| Original Article | ||
Open Vet. J.. 2022; 12(4): 540-550 Open Veterinary Journal, (2022), Vol. 12(4): 540–550 Original Research Left lateral flank approach for spaying in catsMohammad Raguib Munif*, Mst. Sanjida Safawat and Abdul HannanDepartment of Surgery and Obstetrics, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, Bangladesh *Corresponding Author: Mohammad Raguib Munif. Department of Surgery and Obstetrics, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, Bangladesh. Email: md.raguibmunif [at] gmail.com Submitted: 31/05/2022 Accepted: 07/07/2022 Published: 15/08/2022 © 2022 Open Veterinary Journal
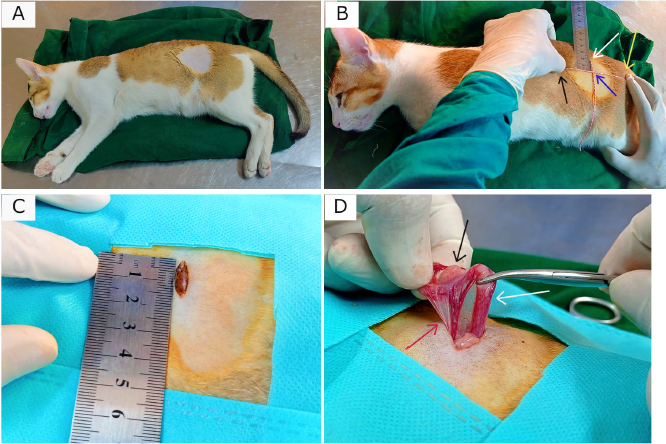
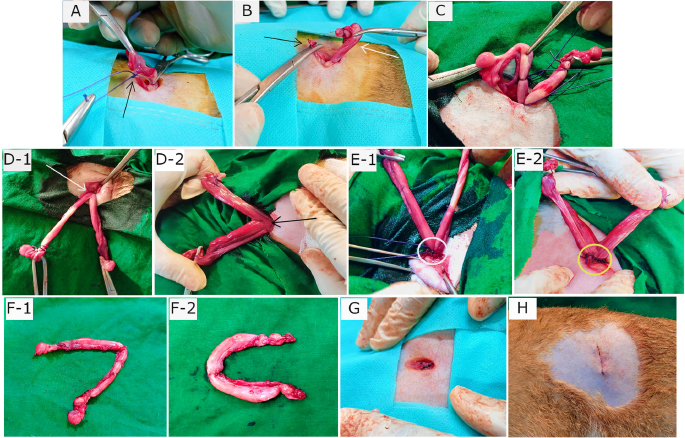
AbstractBackground: Spaying is considered a reliable surgical method for birth control and preventing potential feline reproductive diseases. Aim: This experiment was carried out to evaluate the suitability of the left lateral flank approach for routine spaying in cats. Methods: Twenty-seven queens of 7–24 months old and 1.5–3.5 kg body weight (BW) were spayed through flank laparotomy on the left lateral side of the abdomen. The cats were categorized into two groups: Group A (n=12; cats did not yet give birth) and Group B (n=15; cats gave birth before). The studied variables included age, BW, vaccination history, skin incision length, total surgical duration, the time needed for ligating ovarian pedicles and uterine body, suture materials, postoperative complications, and healing duration. Results: There were no significant differences (p < 0.05) in age, BW, and vaccination history of the cats between the two groups. The mean incision length and total duration of surgery were greater in the case of Group B than in Group A. The ease of entering into the peritoneal cavity, duration of ligating the ovarian pedicles, and transfixing the uterine body did not vary significantly (p < 0.05) between the groups although a longer time was taken for Group B than Group A. 62.96% spayed cats were found with no postoperative complication. The observed complications included wound site infection (7.41%), dehiscence of suture lines (11.11%), bleeding (3.70%), and oozing (14.82%) from wounds which were further treated successfully for complete recovery. Conclusion: Left lateral flank approach can be an effective method of spaying in queens without any life-threatening complications. Keywords: Ovariohysterectomy, Flank celiotomy, Ligation, Postoperative complication, Queens. IntroductionUnwanted domestic and stray cats are a global problem as it gives rise to various issues of cat welfare (Kiani et al., 2014; Roberts et al., 2015). Spaying is the most common surgery performed in female cats as a means of population control (Levy and Wilford, 2013; Murugesan et al., 2020) which involves removing both the ovaries and uterus called ovariohysterectomy, and sometimes only the ovaries are surgically removed (ovariectomy) for sterilization (Omeran et al., 2014). Apart from only birth control, spaying stamps out the incidences of pyometra, metritis, dystocia, uterine torsion, localized or diffuse cystic endometrial hyperplasia, uterine rupture, uterine neoplasia, mammary tumors, and many other reproductive disorders (Stone, 2003; Murugesan et al., 2020). It also decreases the rate of pseudopregnancy and increases the longevity of animals (Michell, 1999; Kiani et al., 2014). Cats usually exhibit their first heat (estrus) around 6 months of age (White, 2012). In cats, the behavioral signs of estrus (i.e., frequent and irritating vocalization, head rubbing to owners, rolling on the floor, tail deviation, treading, nymphomania, and lordosis), very short inter-estrus interval, and chances of conceiving frequently initiate the pet owners to go for ovariohysterectomy (Heffelfinger, 2006; Nelson and Couto, 2014; Oliveria et al., 2014). Elimination of estrus is often required to check vaginal bleeding, behavioral changes, the attraction of males, and undesired mating (Valle and Junior, 1999). Estrus-influenced female sexual behaviors in cats can be completely suppressed by ovariohysterectomy or ovariectomy with or without changes in the nonsexual behaviors (Hart and Eckstein, 1997; Sontas and Ekici, 2007). Usually, feline ovariohysterectomy is performed either through a ventral midline approach or a lateral flank approach (Babu et al., 2018; Murugesan et al., 2020). The flank approach being a substitute for the traditional ventral midline approach for spaying is found to be preferred in the UK, and the midline approach is popular in the USA (Coe et al., 2006; Kiani et al., 2014). The ventral midline approach is recommended when the female cats are pregnant or in various uterine pathological conditions although there may be more hemorrhage and chances of wound infection due to the ventral position, and the wound complications are often difficult to get notified (Babu et al., 2018). Hence, a lateral flank approach is preferred by many veterinarians for ovariohysterectomy in cats. The cost and duration involved in the procedures of ovariohysterectomy largely depend on incision length, closure of the muscles and skin, and for that reason, it is needed to follow such a surgical technique that can be practiced in the least invasive way, e.g., involvement of minimum incision, trauma, and suture materials with short duration. Therefore, this research was conducted to justify the left lateral flank approach as a minimally invasive surgery for regular spaying in cats focusing on better healing with minimum complications. Materials and MethodsThis research work was carried out from May 2021 to March 2022 in the Veterinary Teaching Hospital of Bangladesh Agricultural University to execute spaying in female cats through celiotomy in the left lateral flank. Experimental animalsThis research work included 27 female cats of 1.5–3.5 kg body weight (BW) and aged 7–24 months, which were brought to the Veterinary Teaching Hospital to spay for birth control. Before the surgery, the cats were thoroughly checked whether they had any estrus, pregnancy, or other systemic diseases, and found to be healthy having no such kind of incidences. In addition, the queens were recommended to be fasted for 16 hours before spaying to prevent anesthesia-related complications. Experimental groupsFor this experiment, the queens were categorized into two groups: Group A and Group B. Group A consisted of 12 female cats that did not give birth to kittens yet, whereas 15 cats that gave birth before were included in Group B. Studied particulars of the catsIn both groups, the recorded data from the cats were based on age, BW, and vaccination history for cat flu and rabies. Again, during and after spaying several variables, i.e., length (cm) of given skin incision, total time (minute) needed for surgery, total time (minute) in finding out to ligating the ovarian vascular pedicles, time (minute) involved in ligating uterine body with the frequency of simple and multiple transfixing ligatures, amount of used suture materials, postoperative complications and required time (day) for complete healing were studied to compare within the two groups. Procedure of spayingTo perform spaying, the fasted cats in both groups were premedicated with Atropine sulfate [at] 0.04 mg/kg BW (Atrovet®, Techno Drugs Ltd., Narsingdi, Bangladesh) followed by sedation with Xylazine hydrochloride (HCl) [at] 1.1 mg/kg BW (Xylaxin®, Indian Immunologicals Ltd., Hyderabad, India) through intramuscular (IM) injections. After 10 minutes, Ketamine HCl [at] 16 mg/kg BW (KetalarTM, Popular Pharmaceuticals Ltd., Dhaka, Bangladesh) was used as IM injection to the cats for induction of general anesthesia. Under anesthesia in both groups, each cat was placed in the right lateral recumbency with the upper exposure of left flank (Fig. 1A). After presurgical preparations and proper aseptic procedures, a vertical skin incision (dorsoventral) about 1.5–2.5 cm long depending on animal body and size was given in the left lateral flank region (Fig. 1C). The landmark of this incision was the midpoint of an imaginary straight line (horizontal) from the caudal part of the last rib to the hip joint, about 2 cm below the transverse processes of lumbar vertebrae (Fig. 1B). After skin incision, subcutaneous tissue, abdominal muscles, and peritoneum were exposed through a small stab incision, and thereafter blunt dissection was done by a curved mosquito forceps not heavily tearing the muscle fibers to get entry into the peritoneal cavity. After getting access to the cavity, the jaws of the mosquito forceps were spread to enlarge the opening to get easy approach to the uterine horn of that side (left). Then the uterine horn along with concomitant ovary was pulled out (Fig. 1D) from the cavity by gentle traction through exploring a spay hook. The suspensory ligament of the ovary was ruptured manually by traction, and a large opening was made by tearing the broad ligament (mesovarium). Then, the ovarian pedicle along with associated blood vessels was ligated (Fig. 2A) using Polyglactin 910 of of size 2-0 (VicrylTM, Ethicon, J & J Medical Devices Companies, United States). Small artery forceps (curved) were used to aid in holding the ovarian stump during ligation and detachment from the ovary containing horn (Fig. 2B). Then the severed stump was checked carefully to confirm no hemorrhage and returned to the cavity. Similarly, the contralateral right uterine horn was detected and withdrawn (Fig. 2C) from the cavity by spay hook. This horn was found in a bit easier way as the former detached horn was used as a guide to search this one from the bifurcation. Again, the same procedure of ligation was applied, and the right ovary with horn was detached, and the stump was left back to the cavity. Then the free horns (left and right) were pulled and elevated together to expose the uterine body (Fig. 2D-1 and D-2) while taking care of hemorrhage that could be associated with the rupture of blood vessels within the broad ligament (mesometrium) near the junction of uterine body and horns. The uterine body was grasped with non-crushing forceps to prevent rupture and bleeding, whereas the collateral uterine blood vessels were ligated (if necessary) with VicrylTM (3-0). Transfixing ligatures (single or multiple) using VicrylTM (2-0) were applied to encompass the uterine body (Fig. 2E-1 and E-2) just near to the cervix. After proper ligation, the uterine horns along with a small part of uterine body before the ligature were transected and removed (Fig. 2F-1 and F-2). Finally, the uterine stump was checked carefully to confirm no hemorrhage, and subsequently returned to the cavity. Then a few drops of normal saline (0.9% NaCl) were introduced into the cavity to check evaporative loss. After that, the laparotomic incision was closed with 2–3 stitches by suturing the muscles (Fig. 2G) in a single layer of simple interrupted suture pattern using VicrylTM (2-0). The skin was closed by intradermal suture (Fig. 2H) using VicrylTM (3-0). After completion of spaying, the site of the incision was soaked with 10% povidone-iodine (Viodin® 10% solution, Square Pharmaceuticals Ltd., Dhaka, Bangladesh) to prevent contamination, and Elizabethan collar was recommended to use up to 2 weeks.
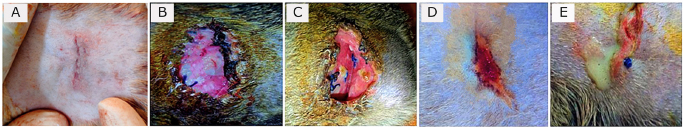
Fig. 1. Surgical landmark to make incision to find out ovary and uterine horn for spaying in cat. (A) Cat in right lateral recumbency with left flank (shaved) upward. (B) Measurement of the point of incision (blue arrowed intersection point of two threads) between the last rib (black arrow) and hip joint (yellow arrow) having 2 cm distance from lumbar transverse processes (white arrow). (C) A skin incision of 1.5 cm. (D) Exteriorized uterine horn (white arrow) with ovary (black arrow) attached to the vascular pedicle (red arrow). Postoperative complications and managementsIn both groups, most of the casts did not show any complication (Fig. 3A) after surgery; however, some were found with wound site infection (Fig. 3B), dehiscence of suture lines (Fig. 3C), bleeding (Fig. 3D), and oozing (Fig. 3E) from the wounds. These postoperative complications were treated successfully by regular dressing of the wounds with Viodin® 10% solution for 5 days. However, Ceftriaxone [at] 35 mg/kg BW (Trizon Vet, ACME Laboratories Ltd., Dhaka, Bangladesh) for 7 days, Ketoprofen [at] 2.2 mg/kg BW (Ketovet, Techno Drugs Ltd., Narsingdi, Bangladesh) for 5 days and Pheniramine Maleate [at] 1 mg/kg BW (Antihista-Vet®, Square Pharmaceuticals Ltd., Dhaka, Bangladesh) for 7 days along with oral Vitamin-C supplements for 14 days were maintained postoperatively for all the cats for better healing and complete recovery. After spaying, the skin wounds of all the cats in both groups were completely healed within 1–3 weeks depending on the absence or presence of complications. Statistical analysisThe data obtained from this experiment were calculated as “mean ± standard error” and percentage (%) for all the cats in both Group A and Group B. Independent Samples t-tests were performed for data analysis using IBM SPSS Statistics (Version 22) to compare the means of parametric variables (i.e., age, BW, skin incision length, total surgical duration, the time needed for ligating ovarian pedicles and uterine body, suture materials, and healing duration) between the groups. p < 0.05 was considered as statistically significant for the tests. Ethical approvalThis experimental methodology was approved by the Animal Welfare and Experimentation Ethics Committee of Bangladesh Agricultural University (Approval No. AWEEC/BAU/2022-12).
Fig. 2. Phases of ligation and transection during the process of ovariohysterectomy along with wound closure. (A) Ligated ovarian vascular pedicle (black arrow). (B) Ovary bearing uterine horn (white arrow) separated from pedicle (black arrow). (C) Externalized contralateral uterine horn with ovary having attachments. (D-1) Exposure of uterine body (white arrow) with extended horns in a cat of Group A. (D-2) Uterine body (black arrow) with horns in a cat of Group B. (E-1) Single transfixing ligature in uterine body (white circle). (E-2) Multiple transfixing ligatures in uterine body (yellow circle). (F-1) Transected uterine horns and ovaries accompanying a little portion of uterine body from a cat of Group A. (F-2) Discarded uterine horns and ovaries with a smaller part of uterine body from a cat of Group B. (G) Closure of muscles. (H) Closure of skin.
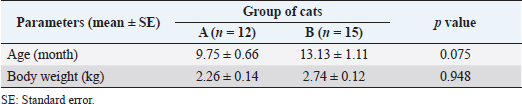
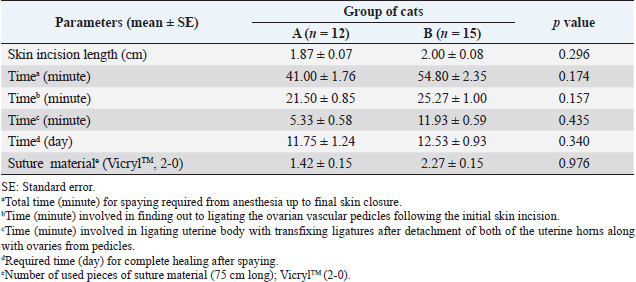
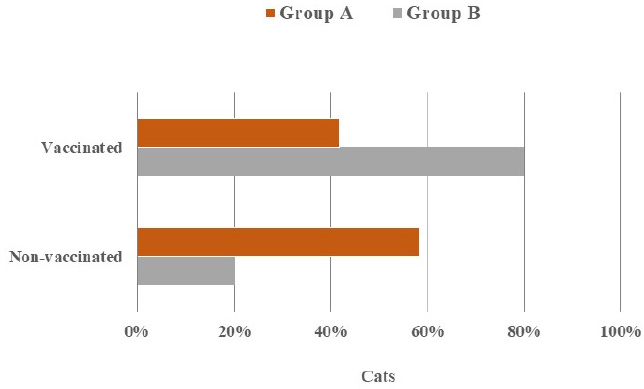
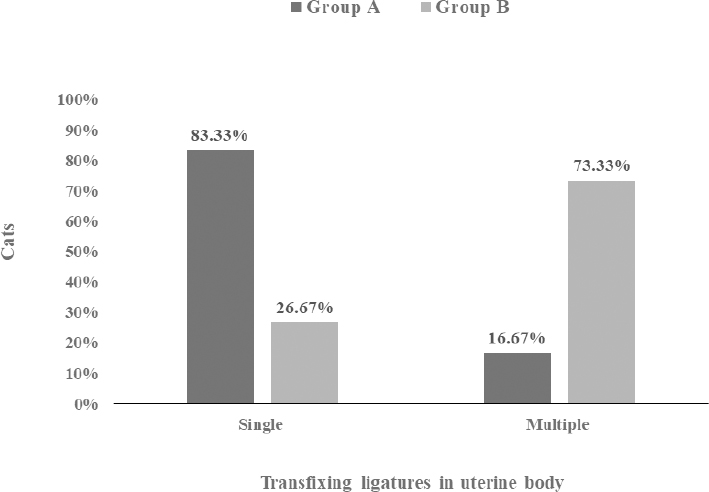
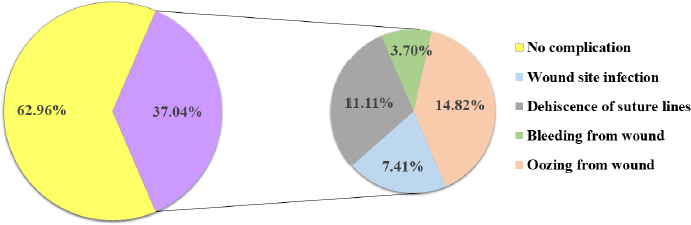
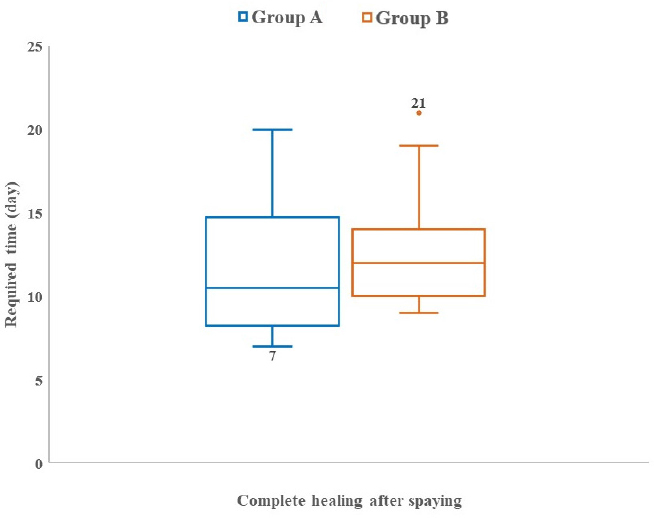
Fig. 3. Absence and presence of postoperative complications. (A) No wound complication toward complete healing. (B) Wound site infection. (C) Dehiscence of suture lines. (D) Bleeding from wound. (E) Oozing from wound. ResultsThe age (month) and BW (kg) of the female cats in both Group A and Group B are presented in Table 1. There were no significant (p < 0.05) differences in the mean values of age and body weight of the cats between the two groups. Although the queens in Group B having kittens were older and heavier than their contemporaries of Group A that did not given birth to any kittens before. The percentages (%) of the vaccinated and non-vaccinated cats against cat flu and rabies in both groups are shown in Figure 4. In Group A, around 42% of cats were vaccinated, whereas 80% of cats were vaccinated in Group B. The times needed for overall spaying, finding out to ligating ovarian vascular pedicles and uterine body, complete healing after surgery; and the required amount of suture materials (i.e., VicrylTM, 2-0 of 75 cm cm length) in the cats of both groups (Groups A and B) are furnished in Table 2. There were no significant (p < 0.05) differences in the mean values of these variables between the groups. However, the cats in Group B were found with comparatively longer skin incision as well as higher operative times for entire spaying involved in a longer duration of finding and ligating the ovarian pedicles and uterine body. In addition, greater requirements of suture materials were found during surgery in Group B cats, and after spaying, these queens took higher time for complete healing than those of Group A. The frequencies of using either single or multiple transfixing ligatures (%) in the uterine body of the cats in both groups during spaying are illustrated in Figure 5. In Group A, during spaying, the feline uterine bodies were ligated mostly with single transfixing ligatures (83.33%), whereas frequent multiple ligatures (73.33%) were observed to be used in Group B for the same purpose. The absence and presence of postoperative complications, irrespective of groups of overall spayed queens (n=27), are embellished in Figure 6. In 62.96% cases, there were no observed complications postoperatively. On the other hand, some cats (37.04%) were found to have few complications, i.e., wound site infection (7.41%), dehiscence of suture lines (11.11%), and bleeding (3.70%) and oozing (14.82%) from wounds after spaying. The durations for complete healing of the female cats after spaying in both groups are shown in Figure 7. In Group B, the queens took a range of days from 9 to 21 for complete healing, whereas 7–20 days were required for the queens to recover in Group A. As the medians are closer to the bottom of the boxes and the whiskers are shorter on the lower ends of the boxes (Fig. 7), the distributions are positively skewed in both groups; which indicates that the most required durations for complete healing of the cats were about 7–11 days and 9–12 days in Group A and Group B, respectively. DiscussionIn the present study, spaying (ovariohysterectomy) in queens was performed through left lateral flank celiotomy in a minimum invasive way. This type of celiotomic procedure for spaying in small animals was also reported by other researchers (McGrath et al., 2004; Coe et al., 2006; Kiani et al., 2014; Babu et al., 2018). This research work is consistent with the reports of others (Janssens and Janssens, 1991; Brooks, 2001; Spain et al., 2004; Kustritz, 2007) who described that spaying means ovariohysterectomy where both the ovaries and uterus are surgically removed. Table 1. Age (month) and body weight (kg) of the female cats.
Table 2. Length of skin incision, required times and suture materials in different phases of spaying in cats.
Fig. 4. Clustered bar chart showing the percentages of vaccinated and non-vaccinated cats in Group A and Group B. For ovariohysterectomy in cats, the recommended length of the incision for the lateral flank approach is 2–3 cm (Coe et al., 2006; Rana, 2007; Babu et al., 2018), and about 1.5–2.5 cm long skin incisions were used for ovariohysterectomy in this research being comparatively shorter than the incision length for ventral midline approach (Ghanawat and Mantri, 1996; Rana, 2007). Freeman et al. (1987) reported that an initial 6 cm long midline incision was used for spaying, whereas Coe et al. (2006) observed relatively smaller incision for the same purpose and stated that it was rarely necessary to extend the incision size in flank approach to find out the uterus, and having longer incision, it would take comparatively a short time for the operation. Although larger incisions may facilitate better exposure for locating the uterine horns and body, it might induce increased possibility of surgical trauma and hemorrhage with the greater requirements of suture materials to deal with the situations. The body weight of the female cats selected for spaying is harmonious with those of the studies of Verstegen et al. (2008) and Murugesan et al. (2020), while the suturing techniques are in compliance with the findings of Kiani et al. (2014), Omeran et al. (2014), Babu et al. (2018), and Murugesan et al. (2020). Left lateral site incision is preferred for flank approach (McGrath et al., 2004), and several incision patterns (i.e., horizontal flank incision, vertical flank incision, and oblique flank incision) are reported for spaying in cats (Hoque, 1991; Babu et al., 2018). Meanwhile, this research observed the vertical incisions applied on the left lateral flank of the cats. Flank approach was selected for the female cats because of several advantages. Holly and Hardie (2004), McGrath et al. (2004), and Kiani et al. (2014) observed that queens with excessive mammary development due to lactation or mammary gland hyperplasia should be considered for the flank approach as it provides the ease of observing the surgical wound from a distance ensuring the prevention of leakage, hemorrhage and inflammation from adjacent tissues after the operation, and reduces the risks of evisceration if wound dehiscence occurs. Hence, these justifications might be the reasons for the occurrences of neither bowel evisceration nor severe wound complications after spaying of the queens in this study followed by the privilege of effective postoperative managements. On the contrary, flank ovariohysterectomy may provoke some definite complications involving the difficulties in exteriorizing and removing the whole uterine body up to the cervix, chances to drop and loss the ovarian pedicle in the cavity before ligating during operation which may be hard to get back again, and the inconveniences to get the exposure of the opposite ovary as well as the uterine bifurcation sometimes (Ghanawat and Mantri, 1996; Fingland, 1998; Hedlund, 2002; Stone, 2003). According to Kaini et al. (2014), the midline approach is usually selectable for spaying in those cases where either the uterus cannot be accessed from the flank approach, or confusions may arise from other technical errors to ascertain an already spayed cat. In case of uterus unicornis being an unusual congenital defect, the flank approach is considered inferior to handle the condition (Murugesan et al., 2020). In addition, Gorelick (1974) found the discoloration of regrown hairs in some cats after pre-surgical clipping or shaving predominantly with flank spaying. However, this study did not observe such kind of complications and difficulties during the surgical procedure of ovariohysterectomy in the queens. This might be due to very skillful procedures to operate the cases, and presence of no anatomical and developmental abnormalities in the cats.
Fig. 5. Stacked bar chart showing single and multiple transfixing ligatures (%) in uterine body of the cats in Group A and Group B.
Fig. 6. Pie chart showing the absence (62.96%) and presence (37.04%) of postoperative complications in the cats of both groups.
Fig. 7. Box and Whisker plots showing the required time (day) for complete healing after spaying in the cats of Group A and Group B. During the surgery, all the cats were generally anaesthetized in the present research, which is in agreements with the reports of other authors (Coe et al., 2006; Kaini et al., 2014; Omeran et al., 2014; Fazio et al., 2015; Costa et al., 2017; Babu et al., 2018; Murugesan et al., 2020). This study revealed that the queens have already given birth to kittens were superior in BW (kg) and age (month) than the queens having no experience of delivery yet. This is due to the normal phenomena of body growth with age (Bellows et al., 2016) and female reproductive consequences (Tsutsui and Stabenfeldt, 1993; Wildt et al., 1998; Brown and Comizzoli, 2018). In addition, the vaccination percentages were greater in the older cats than the younger ones, which might be attributed to the chances of getting immunized over a longer period of time. In this research, comparatively lengthier skin incision was used for spaying in the older and heavier cats of Group B. Moreover, these cats were found to experience increased duration for entire spaying including extended intraoperative and postoperative phases, i.e., searching out the uterine horns and ovaries, ligation of ovarian pedicles and uterine body, and complete recovery after surgery than those of Group A. Likewise, increased usage of suture materials was recorded in the said cats (Group B) than the others (Group A). These findings might be due to the reasons of greater size and anatomical structure of the cats, diameter and thickness of attached structures and organs, excessive intra-abdominal fat causing hindrance to the exposure of contralateral structures during spaying, engorged and dilated blood vessels supplying to the associated organs, patent uterine horns and body, and well-developed ovary with vascular pedicles in the aged cats with the evidence of kitten delivery than the cats had not delivered before. However, in some cases of Group A, it was a bit time-consuming to locate and find out the opposite uterine horn through the small lateral flank incision. This might be because of some facts in which the uterine horn was very thin, tiny, and tightly attached to the opposite ovary and pedicle with broad ligament in the abdominal cavity. Thus, this study included the drawbacks of lateral flank approaches during spaying in queens of a variety of age, body conditions, and anatomic structures of reproductive system. This study included the practice of using multiple transfixing ligatures with the involvement of more suture materials in the uterine body of Group B cats, while only single ligatures were provided in Group A cats for the similar purpose having comparatively less utilization of suture materials. This is due to the requirements of providing more stitches and knots in Group B during ligating the comparatively thickened, flat and enlarged structures; as there might be more patent ovary with pedicles and uterus in the aged cats that gave birth to kittens before, thus having more prominent and well-embedded blood vessels passing through the thickened uterine horns into the wall of the flat and enlarged uterine body, ovarian pedicles, parts of mesovarium and mesometrium, and vice-versa. After spaying, no postoperative complication was seen in more than 60% cases during this research. However, few cats of both groups were suffered from a variety of complications such as wound site infection, dehiscence of suture lines, bleeding, and oozing from incisional wounds, which were further treated successfully. The causes of these complications might be poor hygienic managements involving the contamination of the surgical wounds from environmental and housing fomites, contact with carrier persons, and absence of Elizabethan collar as well as the normal physiological behavior of the cats to lick, bite or scratch the apposed and sutured surgical sites as these might cause a degree of irritation and pain over the healing period. Among these complications, dehiscence of suture lines and oozing from wounds were more frequently observed cases in the cats; which might be because of rubbing the wounds against any object or self-inflicted injuries that cause obstacles to healing resulting in prolonged inflammation, tissue reaction, and discharge of exudates. These findings are consistent with the research of Pollari et al. (1996) and Burrow et al. (2005) who reported that about 6.2%–20.6% surgical complications were associated with spaying in small animals. In addition, Burrow et al. (2005) and Omeran et al. (2014) observed that majority of these complications were moderate including inflammation to the incision sites and gastrointestinal disturbances from prolonged surgery and anesthesia. Some researchers experienced that the lateral flank approach for spaying caused comparatively more discharges from the wounds than the midline approach due to the sequels of seroma and hemorrhage arising from the larger incisions of muscles and dense fat, and/or associated bacterial infections (Murugesan et al., 2020). Furthermore, Kiani et al. (2006) found that the swelling of the wound elevated only after a day of spaying since the suture materials used in spaying cats got recognized as a foreign body by the immune system to elicit foreign body reactions as inflammatory responses. In the present study, it was seen that the spayed queens in both groups completely recovered mostly within 10 days after surgery including the range of 7–21 days. Similar types of early recovery in spayed cats were also observed by several researchers (Brooks, 2001; Davidson et al., 2004; Omeran et al. 2014). The average healing duration was comparatively a little bit longer in the cats of Group B, which might be ascribed to several factors affecting the complete recovery process such as the size of incisional wounds, involvement of muscles, vessels, fatty tissues and other structures during operation, usage of suture materials, total duration of surgery and anesthesia, and postoperative complications (Haley et al., 1985; Nicholson et al., 2002; Burrow et al., 2005). Therefore, smaller incision to skin and muscles in a minimum invasive way while maintaining the least duration of surgery and anesthesia is the prerequisite for faster healing in cats after spaying. The supportive medications provided to the cats in this research during the postoperative period were collateral with the reports of Bauer et al. (1989), Bourguignon et al. (2013), and Schmitt et al. (2019), and these could be the prominent reasons for no further severe complications and minimum incidences of morbidity without any mortality in all the experimented cats that finally got complete recovery over a period of time (1–3 weeks). In conclusion, spaying in queens through left lateral flank can be a successful and quick approach with minimum or no postoperative complications toward promising healing within a reasonable time. Therefore, the veterinarians and small animal practitioners can follow this technique for regular spaying either in breeding or non-breeding cats. AcknowledgementsThe authors are grateful to the authority of Veterinary Teaching Hospital of Bangladesh Agricultural University for the support during this research. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsMRM performed surgery, case follow-up, statistical analysis, and final manuscript writing. MSS and AH performed clinical examinations, review literature and manuscript draft. All authors read and approved the final manuscript. ReferencesBabu, M., Krishnaswamy, A., Nethra, R. and Narasimhamurthy, A. 2018. Simple technique for ovariohysterectomy in the cat. Int. J. Curr. Microbiol. App. Sci. 7, 2554−2561. Bauer, M.S., Remedios, A.M. and Stanley, B.J. 1989. Open wound management for treatment of postoperative infections in eight dogs. Can. Vet. J. 30, 46−49. Bellows, J., Center, S., Daristotle, L., Estrada, A.H., Flickinger, E.A., Horwitz, D.F., Lascelles, B.D.X., Lepine, A., Perea, S., Scherk, M. and Shoveller, A.K. 2016. Aging in cats: common physical and functional changes. J. Feline Med. Surg. 18, 533−550. Bourguignon, E., Matos, L.D.G., Ferreira, T.S. and Favarato, E.S. 2013. Dermatology in dogs and cats. In Insights from veterinary medicine, 1st ed. Ed., Payan-Carreira, R.: London, UK: IntechOpen, pp: 3−34. Brooks, W. 2001. Spaying your female cats–veterinary partner, veterinary information Network®, Inc. Available via https://veterinarypartner.vin.com/doc/?id=4951480&pid=19239 Brown, J.L. and Comizzoli, P. 2018. Female cat reproduction. In Encyclopedia of reproduction, 2nd ed. Ed., Skinner, M.K., Boston, M.A.; Academic Press, Elsevier Inc. Vol. 2, pp: 692−701. Burrow, R., Batchelor, D. and Cripps, P. 2005. Complications observed during and after ovariohysterectomy of 142 bitches at a veterinary teaching hospital. Vet. Rec. 157, 829−833. Coe, R.J., Grint, N.J., Tivers, M.S., Hotston Moore, A. and Holt, P.E. 2006. Comparison of flank and midline approaches to the ovariohysterectomy of cats. Vet. Rec. 159, 309−313. Costa, D., Libardoni, R.N., Schmitt, J.T., Padilha, A.S., Júnior, F.J.S., deAtaíde, M.W., Menezes, F.B., Allievi, K., Brun, M.V., Teixeira, P.P.M. and Silva, M.A.M. 2017. LESS ovariohysterectomy in cats using a new homemade multiport. Cienc. Rural 47, e20161130. Davidson, E.B., Moll, H.D. and Payton, M.E. 2004. Comparison of laparoscopic ovariohysterectomy and ovariohysterectomy in dogs. Vet. Surg. 33, 62−69. Fazio, E., Medica, P., Cravana, C., Pupillo, A. and Ferlazzo, A. 2015. Effects of ovariohysterectomy in dogs and cats on adrenocortical, haematological and behavioural parameters. Acta Sci. Vet. 43, 1339. Fingland, R.B. 1998. Uterus. In Current techniques in small animal surgery, 4th ed. Eds., Bojrab, M.J., Ellison, G.W. and Slocum, B.: Williams & Wilkins, Baltimore, MarylandD: Williams & Wilkins,, pp: 489−502. Freeman, L.J., Pettit, G.D., Robinette, D., Lincoln, J.D. and Person, M.W. 1987. Tissue reaction to suture material in the feline linea alba–a retrospective, prospective and histological study. Vet. Surg. 16, 440−444. Ghanawat, H.G. and Mantri, M.B. 1996. Comparative study of various approaches for ovariohysterectomy in cats. Indian. Vet. J. 73, 987−988. Gorelick, J. 1974. Discolouration of exotic cat’s hair following flank ovario-hysterectomy. Vet. Med. Small. Anim. Clin. 69, 943. Haley, R.W., Culver, D.H. and Morgan, W.M., White, J.W., Emori, T.G. d Hooton, T.M. 1985. Identifying patients at high risk of surgical wound infection: a simple multivariate index of patient susceptibility and wound contamination. Am. J. Epidemiol. 121, 206−215. Hart, B.L. and Eckstein, R.A. 1997. The role of gonadal hormones in the occurrence of objectionable behaviours in dogs and cats. Appl. Anim. Behav. Sci. 52, 331−344. Hedlund, C.S. 2002. Surgery of the reproductive and genital systems. In Small animal surgery, 2nd ed. Ed., Fossum, T.W.: St Louis, MO: Mosby, Elsevier, pp: 610−674. Heffelfinger, D.J. 2006. Ovarian remnant in a 2-year-old queen. Can. Vet. J. 47, 165−167. Holly, M.G. and Hardie, R.J. 2004. Lateral flank approach for ovariohysterectomy in small animals. Vet. Med. Small Anim. Clin. 70, 569−573. Hoque, M. 1991. Comparative study of various approaches to feline ovariohysterectomy. Indian. J. Vet. Surg. 12, 29−30. Janssens, L.A.A. and Janssens, G.H.R.R. 1991. Bilateral flank ovariectomy in the dog: surgical technique and sequelae in 72 animals. J. Small Anim. Pract. 32, 249−252. Kiani, F.A., Kachiwal, A.B., Shah, M.G., Nizamani, Z.A., Khand, F.M., Lochi, G.M., Haseeb, A., Khokhar, A.M., Oad, A. and Ansari, M.I. 2014. Comparative study on midline and flank approaches for ovariohystrectomy in cats. J. Agric. Food. Tech. 4, 21−31. Kustritz, M.V.R. 2007. Determining the optimal age for gonadectomy of dogs and cats. J. Am. Vet. Med. Assoc. 231, 1665−1675. Levy, J.K. and Wilford, C.L. 2013. Management of stray and feral community cats. In Shelter medicine for veterinarians and staff, 2nd ed. Eds., Miller, L. and Zawistowski, S.: Ames, IA: Wiley-Blackwell Publishing, pp: 669−688. McGrath, H., Hardie, R.J. and Davis, E. 2004. Lateral flank approach for ovariohysterectomy in small animals. Compend. Contin. Educ. Small Anim. Pract. 26, 922−930. Michell, A.R. 1999. Longevity of British breeds of dog and its relationships with sex, size, cardiovascular variables and disease. Vet. Rec. 145, 625−629. Murugesan, V., Arunachalam, K., Shanmugam, K. and Palanivel, M. 2020. Comparative study on midline and lateral flank approaches for ovariohysterectomy in cats. Pharma Innov. 9, 191−193. Nelson, R.W. and Couto, C.G. 2014. Reproductive system disorders. In Small animal internal medicine, 5th ed. Eds., Nelson, R.W. and Couto, C.G.: St. Louis, MO: Mosby, Elsevier, pp: 897−962. Nicholson, M., Beal, M., Shofer, F. and Brown, D.C. 2002. Epidemiologic evaluation of postoperative wound infection in clean-contaminated wounds: a retrospective study of 239 dogs and cats. Vet. Surg. 31, 577−581. Oliveria, J.P., Mencalha, R., Sousa, C.A.D.S., Abidu-Figueiredo, M. and Jorge, S.D.F. 2014. Pain assessment in cats undergoing ovariohysterectomy by midline or lateral celiotomy through use of a previously validated multidimensional composite pain scale. Acta Cir. Bras. 29, 633−638. Omeran, B.M., Abdel-Wahed, R.E., El-Kammar, M.H. and Abu-Ahmed, H. 2014. Ovariectomy versus ovariohysterectomy for elective sterilization of female cats. Alex. J. Vet. Sci. 43, 73−81. Pollari, F.L., Bonnett, B.N. and Bamsey, S.C., Meek, A.H. and Allen, D.G. 1996. Postoperative complications of elective surgeries in dogs and cats determined by examining electronic and paper medical records. J. Am. Vet. Med. Assoc. 208, 1882−1886. Rana, M.A. 2007. Comparative study of flank vs midline approach for ovariohysterectomy in cats. M. Phil. (Hons) thesis, UVAS, Lahore, Pakistan, pp: 75−79. Roberts, M.L., Beatty, J.A., Dhand, N.K. and Barrs, V.R. 2015. Effect of age and surgical approach on perioperative wound complication following ovariohysterectomy in shelter-housed cats in Australia. J. Feline Med. Surg. Open. Rep. 1, 1−4. Schmitt, K., Lehner, C., Schuller, S., Schüpbach-Regula, G., Mevissen, M., Peter, R., Müntener, C.R., Naegeli, H. and Willi, B. 2019. Antimicrobial use for selected diseases in cats in Switzerland. BMC. Vet. Res.15, 94−98. Sontas, B.H. and Ekici, H. 2007. Short-term effects of prepubertal ovariohysterectomy on skeletal, physical and behavioural development of dogs up to 24 weeks of age. Acta. Vet. Hung. 55, 379−387. Spain, C.V., Scarlett, J.M. and Houpt, K.A. 2004. Long-term risks and benefits of early-age gonadectomy in dogs. J. Am. Vet. Med. Assoc. 224, 380−387. Stone, E.A. 2003. Ovary and uterus. In Textbook of small animal surgery, 3rd ed. Ed., Slatter, D.H.: Saunders, Philadelphia, PA:, Saunders, pp: 1487−1499. Tsutsui, T. and Stabenfeldt, G.H. 1993. Biology of ovarian cycles, pregnancy and pseudopregnancy in the domestic cat. J. Reprod. Fertil. Suppl. 47, 29−35. Valle, G.R. and Junior, A.P.M. 1999. Endocrinopathology and hormonal therapy of the estrus cycle of the bitch. Caderno Técnico de Veterinária e Zootecnia (Belo Horizonte) 30, 49−74. Verstegen, J., Dhaliwal, G. and Verstegen-Onclin, K. 2008. Mucometra, cystic endometrial hyperplasia, and pyometra in the bitch: advances in treatment and assessment of future reproductive success. Theriogenology 70, 364−374. White, S.C. 2012. Prevention of fetal suffering during ovariohysterectomy of pregnant animals. J. Am. Vet. Med. Assoc. 240, 1160−1163. Wildt, D.E., Brown, J.L. and Swanson, W.F. 1998. Reproduction in cats. In Encyclopedia of reproduction, 1st ed. Eds., Knobil, E., Neill, J.D. and Skinner, M.K.: New York, NY: Academic Press, Vol. 1, pp: 497−510. | ||
| How to Cite this Article |
| Pubmed Style Munif MR, Safawat MS, Hannan A. Left lateral flank approach for spaying in cats. Open Vet. J.. 2022; 12(4): 540-550. doi:10.5455/OVJ.2022.v12.i4.17 Web Style Munif MR, Safawat MS, Hannan A. Left lateral flank approach for spaying in cats. https://www.openveterinaryjournal.com/?mno=42155 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i4.17 AMA (American Medical Association) Style Munif MR, Safawat MS, Hannan A. Left lateral flank approach for spaying in cats. Open Vet. J.. 2022; 12(4): 540-550. doi:10.5455/OVJ.2022.v12.i4.17 Vancouver/ICMJE Style Munif MR, Safawat MS, Hannan A. Left lateral flank approach for spaying in cats. Open Vet. J.. (2022), [cited January 25, 2026]; 12(4): 540-550. doi:10.5455/OVJ.2022.v12.i4.17 Harvard Style Munif, M. R., Safawat, . M. S. & Hannan, . A. (2022) Left lateral flank approach for spaying in cats. Open Vet. J., 12 (4), 540-550. doi:10.5455/OVJ.2022.v12.i4.17 Turabian Style Munif, Mohammad Raguib, Mst. Sanjida Safawat, and Abdul Hannan. 2022. Left lateral flank approach for spaying in cats. Open Veterinary Journal, 12 (4), 540-550. doi:10.5455/OVJ.2022.v12.i4.17 Chicago Style Munif, Mohammad Raguib, Mst. Sanjida Safawat, and Abdul Hannan. "Left lateral flank approach for spaying in cats." Open Veterinary Journal 12 (2022), 540-550. doi:10.5455/OVJ.2022.v12.i4.17 MLA (The Modern Language Association) Style Munif, Mohammad Raguib, Mst. Sanjida Safawat, and Abdul Hannan. "Left lateral flank approach for spaying in cats." Open Veterinary Journal 12.4 (2022), 540-550. Print. doi:10.5455/OVJ.2022.v12.i4.17 APA (American Psychological Association) Style Munif, M. R., Safawat, . M. S. & Hannan, . A. (2022) Left lateral flank approach for spaying in cats. Open Veterinary Journal, 12 (4), 540-550. doi:10.5455/OVJ.2022.v12.i4.17 |