| Original Article | ||
Open Vet. J.. 2022; 12(1): 80-90 Open Veterinary Journal, (2022), Vol. 12(1): 80–90 Original Research Efficacy of synergistic activity of seed oils from Carthamus tinctorius (Safflower) and Nasturtium officinale (Watercress) on the lethality of the cattle tick Hyalomma scupense (Acari: Ixodidae)Dhouha Alimi1*, Azhar Hajri1, Selim Jallouli2, and Hichem Sebai11Laboratory of Functional Physiology and Valorization of Bio-resources (UR17ES27), Higher Institute of Biotechnology of Beja, University of Jendouba, Beja, Tunisia 2Laboratory of Bioactive Substances, Centre of Biotechnology of Borj Cedria, Hammam-Lif, Tunisia *Corresponding Author: Dhouha Alimi. Laboratory of Functional Physiology and Valorization of Bio-resources (UR17ES27), Higher Institute of Biotechnology of Beja, University of Jendouba, Beja, Tunisia. Email: dhouha.enmv [at] gmail.com Submitted: 02/12/2021 Accepted: 10/01/2022 Published: 02/02/2022 © 2022 Open Veterinary Journal
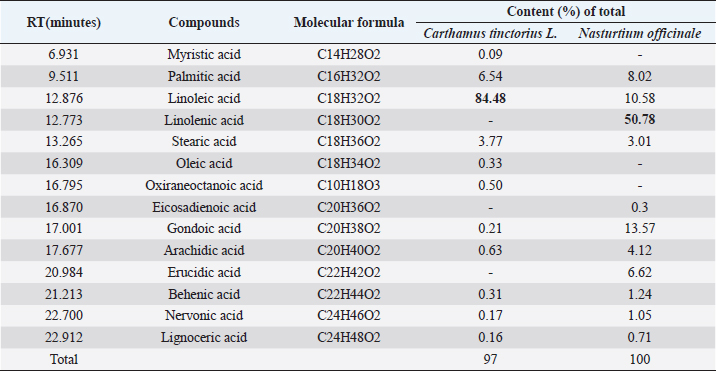
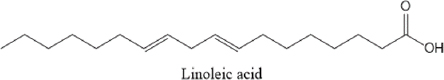
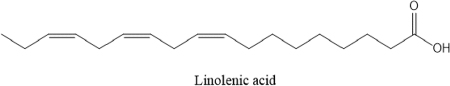
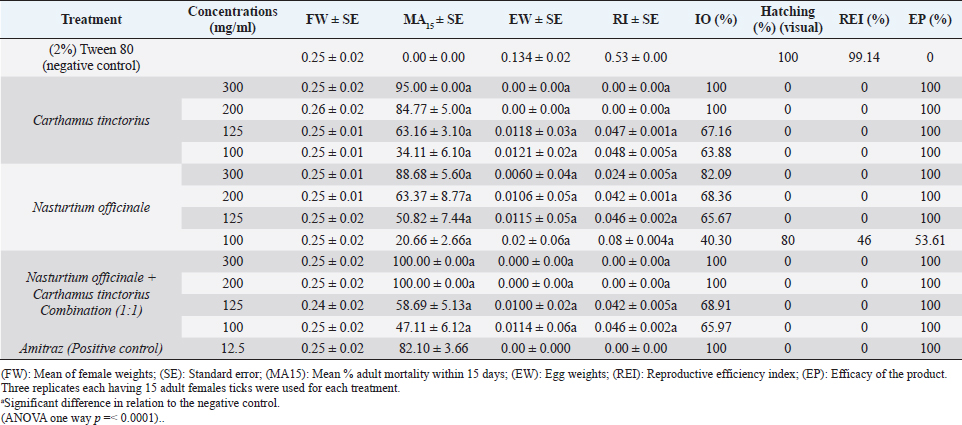
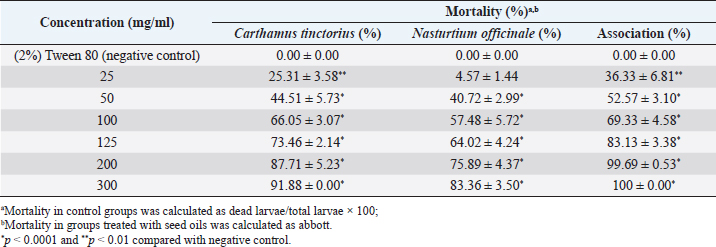
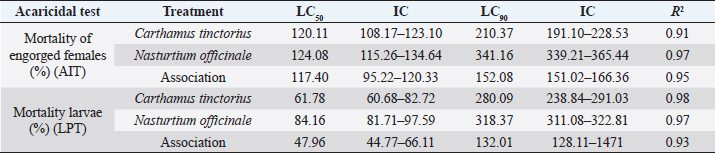
AbstractBackground: Ticks and tick-borne diseases are a severe economic and public-health problem for cattle producers. The emergence of acaricide resistance to synthetic chemical treatments has prompted interest in developing alternative tick control methods. Aim: The main objective of the current research was to identify the chemical structure of Carthamus tinctorius and Nasturtium officinale seed oils and to assess their anti-tick properties against Hyalomma scupense ticks both alone and in combination (1:1). Methods: Analytical methods were used to analyze the chemical components. For in vitro assays, adults of H. scupense were immersed in C. tinctorius and N. officinale seed oils at 100, 125, 200, and 300 mg/ml concentrations; for 5 minutes. Larvae of H. scupense were dipped in 25, 50, 100, 125, 200, and 300 mg/ml doses of seed oils; the mortality percentage was determined after 24 hours. Results: The seed oil safflower was mainly composed of linoleic acid (84.48%), followed by palmitic acid (6.54%) and stearic acid (3.77%). Meanwhile, watercress seed oil was mainly composed of linolenic acid (50.78%), gondoic acid (13.57%), linoleic acid (10.58%), palmitic acid (8.02%), and erucidic acid (6.62%). The Adults Immersion Test showed the sensitivity of ticks to C. tinctorius and N. officinale seed oil: C. tinctorius seed oil caused (95%) mortality of H. scupense at 300 mg/ml, while N. officinale seed oil induced (88.68%) mortality at the same concentration. At a 200 mg/ml concentration, C. tinctorius and N. officinale oil combined caused 100% mortality. Tested oils showed larvicidal efficacy. LC50 values for C. tinctorius and N. officinale seed oils were 84.16 and 61.78 mg/ml, respectively, in 24 hours. LC50 value of oils association (50% C. tinctorius: 50% N. officinale) was 47.96 mg/ml. The mixture of seed oils from two plants tested against H. scupense larvae and adult females at a 1:1 ratio showed synergistic interaction. Conclusion: Seed oils tested alone, and the mixture could be used as an alternative solution in the fight against ticks. Keywords: Carthamus tinctorius, H. scupense, Nasturtium officinale, Acaracidal activity, synergistic. IntroductionIn North Africa, theileriosis, caused by Theileria annulata, represents a major tick-borne protozoan disease and poses serious limitations to the cattle sector (Bouattour et al., 1996; Gharbi and Aziz Darghouth, 2014). In Tunisia, the vector tick is mainly Hyalomma scupense Schulze, 1919 (Gharbi et al., 2013). These arthropods are the most economically important ectoparasites of cattle, causing significant costs in terms of diseases, lower productivity and fertility, and often mortality (Kumar et al., 2020). Many approaches have been used for tick management, such as biological control using pathogens or predators, pheromone-assisted control, herbal pour-on or dip preparations including green manufactured nanoparticles (Banumathi et al., 2017), and vaccination (Labarta et al., 1996). Acaricides and repellents are still regarded as the easiest method for control; however, applications involve several drawbacks like cost, toxicity, waiting times, and acaricide resistance (Quadros et al., 2020). Alternative anti-tick products and or strategies are therefore necessary. Natural products have been investigated for the acaricidal effect (Figueiredo et al., 2018; Alimi et al., 2021; Sbhatu et al., 2021). More recently, oil seeds have garnered the attention of researchers and food scientists (Tanwar and Goyal, 2021). Obviously, seed oils are an excellent source of bioactive compounds used in the food, cosmetic, and pharmaceutical industries, encompassing the prevention and treatment of several diseases (Vergallo, 2020). Carthamus tinctorius Linnaeus, or safflower, commonly called « Bok » (in Tunisia), is a thistle-like herbaceous annual plant belonging to the Asteraceae family (Asgarpanah and Kazemivash, 2013). It is native to Asia and the Mediterranean basin, and it can grow in dry and semi-arid areas with seasonal rainfall. Safflower is a valuable medicinal and fragrant plant utilized as a source of edible additives, natural colors, nutritious drinks, and cosmetics in many regions (Khalid et al., 2017; Jia-Xi et al., 2019). Several pharmacological activities, such as antioxidant (Zemour et al., 2019), antidiabetic (Asgary et al., 2012), antibacterial (Ozkan et al., 2021), analgesic, and anti-inflammatory effects (Alaiye et al., 2020), are exhibited by safflower. Notably, safflower oil is excellent in nutritional value, including 70% polyunsaturated fatty acid (linoleic acid), 10% monounsaturated oleic acid, and trace levels of stearic acid (Zhou et al., 2014; Khémiri et al., 2020). Safflower oil also contains other chemicals. Phenolic molecules, which are found in the unsaponifiable phase of oil and are responsible for its stability and nutritional value, are among them (Khémiri et al., 2020). Nasturtium officinale or watercress, also known as “jarjir” in Tunisia, is a highly uncommon aquatic or semi-aquatic plant endemic to Europe, North Africa, and the Asia Brassicaceae family (Faizy et al., 2021). This herb has well-known nutritional characteristics due to its diverse chemical components, including vitamins B, C, and E, as well as pro-vitamin A, folic acid, carotenoids, glucosinolates, and a variety of minerals such as calcium, iron, and sulfur. Furthermore, Glucosinolates, isothiocyanates, polyphenols (flavonoids, phenolic acids, proanthocyanidins), and terpenoids, including carotenoids, are the primary components discovered in the N. officinale plant (Afsharypuor and Salehi 2008; Jeon et al., 2017). The European Food Safety Authority has approved N. officinale as a safe food plant, and it is listed in the monographs on “Leaf vegetables, herbs, and edible flowers”. Watercress is considered an important medicinal plant largely used in traditional medicine (Teixidor-Toneu et al., 2016). Moreover, N. officinale oil revealed a broad spectrum of pharmacological activities, including anticancer (Hecht et al., 1995), antioxidant (Bahramikia and Yazdanparast, 2010; Zeb, 2015; Ramezani et al., 2021), antibacterial (Bahramikia and Yazdanparast, 2008), tuberculosis (Halberstein, 2005), and cardioprotective actions (Pandey et al., 2018). According to the literature, any previous investigations were found about the oils of C. tinctorius and of N. officinale, or seed oils generally, having any anti-tick activity on H. scupense. Therefore, the major goal of this study was to characterize the chemical structure of C. tinctorius and N. officinale seed oils, as well as to evaluate their anti-tick activities against H. scupense larvae and engorged female ticks individually and in association (1:1). Materials and MethodsPlant materialCollection of plantSamples of C. tinctorius and N. officinale seeds were collected during the month of June 2020 from the village of Aïn Draham in the gouvernate of Jendouba (Northwestern of Tunisia, alt 800 m; 36° 46′ 34″ N, 8° 41′ 05″ E). The plants’ species were identified as C. tinctorius L and N. officinale by the Department of Plant Biotechnology, Higher Institute of Biotechnology of Beja, Jendouba University, Tunisia, according to the flora of Tunisia (Cuénod, 1954). Seed oils extractionSeeds were mechanically separated, rinsed in clean water, and air-dried at room temperature (20°C–25°C). To preserve their constituents’ quality, both oils were extracted naturally by cold pressing using an oil press machine (SMIR, MUV2 65) without any chemical treatment. After filtration, seeds oils were preserved in labeled dark glass bottles at room temperature until analysis. Fatty acid methyl esters analysis and preparation from samplesOne ml of the oils were dissolved in 20 ml petroleum ether, and 2 ml of methanolic potassium hydroxide (KOH) (2M) was added for fatty acid methyl esterification. The mixture was allowed to sit for 10 minutes after being agitated for 2 minutes. The top layer, high in fatty acid methyl esters, was removed, washed with water, and examined using gas chromatography/mass spectrometry (GC/MS) (Nickavar et al., 2003). An Agilent GC-MS was used to analyze C. tinctorius and N. officinale (Agilent Technologies, Wilmington, DE, 7890) equipped with a gas chromatograph and a 5975C quadrupole mass selective detector. A fused silica capillary column from HP-5MS (30 m 0.25 mm i.d. 0.25 m film thickness) was employed. The carrier gas was helium, with a 1 ml/mn constant flow rate. A 1.0 µl sample was injected into a split 1/120 injector at 250°C. The ion source had a temperature of 230°C, whereas the quadrupole had a temperature of 150°C. The oven temperature was firstly kept at 150°C for 1 minute, then increased at a rate of 15°C/minute to 200°C for 3 minutes, then elevated to 280°C at a rate of 3°C/minute for 10 minutes, then maintained to 300°C at 15°C/minute for 10 minutes, and finally held for 10 minutes. The methyl esters of standard fatty acids were run under the same conditions (Freese et al., 1973). Their mass spectra were compared to the Wiley 275 and NIST mass spectra data bases to identify and authenticate substances. Quantitative data was obtained using Peak’s area percents. Hyalomma scupense ticksTicks collectionAdult-engorged females of H. scupense were handpicked from naturally infected cattle, without chemical acaricidal treatment, and in the vicinity of cattle pensin a rural farm in the hamlet of Soliman (North-East of Tunisia, gouvernate of Nabeul). To allow for improved ventilation, ticks were delivered to the laboratory in perforated bottles (about 1 mm in diameter for each entire). Ticks were washed, weighted (with an average weight of 0.25 g), dried, and chosen for vitality and movement. A total of 225 adult engorged female ticks were used for the present study. Out of this, 15 ticks were separated and were held individually at 28°C ± 1°C and 85% ± 5% relative humidity in a labeled glass bottle with the mouth covered by muslin cloth for oviposition. The eggs were allowed to hatch to larvae in 18–25 days under similar incubation conditions. The larvae were used for performing a “larval packet test” (LPT). The remaining 210 ticks were gathered into three groups (one for each plant and combination), each of 60 ticks and 30 ticks for two controls. Each comprising of 15 ticks as 5 ticks each in 3 replicates. Each group of ticks was used to estimate the acaricidal effects of the respective concentration of plant by adult immersion test (AIT). AIT and evaluation of synergismDrummond et al. (1973) described the immersion protocol to evaluate acaricide activity against adult ticks. Seeds oils from C. tinctorius and N. officinale were tested individually and in association at a 1:1 ratio. Ticks were dipped in increasing doses of oils for 5 minutes, while control ticks were immersed in a solution of 2% Tween-80 (negative control). This control solution was used to prepare a series of C. tinctorius and N. officinale oils solutions at different concentrations: 100, 125, 200, and 300 mg/ml. The same concentrations were used for combined seed oils. At doses of 0.0125 mg/ml, a commercial product containing amitraz was employed as a control sample. The ticks were then placed on Petri dishes over Whatman filter paper 1. All the Petri dishes with treated ticks were kept at room temperature for 24 hours. After 24 hours, ticks were transferred to glass vials covered with muslin cloth and kept in desiccators having 85% ± 2% relative humidity and placed in an incubator at 28°C ± 2°C. These ticks were observed for oviposition and death up to 15 days. The percent adult tick mortality and the weight of the eggs laid by the treated ticks were recorded compared to the control. The eggs were incubated at the same condition, and the percentage of hatched eggs was estimated visually. To evaluate reproductive and percentage inhibition of fecundity, the following equations were used (Drummond et al., 1973; Ribeiro et al., 2008; Matos et al., 2019): Reproductive Index (RI)=average weight of eggs laid (mg)/average weight of females before treatment (mg). Inhibition of Oviposition (IO%)=RI (control group)—RI (treated group)/ RI (control group) × 100. Reproduction efficiency (RE)=egg weight × % hatchability × 20.000*/ weight of females *Constant indicating the number of eggs present in 1 g of egg-laying RI (%)=RE (control)-RE (treated)/RE (control) × 100 Effectiveness of the product (PE)=RE (control group)—RE (treated group) /RE (control group) × 100 The synergistic factor (SF) was calculated using the formula described by Kalyanasundaram and Das (1985) with a slight modification. A SF > 1 indicates synergistic effect, whereas the value of SF < 1 shows antagonism. SF value=1 indicates that there is no substantial effect. SF=LC50 value of the individual plant extract/LC50 value of the combined plant extract Larval packet test (LPT)The H. scupense larvae packet test was used for each treatment, according to Stone and Haydock (1962). Filter paper sheets (2 × 2 cm) were impregnated with 1 ml of C. tinctorius, N. officinale, and their combination oil at concentrations of 300, 200, 125, 100, 50, and 25 mg/ml. About one hundred larvae, 14–21 days old, were deposited on each sheet impregnated with the solution. After 24 hours of impregnation, larvae were put inside the packets and subsequently incubated (27°C and RH > 80%) (Figueiredo et al., 2018). After 24 hours, an assessment of percent larval mortality was performed. Three replicates were performed for each concentration, as well as for the controls groups, which were composed of: 2% Tween 80 in sterile distillate water (negative control) and amitraz (0.0125 mg/ml) (positive control). The percentage mortality of H. scupense was calculated with Abbott’s correction. Statistical analysesTo see any significant variations in larval and adult mortality rates between the treatments, analysis of variance (ANOVA) was used, followed by Fisher’s PLSD tests. If the p-value was less than 0.05, it was considered to be significant. The mean and standard error of the mean was used to represent the data. Probit analysis with GraphPad Prism 9.0 software was used to compute the lethal concentration of the fixed oil for 50% (LC50) and 90% (LC90) of the tick population with a 95% confidence interval. The Statview v.5.0.1 program conducted all of these statistical studies (SAS Institute, Cary, NC). Ethical approvalAt the Department of Comparative Medicine, parasites were kept in a pathogen-free environment. All tests were carried out in compliance with IACUC procedure No. (NIH publication 86-23 modified 1985) USA (National Ethics Committee of Tunis University). ResultsSeed oils analysisTable 1 shows the various oil compositions. Linolenic acid (84.48%) was the most abundant ingredient in C. tinctorius seed oil (Fig. 1), followed by palmitic acid (6.54%) and stearic acid (3.77%). Other fatty acids such as myristic acid, oleic acid, oxiraneoctanoic acid, arachidic acid, gondoic acid, behenic acid, nervonic acid, lignoceric acid, and lignoceric acid were present in trace amounts and varied between 0.09% and 0.63%. Eleven individual compounds were characterized in N. officinale oil, representing 100% of the total seed oil. The main constituents of the oil were linolenic acid (50.78%) (Fig. 2), gondoic acid (13.57%), linoleic acid (10.58%), palmitic acid (8.02%), and erucidic acid (6.62%). Adult immersion test (AIT)The results of the AIT using C. tinctorius and N. officinale and their mixture oil are shown in Table 2. The efficacy of different treatments was assessed by estimating the percent adult mortality, RI, IO, hatching rate, and efficacity. In the high tested concentration of 300 mg/ml, seed oil from C. tinctorius caused 95% mortality, while at the same concentration, the seed oil of N. officinale showed only 88.68% mortality. The oil mixture of seed oils, at all concentrations tested, exhibited the best inhibition rate of 100% at 200 mg/ml. The results showed that seed oils tested on H. scupense had a dose-dependent effect in all the AIT bio-assays. Compared to the control group, there was a significant difference between all treatments. The positive control amitraz caused 82.10% mortality in engorged females of H. scupense. Table 2 contains further information on tick reproductive efficiency and the efficacy of seed oils as a tick treatment. Our findings revealed that C. tinctorius (LC50=120.11 mg/ml) and the oil combination (LC50=117.40 mg/ml) have the highest oviposition inhibition rate. However, at the highest dose, individual N. officinale oil showed only 82.09% IO. The results were significantly different from the control group (2% Tween 80) (p < 0.001). In all concentrations tested, all treatments used in this study could affect tick reproduction in vitro by inhibiting oviposition and hatchability. However, at a low concentration of 100 mg/ml, seed oil from N. officinale only showed a 46% reproductive efficiency. Table 1. Composition of seeds oils from C. tinctorius and N. officinale obtained by GC-MS.
Fig. 1. The major component of seed oil of C. tinctorius (84.48%).
Fig. 2. The major component of seed oil of N. officinale (50.78%). Table 2. Mean (± SE) standard deviation of biological activity of H. scupense engorged females after exposure to different concentrations of C. tinctorius, N. officinale and combination of seed oils.
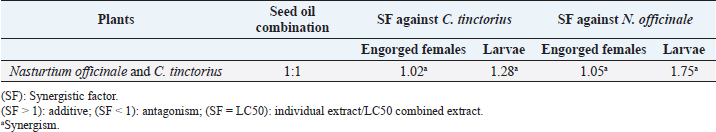
Larval packet test (LPT)The LPT was used to investigate the efficacy of C. tinctorius and N.officinale seed oils against H. scupense larvae (Table 3). Both plants showed larvicide properties. Separately, the lowest mortality value for N. officinale seed oil was observed at 25 mg/ml concentration with 4.57% mortality, while 83.36% tick mortality was achieved at 300 mg/ml concentration (LC50=84.16 mg/ml). Meanwhile, C. tinctorius seed oil had a stronger larvicidal activity, with 91.88% tick mortality at 300 mg/ml (LC50=61.78 mg/ml) and 25.31% tick mortality at a considerably lower dosage of 25 mg/ml (Tables 3 and 4). Tick mortality was 99.69% and 100% at 200 and 300 mg/ml concentrations, respectively, when mixed seed oils. Two seed oils showed a concentration-dependent mortality effect. All of these outcomes differed significantly from the negative control (p < 0.001). No mortality rate was shown for the larvae in the negative control; however, within 24 hours of exposure to amitraz, larvae died at a rate of 77.47%. Additionally, results showed that combined use of these seed oils was highly effective and showed higher acaricidal effects with LC50 value at 24 hours of 47.96 mg/ml (Table 4). The SF value from mixing these seed oils at a 1:1 ratio is presented in Table 5. SFs against C. tinctorius larvae and female adults exhibited a synergistic effect at 1.28 and 1.02, respectively. In addition, a synergistic effect was observed against N. officinale larvae and female adults at 1.75 and 1.05, respectively. This revealed that the interaction of the two plants had a synergistic impact (SF > 1). DiscussionAs demonstrated in Table 1, linoleic acid is the most abundant fatty acid in C. tinctorius seed oil, accounting for 84.48%. Also, safflower seed oil contains appreciable saturated fatty acids, especially palmitic (6.54%) and stearic (3.77%). The oleic acid content in the Tunisian sample was low (0.33%). This finding was completely different from the previous study, which detected the same chemical components in different amounts. Our results differed from those of Carvalho et al. (2006), who obtained 42.80% linoleic acid and 29.09% oleic acid, and Sabzalian et al. (2008) who obtained 45.43% linoleic acid and 28.75% oleic acid. It was also determined that oleic acid (8.0%–21.0%) and linoleic acid (68.0%–83.0%) were the main fatty acids in safflower Turkey oils, according to Orhan et al. (2021). The fatty acid profile of N. officinale analysis by GC/MS in this study showed as major-compounds linolenic acid (50.78%), gondoic acid (13.57%), linoleic acid (10.58%), palmitic acid (8.02%), and erucidic acid (6.62%). The GC-MS analysis of commercial Egyptian watercress revealed two fatty acids: oleic acid (46.44%) and palmitic acid (10.10%) (Alagawany et al., 2018), while oleic acid was the second most abundant fatty acid (17.11%), and palmitic acid was the third most abundant fatty acid (12.89%) in Saudi watercress (Al bratty et al., 2021). According to Ben Moumen et al. (2015), the quality of vegetable oil is determined by several elements, including plant variety, maturity level, pedoclimatic factors, agricultural techniques, oil extraction technology, and storage conditions. Table 3. The activity of seed oils from N. officinale, C. tinctorius and in association on larval mortality of H. scupense.
Table 4. Lethal concentrations required to cause 50% or 90% mortality (LC50 and LC90) of H. scupense engorged females and larvae after exposure to C. tinctorius, N. officinale and their combination with a 95% confidence interval.
Table 5. SF for plant seed oils.
To the authors’ knowledge, no research on the acaricidal effects of combining C. tinctorius and N. officinale seed oils on cow ticks have been reported. The current study revealed the acaricidal effect of C. tinctorius and N. officinale seed oils on different stages of H. scupense. The tick mortality rate was dose-dependent (Tables 2 and 3). Safflower oil had a greater mortality rate of engorged females, attaining 95% mortality at 300 mg/ml and 100% egg hatching at all doses examined than seed oil from N. officinale. Carthamus tinctorius had LC50 and LC90 values of 120.11 and 210.37 mg/ml, respectively. The LC50 of N. officinale was 124.08%, while the LC90 was 341.16%. In addition, The anti-tick action of C. tinctorius oil against larval H. scupense (LC50=61.78 mg/ml) was significantly higher than that of N. officinale oil (LC50=84.16 mg/ml). It is worth noting that there are various papers on the acaricidal activities of volatile oils and extracts from diverse plants for controlling tick populations, all of which are thoroughly reported (Figueiredo et al. 2018; Alimi et al. 2021; Sbhatu et al. 2021). However, reports about the acaricidal properties of fixed oils, to which our results can be compared, are scarce. Nevertheless, Santos et al. (2021) investigated the effects of Mauritia flexuosa and Mauritiella armata seed oils on engorged females and larvae Rhipicephalus (Boophilus) microplus cattle tick; the in vitro tests revealed a typical reduction of the hatched larvae as well as inducing high larval mortality above 80% and reduction in the laying capacity of R. microplus at 5% and 10% concentrations. However, according to Villarreal et al. (2017), the fixed oils of Bertholletia excelsa (Brazil nut) and Helianthus annuus (sunflower seed) had a low effect in vitro in cattle against engorged females of R. microplus, indicating that both fixed oils had low acaricidal activity (39.39% and 58.75%, respectively, at 200 mg/ml). Another study found that the oil from Jatropha curcas seeds had high acaricidal action against R. microplus larvae, with effectiveness levels above 90% (Rizo-Borrego et al., 2019). The fatty acid content of a vegetable oil determines its appropriateness for a certain application, such as nutritional, industrial, or medicinal (Sabzalian et al., 2008). Otherwise, due to their pharmacological usefulness, fatty acids contained in vegetable oils (oleic, linoleic, lignoceric, elaidic, palmitic, palmitelaidic, and stearic) have significant biological activity in animal and human medicine (Asgarpanah and Kazemivash 2013; Orsavova et al., 2015; Jeong et al., 2020). In this study, safflower showed higher effects on ticks than watercress (which contains 10.58% linoleic acids); hence, the anti-tick action (95% mortality) on H. scupense can be associated with this polyunsaturated fatty acids mechanism of action. Many scientific studies have confirmed the effect of seed oils and their major compounds against microbial infections. Khémiri et al. (2020) reported that the safflower seed oil had high antibacterial action against the pathogenic bacterial strains studied (Enterobacter cloacae, Escherichia coli, and Streptococcus agalactiae) with inhibition diameters of 3 and 5 mm. Furthermore, the scientists speculated that safflower seed oil had an antifungal impact on spore germination. Fatty acids have been shown to block certain membrane enzymes, thus killing or inhibiting the development of bacteria (bactericidal activity) (bacteriostatic action). Furthermore, these antimicrobial actions of fatty acids might be associated with those of phytosterols found in safflower oil to improve its efficiency against pathogenic bacteria (Khémiri et al., 2020). Fatty acids have recently been found to block various membrane enzymes, such as glucosyl transferase and stimulate autolytic cell wall enzymes, leading to apoptosis. Furthermore, fatty acids are bacteriostatic and fungistatic action by reducing energy pathway synthesis in mitochondria (Khémiri et al., 2020). According to Marques et al. (2004), each vegetable oil possessing a high content of linoleic acid can be recommended as a therapeutic option in veterinary medicine. On the other hand, multiple studies have shown that the antibacterial properties of safflower are related to its phenolic component, which disrupts cell membranes (Salem et al., 2014). Other investigations have found that secondary metabolites have physiological functions in humans, animals, and microbes (Salem et al., 2011, 2014; Khémiri et al., 2020). Compared to oils tested separately, the combined oil proved to be more toxic to ticks. The main goals of deploying synergistic combinations, according to reports, are to minimize the concentration of each chemical while increasing biological activity against the target organism (Jyoti et al., 2019). Furthermore, the greater the chemical complexity of combinations, the less probable resistant populations are to develop (Arajo et al., 2016a). According to the results, the oil combination showed a significant adulticide action, reaching 100% mortality (LC90=152.08 mg/ml) (Table 4), at a slightly higher concentration than the C. tinctorius seed oil. Also, even at low concentrations, the mixture of oils showed higher larvicide action against H. scupense larvae (LC90=47.96 mg/ml) compared to C. tinctorius (LC90=61.87 mg/ml) and N. officinale (LC90=84.16 mg/ml). Plant mixtures have been shown to have synergistic effects on numerous species, such as bacteria (Didry et al., 1994), fungus (Mugnaini et al., 2012), gastro-intestinal parasites (Ntalli et al., 2011), and insects (Ntalli et al., 2011). Furthermore, the associations are viable for controlling bovine ticks (Vinturelle et al., 2017; De Carvalho Castro et al., 2019; Shezryna et al., 2020). Overall, researchers found that mixing volatile oils showed good action on R. (B.) microplus than testing them alone. Yessinou et al. (2016) studied the use of essential oils in combination as a way to improve efficacy. According to the authors, the combination of Syzygium aromaticum and C. citratus essential oils had higher potential against R. microplus than the oils studied separately. Moreover, the impact of a mixture of main elements of plant-derived essential oils on ticks has been proven (Novato et al., 2015; Arajo et al., 2016b). In addition to concentration, the ratio combination appears to have a role in the efficacy of mixed seed oils. A 1:1 ratio of C. tinctorius and N. officinale exhibited a synergistic effect. Several further studies on different ratios have been published; combining Alpinia galanga with Cymbopogoncitratus volatile oils at different ratios revealed that the 3:7 ratio was the most toxic (Shezryna et al., 2020). Poonia and Kaushik (2013) employed Pongamia pinnata and Kigelia africana plant extracts in three different ratios on Aedes aegypti: 1:1, 1:2, and 2:1. The individual usage of P. pinnata has been proven to have greater toxicity than K. africana. A 2:1 ratio of combination (P. pinnata: K. africana) produced an increased effect, but the 1:2 ratio combination (P. pinnata: K. africana) generated antagonism. Due to reported tick resistance to commercial acaricides and the risks these drugs cause negative impact humans, animals, and the environment, the combination of C. tinctorius and N. officinale is both environmentally-friendly (green pesticide) and cost-effective could be used to control cattle tick infestations. Further study is needed to conduct experiments by isolating the bioactive components to discover which one(s) could produce acaricide activity to better understand the action mechanisms. Both C. tinctorius and N. officinale seed oils were effective against H. scupense larvae and engorged females in the current investigation. A 1:1 mixture of these seed oils, on the other hand, had a synergistic effect (SF > 1). It may be concluded that C. tinctorius and N. officinale in individual usage and a 1:1 mixture of these seed oils can be recommended as a feasible synthetic acaricide alternative. Isolation and purification of bioactive chemicals might be important in developing alternative acaricidal drugs. AcknowledgmentsHigher Institute of Biotechnology of Beja, Jendouba University, Tunisia, Laboratory of Functional Physiology and Valorization of Bio-resources (UR17ES27), and Laboratory of Bioactive Substances, Centre of Biotechnology of Borj Cedria, Tunisia, provided financial support for this research. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAfsharypuor, S. and Salehi, M. 2008. Volatile constituents of leaves and stems of Nasturtium officinale R. Br. J. Essent. Oil Res. 20(6), 517–518. Alagawany, M., Abd El-Hack, M., Al-Sagheer, A., Naiel, M., Saadeldin, I. and Swelum, A. 2018. Dietary Cold Pressed Watercress and Coconut Oil Mixture Enhances Growth Performance, Intestinal Microbiota, Antioxidant Status, and Immunity of Growing Rabbits. Animals 8, 212. Alaiye, A., Kaya, E., Pınarbaşlı, M.Ö., Harmancı, N., Yıldırım, C., Dönmez, D.B. and Cingi, C. 2020. An Experimental comparison of the analgesic and anti-inflammatory effects of safflower oil, benzydamine hcl, and naproxen sodium. J. Med. Food. 23(8), 862–869. Alimi, D., Hajri, A., Jallouli, S. and Sebai, H. 2021. In vitro acaricidal activity of essential oil and crude extracts of Laurus nobilis, (Lauraceae) grown in Tunisia, against arthropod ectoparasites of livestock and poultry: Hyalomma scupense and Dermanyssus gallinae. Vet. Parasitol. 298, 109507. Al bratty, M., Alhazmi, H.A. and Thangavel, N. 2021. GC–MS profiling and in silico prediction of MAPK receptor activation by fatty acids of watercress oil for hair growth marketed in Saudi Arabia. J. Saudi Chem. Soc. 25(2), 1-10. Araújo, L.X., Novato, T.P.L., Zeringota, V., Maturano, R., Melo, D., Da Silva, B.C., Daemon, E., De Carvalho, M.G. and Monteiro, C.M.O. 2016. Synergism of thymol, carvacrol and eugenol in larvae of the cattle tick, Rhipicephalus microplus, and brown dog tick, Rhipicephalus sanguineus: Synergism of plant compounds in ticks. Med. Vet. Entomol. 30, 377–382. Asgarpanah, J. and Kazemivash, N. 2013. Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chin. J. Integr. Med. 19(2), 153–159. Asgary, S., Rahimi, P., Mahzouni, P. and Madani, H. 2012. Antidiabetic effect of hydroalcoholic extract of Carthamus tinctorius L. in alloxan-induced diabetic rats. J. Res. Med. Sci. 17(4), 386–392. Bahramikia, S. and Yazdanparast, R. 2008. Effect of hydroalcoholic extracts of Nasturtium officinale leaves on lipid profile in high-fat diet rats. J. Ethnopharmacol. 115(1), 116–121. Bahramikia, S. and Yazdanparast, R. 2010. Antioxidant efficacy of Nasturtium officinale extracts using various in vitro assay systems. J. Acupunct. Meridian Stud. 3(4), 283–290. Banumathi, B., Vaseeharan, B., Rajasekar, P., Prabhu, N.M., Ramasamy, P., Murugan, K., Canale, A. and Benelli, G. 2017. Exploitation of chemical, herbal and nanoformulated acaricides to control the cattle tick, Rhipicephalus (Boophilus) microplus—a review. Vet. Parasitol. 244, 102–110. Ben Moumen, A., Mansouri, F., Richard, G., Abid, M., Fauconnier, M.L., Sindic, M., El Amrani, A. and Serghini Caid, H. 2015. Biochemical characterisation of the seed oils of four safflower (Carthamus tinctorius) varieties grown in north-eastern of Morocco. Int. J. Food Sci. Technol. 50(3), 804–810. Bouattour, A., Darghouth, M.A. and Miled, L.B. 1996. Cattle infestation by Hyalomma ticks and prevalence of Theileria in H. detritum species in Tunisia. Vet. Parasitol. 65(3–4), 233–245. Carvalho, I.S., Miranda, I. and Pereira, H. 2006. Evaluation of oil composition of some crops suitable for human nutrition. Ind. Crops Prod. 24, 75–78. Cuénod, A. 1954. Flore de la Tunisie : Cryptogames Vasculaires, Gymnospermes et Monocotylédones. Office de l’Expérimentation et de la Vulgarisation Agricoles de Tunisie, Tunis. 1(39), pp: 287. De Carvalho Castro, K.N., Costa-Júnior, L.M., Lima, D.F., Canuto, K.M., Sousa De Brito, E., De Andrade, I.M., Teodoro, M.S., Oiram-Filho, F., Dos Santos, R.C. and Mayo, S.J. 2019. Acaricidal activity of cashew nut shell liquid associated with essential oils from Cordia verbenacea and Psidium guajava on Rhipicephalus microplus. J. Essent. Oil Res. 31, 297–304. Didry, N., Dubreuil, L. and Pinkas, M. 1994. Activity of thymol, carvacrol, cinnamaldehyde and eugenol on oral bacteria. Pharm. acta Helvet. 69, 25–28. Drummond, R.O., Ernst, S.E., Trevino, J.L., Gladney, W.J. and Graham, O.H. 1973. Boophilus annulatus and B. microplus: Laboratory Tests of Insecticides13. J. Econ. Entomol. 66, 130–133. Faizy, H.S., Esmail, L.S. and Mahdi, H.S. 2021. Phytochemicals analysis in Watercress (Nasturtium officinale) plant extracts. IOP Conf. Ser.: Earth. Environ. Sci. 761(1), 012042. Figueiredo, A., Nascimento, L.M., Lopes, L.G., Giglioti, R., Albuquerque, R.D.D.G., Santos, M.G., Falcão, D.Q., Nogueira, J.A.P., Rocha, L. and Chagas, A.C.S. 2018. First report of the effect of Ocotea elegans essential oil on Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 252, 131–136. Freese, E., Shew, C.W. and Galliers, E. 1973. Function of lipophilic acids as antimicrobial food additives. Nature 241, 321–325. Gharbi, M. and Aziz Darghouth, M. 2014. A review of Hyalomma scupense (Acari, Ixodidae) in the Maghreb region: from biology to control. Parasite 21, 2. Gharbi, M., Hayouni, M.E., Sassi, L., Dridi, W. and Darghouth, M.A. 2013. Hyalomma scupense (Acari, Ixodidae) in northeast Tunisia: seasonal population dynamics of nymphs and adults on field cattle. Parasite 20, 12. Halberstein, R.A. 2005. Medicinal plants: historical and cross-cultural usage patterns. Ann. Epidemiol. 15(9), 686–699. Hecht, S.S., Chung, F.L., Richie, J.P., Akerkar, S.A., Borukhova, A., Skowronski, L. and Carmella, S.G. 1995. Effects of watercress consumption on metabolism of a tobacco-specific lung carcinogen in smokers. Cancer Epidemiol. Biomarkers Prev. 4(8), 877–884. Jeon, J., Bong, S.J., Park, J.S., Park, Y.-K., Arasu, M.V., Al-Dhabi, N.A. and Park, S.U. 2017. De novo transcriptome analysis and glucosinolate profiling in watercress (Nasturtium officinale R. Br.). BMC Genom. 18(1), 401. Jeong, E.H., Yang, H., Kim, J.-E. and Lee, K.W. 2020. Safflower Seed Oil and Its Active Compound Acacetin Inhibit UVB-Induced Skin Photoaging. J. Microbiol. Biotechnol. 30, 1567–1573. Jia-Xi, L., Chun-Xia, Z., Ying, H., Meng-Han, Z., Ya-Nan, W., Yue-Xin, Q., Jing, Y., Wen-Zhi, Y., Miao-Miao, J. and De-An, G. 2019. Application of multiple chemical and biological approaches for quality assessment of Carthamus tinctorius L. (safflower) by determining both the primary and secondary metabolites. Phytomedicine 58, 152826. Jyoti, N.K., Singh, H., Mehta, N. and Rath, S.S. 2019. In vitro assessment of synergistic combinations of essential oils against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Exp. Parasitol. 201, 42–48. Kalyanasundaram, M. and Das, P.K. 1985. Larvicidal and synergestic activity of plant extracts for mosquito control. Indian J. Med. Res. 82, 19–23. Khalid, N., Khan, R.S., Hussain, M.I., Farooq, M., Ahmad, A. and Ahmed, I. 2017. A comprehensive characterisation of safflower oil for its potential applications as a bioactive food ingredient—a review. Trends Food Sci. Technol. 66, 176–186. Khémiri, I., Essghaier, B., Sadfi-Zouaoui, N. and Bitri, L. 2020. Antioxidant and antimicrobial potentials of seed oil from Carthamus tinctorius L. in the management of skin injuries. Oxid. Med. Cell. Longev. 2020, 1–12. Kumar, B., Manjunathachar, H.V. and Ghosh, S. 2020. A review on Hyalomma species infestations on human and animals and progress on management strategies. Heliyon 6(12), e05675. Labarta, V., Rodríguez, M., Penichet, M., Lleonart, R., Lorenzo Luaces, L. and de la Fuente, J. 1996. Simulation of control strategies for the cattle tick Boophilus microplus employing vaccination with a recombinant Bm86 antigen preparation. Vet. Parasitol. 63(1–2), 131–160. Marques, S.R., Peixoto, C.A., Messias, J.B., Albuquerque, A.R. de and Silva Junior, V.A. da. 2004. The effects of topical application of sunflower-seed oil on open wound healing in lambs. Acta Cir. Bras. 19, 196–209. Matos, R.S., Daemon, E., de Oliveira Monteiro, C.M., Sampieri, B.R., Marchesini, P.B.C., Delmonte, C. and Camargo-Mathias, M.I. 2019. Thymol action on cells and tissues of the synganglia and salivary glands of Rhipicephalus sanguineus sensu lato females (Acari: Ixodidae). Ticks Tick Borne Dis. 10, 314–320. Mugnaini, L., Nardoni, S., Pinto, L., Pistelli, L., Leonardi, M., Pisseri, F. and Mancianti, F. 2012. In vitro and in vivo antifungal activity of some essential oils against feline isolates of Microsporum canis. J. Mycol. Med. 22, 179–184. Nickavar, B., Mojab, F., Javidnia, K. and Roodgaramoli, M.A. 2003. Chemical composition of the fixed and volatile oils of Nigella sativa L. from Iran. Z. Naturforsch. C. 58, 629–631. Novato, T.P.L., Araújo, L.X., de Monteiro, C.M.O., Maturano, R., Senra, T. de O.S., da Silva Matos, R., Gomes, G.A., de Carvalho, M.G. and Daemon, E. 2015. Evaluation of the combined effect of thymol, carvacrol and ( E )-cinnamaldehyde on Amblyomma sculptum (Acari: Ixodidae) and Dermacentor nitens (Acari: Ixodidae) larvae. Vet. Parasitol. 212, 331–335. Ntalli, N.G., Ferrari, F., Giannakou, I. and Menkissoglu-Spiroudi, U. 2011. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece: Terpene interactions and essential oil nematicidal activity against M. incognita. Pest. Manag. Sci. 67, 341–351. Orhan, D.D., Pekacar, S., Ulutaş, O.K., Özüpek, B., Sümmeoğlu, D. and Berkkan, A. 2021. Assessment of Commercially Safflower Oils (Carthami Oleum Raffinatum) In Terms of European Pharmacopoeia Criteria and Their Weight Control Potentials. Turk. J. Pharm. Sci. DOI: 10.4274/tjps.galenos.2021.84484. Orsavova, J., Misurcova, L., Ambrozova, J., Vicha, R. and Mlcek, J. 2015. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 16, 12871–12890. Ozkan, K., Bekiroglu, H., Bayram, Y., Sagdic, O. and Erbas, S. 2021. In vitro bioaccessibility, antioxidant and antibacterial activities of three different safflower (Carthamus tinctorius L.) genotypes. Food Sci. Technol. Ahead of print: 1–7. Pandey, Y., Bhatt, S. and Debbarma, N. 2018. Watercress (Nasturtium officinale): a potential source of nutraceuticals. Int. J. Curr. Microbiol. App. Sci. 7(2), 2685–2691. Poonia, S. and Kaushik, R. 2013. Synergistic activity of a mixture of Pongamia pinnata (Karanj) and Kigelia africana (Sausage tree) leaf extracts against yellow fever mosquito, Aedes aegypti. Pak. Entomol. 35, 1-4. Quadros, D.G., Johnson, T.L., Whitney, T.R., Oliver, J.D. and Oliva Chávez, A.S. 2020. Plant-derived natural compounds for tick pest control in livestock and wildlife: Pragmatism or utopia? Insects 11(8), 490. Ramezani, S., Javadi, I., Kokhdan, E., Omidifar, N., Nikbakht, J., Sadeghi, H., Doustimotlagh, A., Danaei, N., Abbasi, R. and Sadeghi, H. 2021. Protective and therapeutic effects of ethanolic extract of Nasturtium officinale (watercress) and vitamin E against bleomycin-induced pulmonary fibrosis in rats. Res. Pharma Sci. 16(1), 94. Ribeiro, V.L.S., Avancini, C., Gonçalves, K., Toigo, E. and Von Poser, G. 2008. Acaricidal activity of Calea serrata (Asteraceae) on Boophilus microplus and Rhipicephalus sanguineus. Vet. Parasitol. 151, 351–354. Rizo-Borrego, A., Soca-Pérez., M., García-Marrero., D.E., Fuentes-Castillo., A., Giupponi-Cardoso., P., Arece-García., J. and Cepero-Casas, L. 2019. Acaricidal activity of the oil from Jatropha curcas L. seeds on larvae of Rhipicephalus (Boophilus) microplus (Canestrini, 1887) (Acari: Ixodidae). Pastos. Forrajes. 42, 189-193. Sabzalian, M.R., Saeidi, G. and Mirloh, A. 2008. Oil content and fatty acid composition in seeds of three safflower species. J. Am. Oil Chem. Soc. 85, 717–721. Salem, N., Msaada, K., Elkahoui, S., Mangano, G., Azaeiz, S., Ben Slimen, I., Kefi, S., Pintore, G., Limam, F. and Marzouk, B. 2014. Evaluation of antibacterial, antifungal, and antioxidant activities of safflower natural dyes during flowering. BioMed. Res. Int. 2014, 1–10. Salem, N., Msaada, K., Hamdaoui, G., Limam, F. and Marzouk, B. 2011. Variation in Phenolic composition and antioxidant activity during flower development of Safflower (Carthamus tinctorius L.). J. Agric. Food Chem. 59(9), 4455–4463. Santos, G.S.C., Morais-Costa, F., Figueiredo, J.C.G., Cruz, J.P., Arrudas, S.R., Nunes, Y.R.F., Duarte, E.R. and Vasconcelos, V. de O. 2021. Chemical composition and acaricidal activity of seed oils of the palms Mauritia flexuosa and Mauritiella armata in Rhipicephalus microplus (Ixodidae). Res. Soc. Dev. 10(13), e167101321078. Sbhatu, D.B., Abraha, H.B., Gebreyohannes, G. and Demewoz, G.M. 2021. Larvicidal effectiveness of aqueous extracts of Solanum incanum L. (Solanaceae) against Boophilus decoloratus (Acari: Ixodidae) cattle tick larvae. Cogent. Food Agric. 7(1), 1949853. Shezryna, S., Anisah, N., Saleh, I. and Syamsa, R.A. 2020. Acaricidal activity of the essential oils from Citrus hystrix (Rutaceae) and Cymbopogon citratus (Poaceae) on the cattle tick Rhipicephalus (Boophilus) microplus larvae (Acari: Ixodidae). Trop. Biomed. 37, 433–442. Stone, B.F. and Haydock, K.P. 1962. A method for measuring the acaricide-susceptibility of the cattle tick Boophilus microplus (Can.). Bull. Entomol. Res. 53, 563–578. Tanwar, B. and Goyal, A. 2021. Oilseeds: health attributes and food applications. Springer Singapore, Singapore. Teixidor-Toneu, I., Martin, G.J., Ouhammou, A., Puri, R.K. and Hawkins, J.A. 2016. An ethnomedicinal survey of a Tashelhit-speaking community in the High Atlas, Morocco. J. Ethnopharmacol. 188, 96–110. Vergallo, C. 2020. Nutraceutical vegetable oil nanoformulations for prevention and management of diseases. Nanomaterials 10(6), 1232. Villarreal, J.P.V., Santos, P.R. dos, Silva, M.A.M.P. da, Azambuja, R.H.M., Gonçalves, C.L., Escareño, J.J.H., Santos, T.R.B. dos, Pereira, C.M.P. de, Freitag, R.A. and Nascente, P. da S. 2017. Evaluation of phytotherapy alternatives for controlling Rhipicephalus (Boophilus) microplus in vitro. Rev. Bras. Parasitol. Vet. 26, 299–306. Vinturelle, R., Mattos, C., Meloni, J., Nogueira, J., Nunes, M.J., Vaz, I.S., Rocha, L., Lione, V., Castro, H.C. and Chagas, E.F. das. 2017. In Vitro Evaluation of Essential Oils Derived from Piper nigrum (Piperaceae) and Citrus limonum (Rutaceae) against the Tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Biochem. Res. Int. 2017, 1–9. Yessinou, R.E., Akpo, Y., Adoligbe, C., Adinci, J., Assogba, N., Koutinhouin, B., Youssao, I. and Karim, A. 2016. Resistance of tick Rhipicephalus microplus to acaricides and control strategies. J. Entomol. Zool. Stud. 4, 408-414. Zeb, A. 2015. Phenolic profile and antioxidant potential of wild watercress (Nasturtium officinale L.). Springerplus 4(1), 714. Zemour, K., Labdelli, A., Adda, A., Dellal, A., Talou, T. and Merah, O. 2019. Phenol content and antioxidant and antiaging activity of safflower seed oil (Carthamus tinctorius L.). Cosmetics 6(3), 55. Zhou, X., Tang, L., Xu, Y., Zhou, G. and Wang, Z. 2014. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: a phytochemical and pharmacological review. J. Ethnopharmacol. 151(1), 27–43. | ||
| How to Cite this Article |
| Pubmed Style Alimi D, Hajri A, Jallouli S, Sebai H. Efficacy of synergistic activity of seed oils from Carthamus tinctorius (Safflower) and Nasturtium officinale (Watercress) on lethality of the cattle tick Hyalomma scupense (Acari: Ixodidae). Open Vet. J.. 2022; 12(1): 80-90. doi:10.5455/OVJ.2022.v12.i1.10 Web Style Alimi D, Hajri A, Jallouli S, Sebai H. Efficacy of synergistic activity of seed oils from Carthamus tinctorius (Safflower) and Nasturtium officinale (Watercress) on lethality of the cattle tick Hyalomma scupense (Acari: Ixodidae). https://www.openveterinaryjournal.com/?mno=35987 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i1.10 AMA (American Medical Association) Style Alimi D, Hajri A, Jallouli S, Sebai H. Efficacy of synergistic activity of seed oils from Carthamus tinctorius (Safflower) and Nasturtium officinale (Watercress) on lethality of the cattle tick Hyalomma scupense (Acari: Ixodidae). Open Vet. J.. 2022; 12(1): 80-90. doi:10.5455/OVJ.2022.v12.i1.10 Vancouver/ICMJE Style Alimi D, Hajri A, Jallouli S, Sebai H. Efficacy of synergistic activity of seed oils from Carthamus tinctorius (Safflower) and Nasturtium officinale (Watercress) on lethality of the cattle tick Hyalomma scupense (Acari: Ixodidae). Open Vet. J.. (2022), [cited January 25, 2026]; 12(1): 80-90. doi:10.5455/OVJ.2022.v12.i1.10 Harvard Style Alimi, D., Hajri, . A., Jallouli, . S. & Sebai, . H. (2022) Efficacy of synergistic activity of seed oils from Carthamus tinctorius (Safflower) and Nasturtium officinale (Watercress) on lethality of the cattle tick Hyalomma scupense (Acari: Ixodidae). Open Vet. J., 12 (1), 80-90. doi:10.5455/OVJ.2022.v12.i1.10 Turabian Style Alimi, Dhouha, Azhar Hajri, Selim Jallouli, and Hichem Sebai. 2022. Efficacy of synergistic activity of seed oils from Carthamus tinctorius (Safflower) and Nasturtium officinale (Watercress) on lethality of the cattle tick Hyalomma scupense (Acari: Ixodidae). Open Veterinary Journal, 12 (1), 80-90. doi:10.5455/OVJ.2022.v12.i1.10 Chicago Style Alimi, Dhouha, Azhar Hajri, Selim Jallouli, and Hichem Sebai. "Efficacy of synergistic activity of seed oils from Carthamus tinctorius (Safflower) and Nasturtium officinale (Watercress) on lethality of the cattle tick Hyalomma scupense (Acari: Ixodidae)." Open Veterinary Journal 12 (2022), 80-90. doi:10.5455/OVJ.2022.v12.i1.10 MLA (The Modern Language Association) Style Alimi, Dhouha, Azhar Hajri, Selim Jallouli, and Hichem Sebai. "Efficacy of synergistic activity of seed oils from Carthamus tinctorius (Safflower) and Nasturtium officinale (Watercress) on lethality of the cattle tick Hyalomma scupense (Acari: Ixodidae)." Open Veterinary Journal 12.1 (2022), 80-90. Print. doi:10.5455/OVJ.2022.v12.i1.10 APA (American Psychological Association) Style Alimi, D., Hajri, . A., Jallouli, . S. & Sebai, . H. (2022) Efficacy of synergistic activity of seed oils from Carthamus tinctorius (Safflower) and Nasturtium officinale (Watercress) on lethality of the cattle tick Hyalomma scupense (Acari: Ixodidae). Open Veterinary Journal, 12 (1), 80-90. doi:10.5455/OVJ.2022.v12.i1.10 |