| Case Report | ||
Open Vet. J.. 2022; 12(5): 768-773 Open Veterinary Journal, (2022), Vol. 12(5): 768–773 Original Research Identification of an ectopic periorbital lymph node in two horsesFlorine Narinx1*, Sébastien Monclin1, Aurélie Sauvage1, Eline Vercruysse1, Marianne Heimann2, Elizabeth Alloway3, Maxime Vandersmissen4 and Magda Grauwels11Department of Ophthalmology, Veterinary Teaching Hospital, University of Liège, Liège, Belgium 2Anapet sprl-Synlab, Heppignies, Belgium 3Synlab, VPG Histology, Bristol, UK 4Department of Medical Imaging, Veterinary Teaching Hospital, University of Liège, Liège, Belgium *Corresponding Author: Florine Narinx. Department of Ophthalmology, Veterinary Teaching Hospital, University of Liège, Liège, Belgium. Email: f.narinx [at] uliege.be Submitted: 25/05/2022 Accepted: 18/09/2022 Published: 12/10/2022 © 2022 Open Veterinary Journal
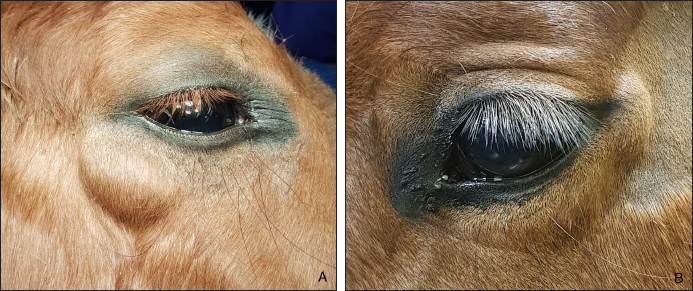
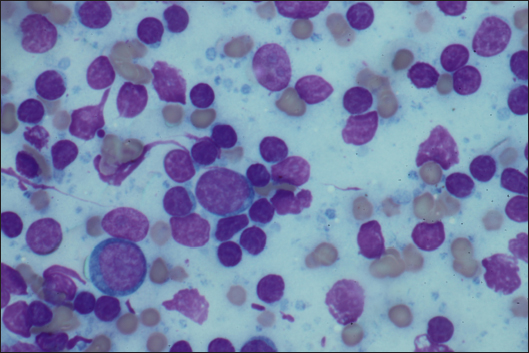
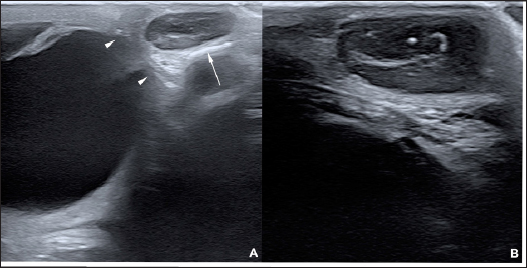
AbstractBackground: To describe the clinical presentation and treatment of an ectopic periorbital lymph node in two young horses. Case Description: Two warm-blood horses were presented at the equine clinic of the University of Liège with a periorbital non-painful mass. Differential diagnosis included neoplasm (lymphoma), (sterile) abscess, cyst, ectopic lacrimal gland tissue, hematoma, adipose tissue, or ectopic lymphoid tissue. Information collected included physical and ophthalmic examination findings, results of the ocular and periocular ultrasound, surgical procedure, histopathology, and follow-up. Masses of 2 × 2 cm and 3 × 2 cm subcutaneous, ovoid, smooth, and firm mass were observed in the zygomatic region of the head. On ultrasound, the mass appeared as a hypoechoic subcutaneous structure. Cytology showed a mixed lymphocytic cell population. No abnormal mitotic activity was observed. Histopathology revealed a chronic markedly reactive lymph node. Conclusion: To the authors’ knowledge, this is the first description of periorbital ectopic lymph nodes in veterinary medicine. Ectopic lymph nodes should therefore be included in the differential diagnosis of periocular masses in animals. Surgery was easily performed and was curative. Keywords: Ectopic lymph node, Eye, Eyelid, Horse, Mass. IntroductionLymphoid organs are classified as primary, secondary, and tertiary structures. Primary lymphoid organs in mammals include bone marrow and thymus, where T and B lymphocytes are developed and selected. The lymph nodes, spleen, and mucosal-associated lymphoid tissues (MALT) represent secondary lymphatic organs. They develop during the embryonic phase and provide a place for lymphocyte activation and differentiation into regulatory or effector cells. Tertiary lymphoid tissues, also known as ectopic lymphoid tissues, develop after birth in response to chronic inflammation, chronic infection, or autoimmunity (Aloisi and Pujol-Borrell, 2006). These tissues can develop in nearly every organ in the body without predictable sites. They are characterized by the absence of a capsule, unlike lymph nodes, and do not resemble typical sub-mucosal tissues like MALT. Instead, they are often embedded in non-lymphoid organs at sites of inflammation or infection (Carragher et al., 2008). Although the orbit is normally devoid of lymph nodes, lymphatics vessels have been reported in the eyelids, conjunctiva, lacrimal glands, and optic nerve sheath in humans (Dickinson and Gausas, 2006). Their presence in the extraocular muscles is controversial. Lymphatics can also be induced in the cornea after inflammatory, infectious, traumatic, chemical, or toxic insults (Chen, 2009). In dogs, lymphatic vessels have been reported in the eyelids, conjunctiva (Eichenbaum et al., 1987), and inflamed cornea. Conjunctiva and eyelids are mainly drained to the parotid and mandibular lymph nodes (Stades and Van der Woerdt, 2021). As there is a paucity of lymphatics vessels draining the intraocular tissues, the majority of immune cells leave the eye via blood, making the spleen the main lymphoid organ (English and Gilger, 2021). This case report describes the presence of a periorbital secondary lymphoid structure (lymph node) in two unrelated horses. Case DetailsHistoryTwo unrelated warm-blood horses, a 10-month-old filly and a 2-year-old gelding, were presented at the equine clinic of the University of Liège, Belgium, for evaluation of a subcutaneous mass. In the first case, the mass was located ventral to the lateral angle of the right eye, in the cranial zygomatic region of the head (Fig. 1A), and immediately caudal to the lateral angle of the left eye, and in the second case, in the mid zygomatic region of the head (Fig. 1B). The mass has been observed for 1 month in the first case, and for 2 months in the second case. Both horses were born in Belgium and were up to date regarding vaccinations and deworming. The foaling of both cases was unremarkable and there was no history of head trauma or infection. Initial examinationCase 1 Excepting the presence of a periorbital subcutaneous mass, the remainder of the general physical examination was normal. After evaluating the visual tests and reflexes, the rest of the ophthalmic examination was performed under sedation using intravenous detomidine (10 µg/kg, Domidine ®, Dechra). A gross ophthalmic examination of the periocular region revealed a 3 × 2 cm, non-painful, subcutaneous, non-adherent, ovoid, well-delineated, smooth, and firm mass located ventro-temporal to the right orbital rim. The skin overlying this mass appeared normal and there was no evidence of bone deformation of the zygomatic arch. The eyes and adnexa were examined by slit-lamp biomicroscopy (SL-17 Portable Slit Lamp, Kowa) and binocular indirect ophthalmoscopy (Keeler, Vantage Plus). No abnormality was reported. Ophthalmic examination of the left eye was unremarkable. Fluorescein staining was negative for both eyes. Case 2 Initial examination was similar to Case 1 except that the mass was located temporal to the left orbital rim and was slightly smaller in size: 2 × 2 cm. Differential diagnosisDifferential diagnosis included neoplasia (lymphoma), (sterile) abscess, cyst, inflammatory process, ectopic lacrimal or salivary gland tissue, hematoma, adipose tissue or lipoma, and ectopic lymphoid tissue. Medical workupCase 1 Complete blood count and biochemical profile were within normal limits. Fine needle aspiration was performed and submitted for cytology (Anapet Laboratory, Belgium). The smears showed a mixed lymphocytic cell population made up of small, intermediate, and large-sized lymphocytes within the normal distribution for a balanced adenogram except for a mild increase of the intermediate cells (Fig. 2). Occasional individual, normal, mastocytes were present. Diagnostic hypotheses included: a nodular lymphocytic follicular dermatitis, panniculitis, and an ectopic lymph node with mild hyperplasia. On ultrasound examination, the subcutaneous mass appeared homogenously hypoechoic and lobulated. Case 2 Fine needle aspiration was performed by the referring veterinarian and submitted for cytology (Lab for Vet Laboratory, Belgium). The smear showed a lymphoid cell population, rich in intermediated-sized and reactive lymphocytes, without notable atypia. Rare small lymphocytes, centroblasts, and lymphoblasts were present. Eosinophils were regularly found (about 4%). Cytology was in favor of reactive lymphadenitis with a slight eosinophilic component. On ultrasound examination, a roughly ovoid (18 × 17 × 9 mm) delineated subcutaneous mass was visible immediately caudal to the lateral angle of the left eye, superficial to the zygomatic arch. This mass was of intermediate and heterogenous echogenicity with a hypoechoic center circumscribed by thin concentric hyperechoic lines (Fig. 3). No bone lesion nor adhesion between the mass and the osseous surface was identified. The left eye was within normal limits. The parotid gland and parotid lymph nodes had a physiological position and a normal ultrasonographic appearance.
Fig. 1. A non-painful, subcutaneous, less-adherent, ovoid, smooth, and firm mass located (A) ventro-temporal to the right orbital rim in Case 1 and (B) temporal to the left orbital rim in Case 2.
Fig. 2. FNA of the mass of Case 1. A mixed lymphocytic cell population with centroblasts, immunoblasts, and centrocytes (×1,000).
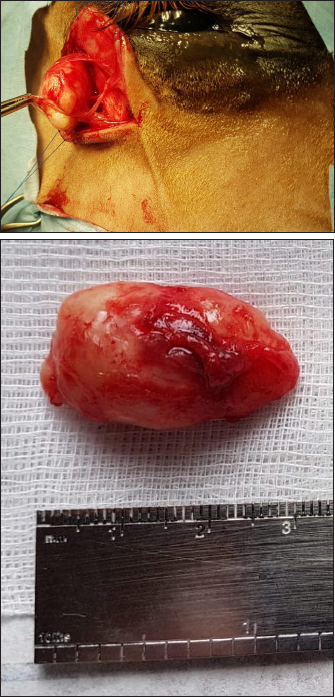
Fig. 3. Ultrasonographic images obtained in Case 2 in a longitudinal (temporo-nasal) orientation. The temporal side is on the right of each image. (A) General view showing the relationship with the globe (white arrowheads) and the zygomatic arch (white arrow). (B) Close-up view showing the described architecture and echogenicity. Surgical procedure and postoperative treatmentCase 1 The filly was hospitalized for surgical excision and histopathological analysis of the mass (VPG Histology, Synlab Laboratory, UK). Premedication was carried out with intramuscular acepromazine (0.1 mg/kg, Placivet®, Kela) and intravenous xylazine (0.6 mg/kg, Proxylaz®, Prodivet). General anesthesia was induced with an intravenous combination of ketamine (2.2 mg/kg, Ketamidor®, Richter Pharma) and midazolam (0.06 mg/kg, Midazolam®, Mylan) and was maintained on isoflurane (Isoflo®, Zoetis). The filly received intravenous flunixin meglumine (1.1 mg/kg, Emdofluxin®, Emdoka) and trimethoprim-sulfadiazine (30 mg/kg, Emdotrim®, Emdoka) preoperatively. A 5 cm longitudinal skin incision was made centered on the mass and subcutaneous tissues were dissected from the mass using Stevens scissors (Fig. 4). The dissection was uncomplicated as the mass was well encapsulated and there was no adherence to the adjacent tissue. The deep and subcutaneous tissues were closed in a continuous pattern with 5–0 polylactic acid (Vicryl, Ethicon) and the skin was in a simple interrupted pattern with 4–0 polyethylene (Ethilon, Ethicon). Postoperative care consisted of oral administration of trimethoprim-sulfadiazine (30 mg/kg, Emdotrim®, Emdoka) twice a day for 5 days, meloxicam (0.6 mg/kg, Metacam®, Boehringer Ingelheim) once a day for 3 days, and omeprazole (1 mg/kg, Ulcergold®, Norbrook Lab) once a day for 5 days.
Fig. 4. Well encapsulated mass, easily dissected with no adherence to the adjacent tissues (Case 1). Case 2 The surgical procedure was similar but performed on a standing horse. The sedation was performed with a bolus of detomidine (10 µg/kg, Domidine®, Dechra) and morphine (0.1 mg/kg, Morphine®, Sterop). To maintain adequate sedation depth, a constant rate infusion was prepared with 10 mg of detomidine and 50 mg of morphine, added to 1 l of NaCl solution, in order to obtain a rate of 50–100 and 10–15 µg/kg/h, respectively. The rate of infusion was altered according to the Ghent Sedation Algorithm (Schauvliege et al., 2019). The gelding was administered intravenous flunixin meglumine (1.1 mg/kg, Emdofluxin®, Emdoka) and trimethoprim-sulfadiazine (30 mg/kg, Emdotrim®, Emdoka) preoperatively. An auriculopalpebral block was performed with 1 ml of 2% lidocaine (Xylocaine® 2%, Astrazeneca). Regional anesthesia was performed with 5 ml of 2% lidocaine, infiltered in the subcutaneous tissue around the mass. The mass was slightly more adherent to the underlying tissue compared to Case 1. Histopathological findingsBoth masses were fixed in 10% neutral buffered formalin. They were routinely processed for histopathology and stained with hematoxylin and eosin (Fig. 5). Case 1 The tissue was composed of a thickly encapsulated, markedly enlarged lymph node. A marked, diffuse hyperplasia of lymphoid follicles, with robust germinal center formation, was present and indicated its reactivity. In the majority of the follicles, antigen-related polarity was retained, with the presence of light and dark poles. There was a mild, multifocal expansion of the paracortex by small lymphocytes. The trabecular stroma was moderate to markedly and multifocally thickened, resulting in the isolation of large aggregates of lymphoid follicles and a multi-lobulated appearance. The vascular endothelial cells (particularly high endothelial venules) throughout the lymph node were moderate to markedly hypertrophied. Some small to medium-caliber lymphatic vessels were present in the tissue. Within the paracortical and medullary sinuses, there were mildly increased numbers of small lymphocytes and eosinophils. Analysis was consistent with a markedly chronically reactive lymph node. Case 2 The tissue was ovoid and surrounded by a thick fibrous capsule, multifocally infiltrated by small clusters of small lymphocytes and plasma cells. The mass was composed of numerous, closely packed, and sometimes coalescing nodular aggregates of lymphoid cells separated by anastomosing bands of the dense fibrovascular stroma. The majority of the lymphoid aggregates exhibited a large germinal center consisting of large lymphoid cells with large nuclei that contained reticular chromatin and 1–2 distinct basophilic nucleoli. There was an increased mitotic rate within these germinal centers, with up to 12 mitotic figures per high power field. Few small blood vessels were noted between the cells in the germinal centers. Few tangible body macrophages were observed. Sheets of small- and medium-sized lymphocytes surrounded the germinal centers along with rare plasma cells and mast cells. The findings were consistent with a chronically activated lymph node.
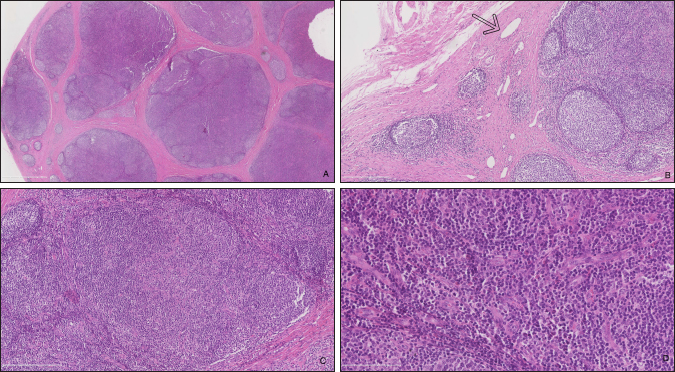
Fig. 5. Histopathologic analysis of case 1. (A) General overview of the lymph node: marked capsular sclerosis and lymphoid follicle hyperplasia. (B) Small lymphatic vessels (arrow) surrounding the hyperplastic lymphoid follicles. (C) Hyperplastic lymphoid follicles. (D) Eosinophilic infiltrates. Follow-upCase 1 The surgical site healed without complication and the filly was discharged after 5 days. At 18 months follow-up, the owner reported there was no evidence of a mass at the surgery site. Case 2 The gelding was discharged the day after surgery. No recurrence was noticed after an 8-months follow-up. DiscussionNeoplasms of the eye and adnexa are commonly described in horses. The most common periocular neoplasms include sarcoid and squamous cell carcinoma, followed by papilloma, lymphosarcoma, and melanoma (Giuliano, 2010). Based on histopathology, these neoplasms were excluded in the two horses reported here. The orbit is normally devoid of lymph nodes. Parotid lymph nodes in horses are located caudal to the ramus of the mandible, ventral to the temporo-mandibular joint, and deep to the cranial margin of the parotid salivary gland. While not described, a developmental migration of a parotid lymph node along the path of the transverse facial artery was considered a possibility in our cases. This hypothesis is difficult to confirm as the parotid lymph nodes are a cluster of multiple small nodes. In the second case, the ultrasound showed the presence of several parotid lymph nodes in their normal localization. The migration of one of them could not be excluded. On histopathology, small- to medium-caliber lymphatic vessels are observed in the first mass. The source of these lymphatic vessels is not clear: they may originate from elsewhere on the head or the orbit. The histologic features of the lymph nodes, including the prominent follicle formation and trabecular and capsular fibrosis, are indicative of chronic, reactive hyperplasia. The cause of lymph node reactivity remains unknown. Eosinophilic infiltration is not a typical feature of reactive lymph nodes: this inflammation may suggest a response to parasitism or allergy. However, previous focal infection or trauma was not reported and no (peri)ocular disease or systemic involvement was present at the time of the examination. In horses, focal eosinophilic inflammation is most commonly observed with eosinophilic granuloma, insect bites, parasites (such as Habronema, Thelazia, Onchocerca, Gasterophilus or a chronic arthropod infestation), and in idiopathic cases. In these horses, no evidence of parasitic infection was present. A thorough examination of the oral and nasal cavities by endoscopy would have been worthwhile as these structures are closely related to lymph nodes. Publications on ectopic lymph nodes are sparse. To the best of our knowledge, one case has been described in the veterinary literature. Necropsy of an 8-month-old heifer revealed an intracranial, extraneural ectopic lymph node (Clancy et al., 2018). It died following an acute onset of lethargy, dyspnea, and anorexia. A congenital abnormality was suspected. In humans, a 47-year-old female had an ectopic lymph node localized in the lacrimal gland region for 20 years (Wolter and Roosenberg, 1977) and a 24-year-old female had an inflamed lymph node localized at the lateral side of the upper eyelid (Ahn et al., 2018). Similar to our cases, there was no inflammatory process or systemic illness in the 47-year-old female. Surgical excision was curative for these patients and recurrence was not reported. ConclusionTo the authors’ knowledge, this is the first description of a periorbital ectopic lymph node in animals. Ectopic lymph nodes should therefore be included in the differential diagnosis of periocular masses in animals. Surgical excision was uncomplicated in these two cases. Based on these two animals, the prognosis for successful treatment of periorbital ectopic lymph nodes following surgical excision appears to be excellent in horses. Conflict of interestThe author declares that there is no conflict of interest Authors’ contributionsFlorine Narinx performed all the examinations, participated in both surgeries, drafted the manuscript, and revised the manuscript. Sébastien Monclin provided critical review. Aurélie Sauvage performed the examination of Case 1 and supervised the surgery of Case 2. Eline Vercruysse performed the first surgery. Marianne Heimann interpreted cytology (Case 1). Elizabeth Alloway interpreted the histopathology (Case 1). Maxime Vandersmissen performed both ultrasounds. Magda Grauwels was the clinician responsible for the entire supervision and critical review. ReferencesAhn, J., Park, M.H., Yoo, J.M. and Seo, S.W. 2018. A case of orbital lymph node misdiagnosed as a dermoid. J. Korean Ophthalmol. Soc. 59, 672–675. Aloisi, F. and Pujol-Borrell, R. 2006. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 6, 205–217. Carragher, D.M., Rangel-Moreno, J. and Randall, T.D. 2008. Ectopic lymphoid tissues and local immunity. Semin. Immunol. 20, 26–42. Chen, L. 2009. Ocular lymphatics: state-of-the-art review. Lymphology 42, 66–76. Clancy, C.S., Van Wettere, A.J. and Hullinger, G.A. 2018. Intracranial, extraneural ectopic lymph node in a bovine (Bos taurus). Anat. Histol. Embryol. 47, 385–388. Dickinson, A.J. and Gausas, R.E. 2006. Orbital lymphatics: do they exist? Eye 20, 1145–1148. Eichenbaum, J.D., Lavach, J.D., Severin, G.A. and Paulsen, M.E. 1987. Immunology of the ocular surface. Compend. Contin. Educ. Vet. 9, 1101–1109. English, R. and Gilger, B.C. 2021. Ocular immunology. In Veterinary ophthalmology, 6th ed. Eds., Gelatt, K.N., Ben-Shlomo, G., Gilger, B.C., Hendrix, D.V.H., Kern, T.J. and Plummer, C.E. MI: Wiley Blackwell, pp: 263–292. Giuliano, E.A. 2010. Equine periocular neoplasia: current concepts in aetiopathogenesis and emerging treatment modalities. Equine Vet. J. Suppl. 37, 9–18. Schauvliege, S., Cuypers, C., Michielsen, A., Gasthuys, F. and Gozalo-Marcilla, M. 2019. How to score sedation and adjust the administration rate of sedatives in horses: a literature review and introduction of the Ghent Sedation Algorithm. Vet. Anaesth. Analg. 46, 4–13. Stades, F.C. and Van der Woerdt, A. 2021. Diseases and surgery of the canine eyelid. In Veterinary ophthalmology, 6th ed. Eds., Gelatt, K.N., Ben-Shlomo, G., Gilger, B.C., Hendrix, D.V.H., Kern, T.J. and Plummer, C.E. MI: Wiley Blackwell, pp: 923–987. Wolter, J. and Roosenberg, R. 1977. Ectopic lymph node of the orbit simulating a lacrimal gland tumor. Am. J. Ophthalmol. 83, 908–914. | ||
| How to Cite this Article |
| Pubmed Style Narinx F, Vercruysse E, Sauvage A, Grauwels M. Identification of an ectopic periorbital lymph node in two horses. Open Vet. J.. 2022; 12(5): 768-773. doi:10.5455/OVJ.2022.v12.i5.23 Web Style Narinx F, Vercruysse E, Sauvage A, Grauwels M. Identification of an ectopic periorbital lymph node in two horses. https://www.openveterinaryjournal.com/?mno=47568 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i5.23 AMA (American Medical Association) Style Narinx F, Vercruysse E, Sauvage A, Grauwels M. Identification of an ectopic periorbital lymph node in two horses. Open Vet. J.. 2022; 12(5): 768-773. doi:10.5455/OVJ.2022.v12.i5.23 Vancouver/ICMJE Style Narinx F, Vercruysse E, Sauvage A, Grauwels M. Identification of an ectopic periorbital lymph node in two horses. Open Vet. J.. (2022), [cited January 25, 2026]; 12(5): 768-773. doi:10.5455/OVJ.2022.v12.i5.23 Harvard Style Narinx, F., Vercruysse, . E., Sauvage, . A. & Grauwels, . M. (2022) Identification of an ectopic periorbital lymph node in two horses. Open Vet. J., 12 (5), 768-773. doi:10.5455/OVJ.2022.v12.i5.23 Turabian Style Narinx, Florine, Eline Vercruysse, Aurélie Sauvage, and Magda Grauwels. 2022. Identification of an ectopic periorbital lymph node in two horses. Open Veterinary Journal, 12 (5), 768-773. doi:10.5455/OVJ.2022.v12.i5.23 Chicago Style Narinx, Florine, Eline Vercruysse, Aurélie Sauvage, and Magda Grauwels. "Identification of an ectopic periorbital lymph node in two horses." Open Veterinary Journal 12 (2022), 768-773. doi:10.5455/OVJ.2022.v12.i5.23 MLA (The Modern Language Association) Style Narinx, Florine, Eline Vercruysse, Aurélie Sauvage, and Magda Grauwels. "Identification of an ectopic periorbital lymph node in two horses." Open Veterinary Journal 12.5 (2022), 768-773. Print. doi:10.5455/OVJ.2022.v12.i5.23 APA (American Psychological Association) Style Narinx, F., Vercruysse, . E., Sauvage, . A. & Grauwels, . M. (2022) Identification of an ectopic periorbital lymph node in two horses. Open Veterinary Journal, 12 (5), 768-773. doi:10.5455/OVJ.2022.v12.i5.23 |