All autogenous vaccines are made from the inactivated microbial strain isolated from the flock where the vaccine has to be used and/or in surrounding farms where the same pathogen was isolated. The use of an autogenous vaccine is allowed if no licensed vaccine is available or if it is ineffective against the pathogen of interest. As emergency tools, autogenous vaccines can be modulated or modified as required, to reduce adverse outcomes in the target species.
Fig. 2. Schematic representation of the outbreak management. The aluminum hydroxide used for the production of the first vaccine batch is a classical adjuvant, which allows the antigen to be “retained” at the injection point to recall the immune system cells. Even if the inoculation of aluminum hydroxide-based adjuvant vaccine is well known and safe, it can cause local adverse reactions, such as erythema, subcutaneous nodules, contact hypersensitivity, and granuloma (He et al., 2015). In some cases, vaccines containing aluminum salts (characterized by acid pH) can cause pain, redness, and swelling at the injection site, which generally resolve on its own after a few hours without compromising the vaccine efficacy and without severe adverse outcome. In this case, the appearance of transient local swelling, with neck stiffness and fever, suggested the inadequacy of alum hydroxide adjuvant for the equine species and the need of finding less aggressive but still effective new generation adjuvant replacement to be used. Intended for the described outbreak caused by Salmonella abortus equi, a new vaccine batch was produced. We adjuvanted the bacterial culture with Montanide™ Seppic IMS1313, a combination of microemulsions and an immunostimulating compound. Since this adjuvant is composed of an aqueous phase, it is suitable as a mucosal delivery vehicle. Montanide™ Seppic IMS1313 was tested in different formulation vaccines for different animal species and there was absence of any severe injection site reactions (Dhakal and Renukaradhya, 2019). It is also eligible for oral delivery mass vaccination too (Riffault et al., 2010). Moreover, in vivo experimental infections demonstrated the ability of Montanide™ Seppic IMS1313 to enhance immune protection in different animal species (Jang et al., 2011). Here, we observed that Montanide™ Seppic IMS1313 adjuvant at 20% v/v was able to minimize possible adverse effects in horses. Particularly, after the second immunization dose, no animal showed clinical adverse effects and the infection was successfully controlled. Remarkably, serological data collected after the second immunization suggested a high humoral protection elicited in mares and a huge passive immunity conferred to newborn foals. Vaccination is also useful to prevent the massive antibiotic use. As a result, it can have a major role in fighting antimicrobial resistance. In conclusion, we support the use of the Montanide™ Seppic IMS1313-adjuvanted vaccine as a safe, well-tolerated, and highly immunogenic tool for preventing Salmonella abortus equi abortion. Despite the combined protocol effect on resolution of the infection in the stall and its 1-year lasting protection, we can consider to adopt other modifications to the vaccination protocol in other possible future outbreaks. An autogenous vaccine is a flexible tool and it can be customized depending on the epidemiological scenario and overall with the aim to minimize animal stress, particularly in pregnant mares, or avoid possible adverse reactions. To the best of authors’ knowledge, this is the first reported case of immunization intervention in horses with an autogenous vaccine adjuvanted with Montanide™ Seppic IMS1313 against Salmonella abortus equi. Conflict of interestThe authors declare no potential conflicts of interest for the research, authorship, and/or publication of this article. Authors’ contributionsSM, PM, and DPA: Conceptualization; DPA, RE, SM, and PF: Data curation; RE, SM, PF, PM, SM; SM, RE, SM, and PF: Formal analysis; RE, SM, PF, and PM: Investigation; SM, SG, and PF: Supervision; SM, PM, DPA, RE, SM, PF, and SG: Writing, review, and editing. FundingThe authors received no financial support for the research, authorship, and/or publication of this article. ReferencesBustos, C.P., Gallardo Retamar, G., Lanza, N.S., Falzoni, E, Caffer, M.I., Picos, J., Munos, A.J., Perez, A., Moras, E.V., Mesplet, M. and Guida, N. 2016. Salmonella enterica serovar abortus equi as an emergent pathogen causing equine abortion in Argentine. J. Equine Vet. Sci. 39(Suppl), S58–S59. Chandra, M. and Kaur, G. 2018. An update on Equine Salmonellosis. E.C. Vet. Sci. 3, 348-353. DECRETO LEGISLATIVO 4 marzo 2014, n. 26. Attuazione della direttiva 2010/63/UE sulla protezione degli animali utilizzati a fini scientifici. Dhakal, S. and Renukaradhya, G.J. 2019. Nanoparticle-based vaccine development and evaluation against viral infections in pigs. Vet. Res. 50, 90. EU Directive 2010/63/EU of the European Parliament “on the protection of animals used for scientific purposes” and is one of the most stringent ethical and welfare standards worldwide. 2010. 102(1), 83–86. European Pharmacopoeia. 2019. Test for sterility, 10th ed., Chapter 2.6.1. Published by European Directorate for the Quality of Medicines & HealthCare, Council of Europe, Avenue de l’Europe F-67075 Strasbourg Cedex, France. Grandolfo, E., Parisi, A., Ricci, A., Lorusso, E., de Siena, R., Trotta, A., Buonavoglia, D., Martella, V. and Corrente, M. 2018. High mortality in foals associated with Salmonella enterica subsp. enterica abortus equi infection in Italy. J. Vet. Diagn. Invest. 30(3), 483–485. He, P., Zou, Y. and Hu, Z. 2015. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Human Vac. Immunother. 11(2), 477–488. Jajere, S.M. 2019. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 12(4), 504–521. Jang, S.I., Lillehoj, H.S., Lee, S.H., Lee, K.W., Lillehoj, E.P., Bertrand, F., Dupuis, L. and Deville, S. 2011. Montanide IMS 1313 N VG PR nanoparticle adjuvant enhances antigen-specific immune responses to profilin following mucosal vaccination against Eimeria acervulina. Vet. Parasitol. 182(2–4), 163–170. Llorente, L., Ivanissevich, A., Camiña, S., Marco, L., Vissani, A., Olguin, P.C., Herrera, M. and Barrandeguy, M.E. 2016. Occurrence of multiple abortions due to Salmonella enterica serovar abortus equi infection. J. Equine Vet. Sci. 39(Suppl), 58. Marenzoni, M.L., Lepri, E., Casagrande, P.P., Bietta, A., Coletti, M., Timoney, P.J. and Passamonti, F. 2012. Causes of equine abortion, stillbirth and neonatal death in central Italy. Vet. Rec. 170(10), 262. Maxie, M.G. 2016. Jubb, Kennedy and Palmer’s. Pathology of domestic animals, 6th ed., Chapter 1: Alimentary system, Elsevier, 11830 Westline Industrial Drive, St. Louis, MO 63146, United States of America, pp: 167–168–172. Riffault S., Meyer, G., Deplanche, M., Dubuquoy, C., Durand, G., Soulestin, M., Castagné, N., Bernard, J., Bernardet, P., Dubosclard, V., Bernex, F., Petit-Camurdan, A., Deville, S., Schwartz-Cornil I. and Eléouët, J.F. 2010. A new subunit vaccine based on nucleoprotein nanoparticles confers partial clinical and virological protection in calves against bovine respiratory syncytial virus. Vaccine 28, 3722–3734. Wang, H., Liu, K.J., Sun, Y.H., Cui, L.Y., Meng, X., Jiang, G.M., Zhao, F.W. and Li, J.J. 2019. Abortion in donkeys associated with Salmonella abortus equi infection. Equine Vet. J. 51(6), 756–759. | ||
| How to Cite this Article |
| Pubmed Style Stazi M, Pellegrini M, Rampacci E, Sforna M, Passamonti F, Paolo AD, Severi G. A new Montanide Seppic IMS 1313-adjuvanted autogenous vaccine as useful emergency tool to resolve a Salmonella enterica subsp. enterica serovar abortusequi abortion outbreak in mares.. Open Vet. J.. 2022; 12(2): 303-307. doi:10.5455/OVJ.2022.v12.i2.19 Web Style Stazi M, Pellegrini M, Rampacci E, Sforna M, Passamonti F, Paolo AD, Severi G. A new Montanide Seppic IMS 1313-adjuvanted autogenous vaccine as useful emergency tool to resolve a Salmonella enterica subsp. enterica serovar abortusequi abortion outbreak in mares.. https://www.openveterinaryjournal.com/?mno=35151 [Access: January 26, 2026]. doi:10.5455/OVJ.2022.v12.i2.19 AMA (American Medical Association) Style Stazi M, Pellegrini M, Rampacci E, Sforna M, Passamonti F, Paolo AD, Severi G. A new Montanide Seppic IMS 1313-adjuvanted autogenous vaccine as useful emergency tool to resolve a Salmonella enterica subsp. enterica serovar abortusequi abortion outbreak in mares.. Open Vet. J.. 2022; 12(2): 303-307. doi:10.5455/OVJ.2022.v12.i2.19 Vancouver/ICMJE Style Stazi M, Pellegrini M, Rampacci E, Sforna M, Passamonti F, Paolo AD, Severi G. A new Montanide Seppic IMS 1313-adjuvanted autogenous vaccine as useful emergency tool to resolve a Salmonella enterica subsp. enterica serovar abortusequi abortion outbreak in mares.. Open Vet. J.. (2022), [cited January 26, 2026]; 12(2): 303-307. doi:10.5455/OVJ.2022.v12.i2.19 Harvard Style Stazi, M., Pellegrini, . M., Rampacci, . E., Sforna, . M., Passamonti, . F., Paolo, . A. D. & Severi, . G. (2022) A new Montanide Seppic IMS 1313-adjuvanted autogenous vaccine as useful emergency tool to resolve a Salmonella enterica subsp. enterica serovar abortusequi abortion outbreak in mares.. Open Vet. J., 12 (2), 303-307. doi:10.5455/OVJ.2022.v12.i2.19 Turabian Style Stazi, Marica, Martina Pellegrini, Elisa Rampacci, Monica Sforna, Fabrizio Passamonti, Antonella Di Paolo, and Giulio Severi. 2022. A new Montanide Seppic IMS 1313-adjuvanted autogenous vaccine as useful emergency tool to resolve a Salmonella enterica subsp. enterica serovar abortusequi abortion outbreak in mares.. Open Veterinary Journal, 12 (2), 303-307. doi:10.5455/OVJ.2022.v12.i2.19 Chicago Style Stazi, Marica, Martina Pellegrini, Elisa Rampacci, Monica Sforna, Fabrizio Passamonti, Antonella Di Paolo, and Giulio Severi. "A new Montanide Seppic IMS 1313-adjuvanted autogenous vaccine as useful emergency tool to resolve a Salmonella enterica subsp. enterica serovar abortusequi abortion outbreak in mares.." Open Veterinary Journal 12 (2022), 303-307. doi:10.5455/OVJ.2022.v12.i2.19 MLA (The Modern Language Association) Style Stazi, Marica, Martina Pellegrini, Elisa Rampacci, Monica Sforna, Fabrizio Passamonti, Antonella Di Paolo, and Giulio Severi. "A new Montanide Seppic IMS 1313-adjuvanted autogenous vaccine as useful emergency tool to resolve a Salmonella enterica subsp. enterica serovar abortusequi abortion outbreak in mares.." Open Veterinary Journal 12.2 (2022), 303-307. Print. doi:10.5455/OVJ.2022.v12.i2.19 APA (American Psychological Association) Style Stazi, M., Pellegrini, . M., Rampacci, . E., Sforna, . M., Passamonti, . F., Paolo, . A. D. & Severi, . G. (2022) A new Montanide Seppic IMS 1313-adjuvanted autogenous vaccine as useful emergency tool to resolve a Salmonella enterica subsp. enterica serovar abortusequi abortion outbreak in mares.. Open Veterinary Journal, 12 (2), 303-307. doi:10.5455/OVJ.2022.v12.i2.19 |