| Original Article | ||
Open Vet. J.. 2020; 10(1): 86-93 doi: 10.4314ovj.v10i1.14 Open Veterinary Journal, (2020), Vol. 10(1): 86–93 Original Article DOI: http://dx.doi.org/10.4314/ovj.v10i1.14 Validation of an optical, computer-assisted technique for intraoperative tracking of 3-dimensional canine stifle joint motionCecilia Signorelli1, Filippo Cinti2*, Stefano Zaffagnini3, Luciano Pisoni4, and Nicola Francesco Lopomo51Laboratorio di Biomeccanica e Innovazione Tecnologica, IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy 2Eastcott Referral Hospital, Swindon, Wiltshire, UK 3Clinica Ortopedica e Traumatologica II, IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy 4Dipartimento di Scienze Mediche Veterinarie, Università degli studi di Bologna, Bologna, Italy 5Dipartimento di Ingegneria dell’Informazione, Università degli Studi di Brescia, Brescia, Italy *Corresponding Author: Filippo Cinti. Eastcott Referral Hospital, Swindon, Wiltshire, UK. Email: filippocinti [at] libero.it Submitted: 01/08/2019 Accepted: 05/03/2020 Published: 22/03/2020 © 2020 Open Veterinary Journal
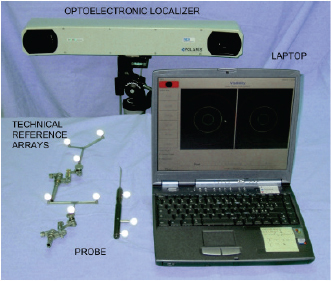
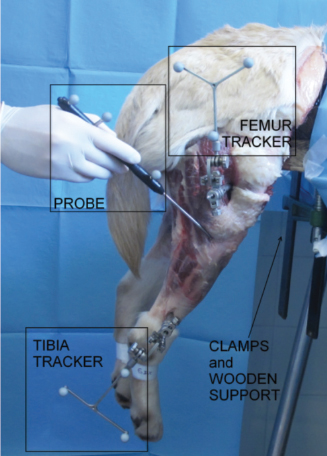
AbstractBackground: Cranial cruciate ligament (CCL) rupture is the most common orthopedic pathology in dog and in men. In human, optical computer-assisted technique is considered as a repeatable and reliable method for the biomechanical assessment of joint kinematics and laxity in case of CCL surgery. Aim: To evaluate the repeatability and reliability afforded by clinical tests in terms of laxity measured by means of a computer-assisted tracking system in two canine CCL conditions: CCL-Intact, CCL-Deficient. Methods: Fourteen fresh frozen canine stifles were passively subjected to Internal/External (IE) rotation at 120° of flexion and Cranial drawer test (CC). To quantify the repeatability and the reliability, intra-class correlation coefficient (ICC) and the mean percent error were evaluated (Δ r %). Results: The study showed a very good intra-class correlation, before and after CCL resection for kinematics tests. It was found a minimum ICC=0.73 during the IE rotation in CCL-Intact and a maximum value of ICC=0.97 for the CC displacement in CC-Deficient. IE rotation with CCL-Intact is the condition with the greatest Δ r %=14%, while the lowest Δ r %=6% was obtained for CC displacement in CCL-Deficient. Conclusion: The presented work underlined the possibility of using a computer-assisted method also for biomechanical studies concerning stifle kinematics and laxity. Keywords: Computer-assisted technique, Cranial cruciate ligament, Dog, Stifle joint. IntroductionThis stifle joint contains two cruciate ligaments: the Caudal Cruciate Ligament and the cranial cruciate ligament (CCL), which specifically presents the important biomechanical function of limiting internal rotation, hyperextension, and cranial tibial translation (De Rooster et al., 2006). In medium-large breed dogs, CCL insufficiency is one of the most frequent canine stifle pathologies (Duval et al., 1999). Literature reports the presence of different risk factors which may predispose dogs to the CCL rupture, such as breed, sex, age at diagnosis, and body weight (Harasen, 1995; Duval et al., 1999; Griffon, 2010). CCL injury can be treated either involving or not involving surgery (Griffon, 2010). Analogously to anterior cruciate ligament (ACL) reconstruction in human surgery, several surgical techniques have been developed including placement of intra-articular grafts (Arnoczky et al., 1979), insertion of suture material and/or advancement of periarticular structures outside the joint (extracapsular) (Flo, 1975; Cook et al., 2010; Tonks et al., 2011), and tibial osteotomies that alter joint mechanics (Slocum and Devine, 1984; Montavon et al., 2002). In ACL reconstruction surgery, new perspective were achieved due to the increased accuracy of the surgical reconstruction using computer-assisted tracking system (Sati et al., 2002; Mushal et al., 2003). The same result seems to be desirable even in the CCL surgery. Currently, the literature fails to prove the superiority of one technique compared to the others, thus leaving technique selection a pure matter of surgeon’s experience (Griffon, 2010; Tonks et al., 2011). In clinical practice, CCL laxity is evaluated using clinical tests without the support of any tool that allows for quantitative evaluation. Unfortunately, these qualitative measures have been found to be unreliable within the same case and are difficult to compare across different trials (Muir, 2010). A significant improvement in CCL surgery, from both clinical and biomechanical point of view, could be achieved by quantifying the kinematic evaluation of graft performance, thus to allow the evaluation and documentation of the effect of CCL reconstruction on stifle laxity at time-zero immediately after the fixation of the implant. Moreover, a quantitative evaluation of residual instability would be extremely helpful during the CCL reconstruction procedure. D’Amico et al. (2013) attempted to quantify the laxity level of the stifle joint during the most common clinical tests in three different conditions: CCL-intact, CCL-deficient, and CCL-stabilized. In the study, the quantification was performed by an electromagnet tracking system. Chailleux et al. (2007) tested 10 hindlimbs in order to asses the time zero postoperative effect of two corrective surgical techniques for CCL reconstruction. Even Tonks et al. (2010) quantify the effects of two different stifle stabilization in an in vitro study using a pressure sensor (Tonks et al., 2010). Unfortunately, the previous studies used testing machines which are impossible to apply during in vivo surgery. Moreover, they removed all the muscles complicating the applicability of their results to an in vivo condition. The goal of this study was to validate a quantitative kinematic method, based on a computer-assisted tracking system for assessment of joint laxity. This method was specifically applied to the assessment of the joint laxity in canine CCL injury. The main hypothesis was that, analogously to what happens in human ACL reconstruction, the proposed system can be extensively used to quantify joint kinematics and laxity even in the veterinary environment, thus providing an optimal tool for the biomechanical analysis of the animal joints. Trying to verify a possible application of the presented methodology also to the clinical practice, this study specifically evaluated both the repeatability and precision afforded by clinical tests in terms of quantitative laxity outcomes in two different conditions: intact (CCL-intact) and after its resection (CCL-deficient). Materials and MethodsSpecimensFourteen canine stifles from seven fresh-frozen medium-large breed cadaveric hemi-corpse specimens (four males and three females, aged 8–15 years, six dog breeds: one Dogue de Bordeaux, one Griffon, two Labradors, one Maremma sheepdogs, one Mongrels and one German Shepherd, 20–40 kg weight) were included in the study. The specimens were sectioned at rump level, thus to have both the complete hind limbs. Before testing, a qualified experienced veterinary surgeon (Dr. Filippo Cinti) examined on inspection and lateral radiographs all limbs to exclude any significant soft tissue pathology, previous surgery, or any gross morphologic abnormality. Limbs were double sealed in plastic bags and immediately frozen at—18°C and all specimens were thawed 24 hours prior to testing. After thawing, the limbs were prepared for testing by complete skin removal but preserved the limb muscles and periarticular tissues of the stifle joint. Each specimen was fixed to a wooden support with threaded Steinmann pins, thus to mimic normal standing position. This wooden support was then fixed to the end of a sturdy table using heavy duty “C” clamps to stabilize the sacrum and pelvis while allowing unrestricted complete motion of the hip and stifle. Each involved dog was euthanatized due to reasons unrelated to the presented study. Following euthanasia, all animals were donated to the Institution for educational purposes. EquipmentA custom-made computer-assisted tracking system, consisting of a commercial optical localizer (POLARIS, NDI, Canada), combined with a dedicated acquisition software (Matlab, The Mathworks Inc., USA), was specifically used to acquire joint kinematics. The optical localizer, used in passive configuration mode, allowed to track the position and orientation of several wireless trackers, which are rigid structures endowed with reflective spherical markers (13 mm diameter). A single marker position has been reported to be identified with a 3D root mean square (RMS) volumetric accuracy of 0.350 mm, and a 3D RMS volumetric repeatability of 0.200 mm (at 20°C) (Wiles et al., 2004). The laptop was interfaced to the optical localizer by means of a RS232 standard serial connection, thus ensuring an acquisition rate of 20 Hz and allowing a correct kinematic analysis. SetupIn order to track the relative motion between the femur and the tibia two, a tracker-holding device equipped with passive optical markers were fixed with 3 mm surgical Steinmann pins, with single trocar and round end (length: 9″–22.86 cm), on the corresponding bones. The femoral tracker-holding device was fixed on the proximal part of the femoral diaphysis about 50 mm distal to femoral head toward the craniolateral part of the femur, whereas the tibial tracker-holding device was fixed on the distal part of tibial diaphysis, about 30 mm proximal to the hock joint, toward the craniolateral part of the tibia. Both the femoral and tibial trackers-holding devices were fixed to the corresponding bones just before the acquisition of the anatomical landmarks and removed after performing the last static laxity test. An additional tracked probe was used to identify specific anatomical landmarks as described in the following section Registration phase. The acquisition of the anatomical landmarks is in fact required in order to transfer the kinematic data from the technical systems of reference to the anatomical ones (Wu et al., 2002; Di Gioia et al., 2005). The optical localizer was placed at almost 2 m away from the patient in the operating room paying particular attention to center the tracking volume within the effective acquisition area. The operating area need to be optimized in order to minimize obstacle along the line-of-sight between the passive markers and the computer-assisted tracking system as well as light reflections. Figure 1 shows the tracking system with the trackers and Figure 2 shows the femoral and tibial tracker as well as the anatomical landmarks acquisition by the tracked probe.
Fig. 1. Navigation system: optical localizer (Polaris, NDI, Canada) and laptop. Tracked markers and probe.
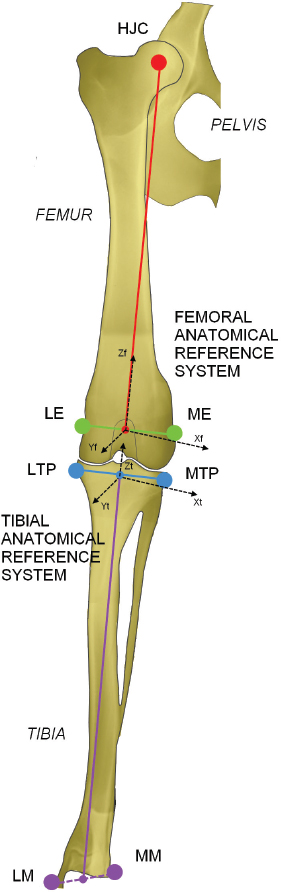
Fig. 2. In vitro setup. Acquisition of anatomalical landmarks and set-up in testing configuration. The proposed equipment for computer-assisted evaluation did not affect any possible CCL reconstruction, which can be performed following the standard surgical procedures and using the standard equipment. Registration phaseThe anatomical references were identified through the palpation of anatomical bony landmarks (medial and lateral malleoli, most medial and lateral points on plateaus, medial and lateral femoral epicondyles) using a probe equipped with passive optical markers. Placing the tracked probe in contact with the anatomical landmarks the software was able to define the coordinates of those points with respects to the femoral and tibial trackers. Functional hip joint center (HJC) was identified through a pivoting motion (Lopomo et al., 2010a) and used to define the anatomical femoral reference only during the acquisitions. The femoral anatomical reference system was defined with the Z-axis (proximal-distal direction) corresponding to the femoral mechanical axis, the X-axis (medial-lateral direction) as the transepicondylar line normalized with respect to the Z-axis and the Y-axis (cranial-caudal direction) as the cross product between the Z-axis and X-axis. The origin of the femoral system of reference is in the middle point between the two femoral epicondyles. Analogously, the tibial anatomical reference system was defined by setting the Z-axis as the tibial mechanical axis, the X-axis as the connecting line between the most lateral and the most medial point of the tibial plateaux normalized by the Z-axis. The Y -axis comes from cross product between Z-axis and X-axis. The origin lies in the middle point between the most lateral and the most medial point of the tibial plateau. Figure 3 reports anatomical landmarks and the corresponding reference systems. Static laxity testsA qualified and trained veterinary surgery performed a set of passive static laxity tests to assess joint kinematics. The surgeon performed the evaluation of stifle laxity as in clinical practice for CCL instability evaluation (Muir, 2010), by applying maximum manual load during the test. The two static laxity tests acquired by the computer-assisted tacking system and used as the reference for this study are:
Testing was conducted before and after CCL resection and repeated three times by the same veterinary surgeon. The acquisition protocol can be summarized with the following steps:
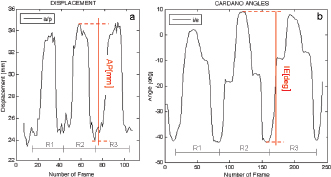
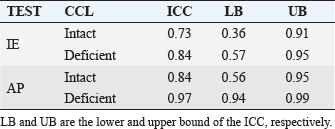
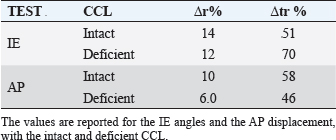
Fig. 3. Anatomical landmarks and reference systems. LE: Lateral Epicondyle, ME: Medial Epicondyle, LTP: Lateral Tibial Plateau, MTP: Medial Tibial Plateau, LM: Lateral Malleolus, MM: Medial Malleolus. The complete excision of the CCL was performed by lateral arthrotomy and complete section was done with a blade number 10. Subsequently, the joint capsule was sutured by a Ethicon Nylon non-absorbable suture three metric (18″–45 cm). During the whole set of tests, the examiner was always the same and he was blinded for test quantitative results (output of navigation system) in order to avoid bias in the acquisitions. The measured variables were total internal/external rotation (around Z-axis) during the first clinical test and craniocaudal displacement (along Y-axis) during the cranial drawer test. All the raw motion data were processed after capture using custom software written in Matlab (The Mathworks Inc. Natick, MA). In particular, the motion (displacement and rotation) of the tibia with respect to the femoral bone was calculated adapting the Grood and Suntay algorithm (1983) to the dog stifle joint. Statistical analysisIn order to verify the test-retest repeatability of the performed motions, the analysis included the patterns of the obtained angles (IE) and cranio/caudal displacement (CC), evaluating intra-class correlation coefficient (ICC), and its confidence interval, among the three repetitions (McGraw and Wong, 1996). The ICC is a quantification of the measurements as describe how strongly measurements in the same group (i.e., specimen) resemble each other. As the relative error compares the absolute error against the size of the variable under measuring it quantifies the precision during the measure. The absolute error, in this case, corresponds to the semidispersion of the data (being the sensitivity of the -assisted tacking system smaller than the semidispersion). The percent error shows the relative error as percentage. Given that, the mean of the percent error (Δr%) calculated for each specimen was used to quantify the precision in the intra-specimen analysis, implementing the equation: where Δri% is the percentage error for the i-th tested paw; n is the number of the tested paw; maxi, mini, meani are the maximum, minimum and mean value obtained in the i-th tested paw; corresponds to the semidispersion for the i-th paw. While the percent error () calculate over the total amount of the specimens was indicative of the precision in the inter-specimen laxity quantification, implementing the equation: where max Δr%, min Δr%, mean Δr% are the maximum, minimum, and mean value among all the Δri% calculated by Equation (1). All the statistical analysis was performed by using Matlab (The Mathworks Inc. Natick, MA). Ethical approvalThe authors follow the guidelines laid down by the International Animal Ethics Committee and Institutional ethics committee, in accordance with local laws and regulations. ResultsThe data obtained with the computer-assisted tracking system were in a range from 8° and 53° for the IE rotation and from 2 to 11 mm for the CC. In particular, concerning the IE rotation has been found a mean value (STD) of 18 (6) deg for the intact condition and 28 (9) deg after CCL resection. While during CC test has been found a displacement of 2.5(0.8) mm and 8(2) mm during the intact and deficient CCL condition, respectively. Figure 4 reports an example of the three repetitions for the two study clinical tests: Cranial Drawer test and Internal-External Rotation, as well. The ICC values for the performed clinical tests before and after the CCL lesion have been calculated (Table 1). The percent errors Δ r % and for the analyzed parameter during the two study clinical test with Intact and Deficient CCL have been reported (Table 2). DiscussionDue to the increasing recognition of the importance of CCL in the stability of stifle joint (De Rooster et al., 2006) different surgical techniques have recently been developed to address this ligament reconstruction (Cook et al., 2010). However, at present, there is no commonly recognized agreement on the best treatment, since a comparison among the data coming from different trials is difficult without a quantitative evaluation method of the surgery outcome. The most important finding of the present work was that it showed how the purposed analysis could be considered a repeatable and reliable method for the biomechanical assessment of joint kinematics and laxity in case of CCL surgery.
Fig. 4. Example of the analyzed laxity parameter: AP displacement [mm] and IE rotation angle [deg] during three repetitions (R1, R2, R3) of the Cranial drawer test (a) and IE rotation test (b). Table 1. ICC values for intact and deficient CCL for the two study tests (IE and AP).
Table 2. Δ r % is the mean of the percent error obtained for each specimens, Δ tr % is the percent error calculate over the total amount of the specimens.
The present study evaluated the performance obtained with a navigation system in quantifying laxity parameters both before and after the CCL lesion, thus analyzing the reliability of the proposed method also in a veterinary environment and trying to provide quantitative parameters in order to evaluate the method also for clinical applications. A similar system has already been intra-operatively validated and in an optimized clinical version is currently extensively used in human ACL surgeries. In particular, Martelli et al. (2007) reported that in 95% of the analyzed cases the repeatability in antero-posterior laxity was better than 2 mm, in internal-external rotation laxity was better than 3° and in varus-valgus laxity was better than 1.5°. Analogously Zaffagnini et al. (2006) reported an average standard deviation of 0.78° for varus-varus laxity tests, 1.83° for internal-external rotation laxity tests and 0.88 mm for antero-posterior laxity tests. The additional average intraoperative time due to navigated kinematic procedure was less than 12 minutes. Moreover, Lopomo et al. (2010b) reported good reliability of the system also in the analysis of dynamic tests. Only one study reported the use of a similar approach in small animal setting, but without reporting the overall reliability (Cinti et al., 2015). According to this literature and to the reliability analysis (Li and Nawar, 2007), this study reported the value of ICC and percentage error for IE rotation as well as CC translation during clinical tests for the assessment of CCL function. The results have shown that there is a very good intra-class correlation both before and after CCL resection for the two study tests. Furthermore, the results have been confirmed by the calculus of the percentage error Δr%. In particular was found a minimum ICC=0.73 during IE Rotation with intact CCL and a maximum value of ICC=0.97 for the CC displacement with deficient CCL. According to the previous results, IE rotation with intact CCL is the condition with the greatest Δ r % while the lowest Δ r % was obtained for CC displacement with deficient CCL. The precision in the inter-specimens analysis is definitively lower, indeed the Δ r % were in a range from 46% and 70%. The higher inter-specimens variability is in accordance with the high degree of inter-breeds as well as inter-cases specificity of the CCL laxity. The latter is considered a risk factor for CCL injuries. Furthermore, biomechanical and histological properties as well as CCL laxity are affected by combined factors including age, overweight, and degenerative changes (Vasseur et al., 1985). Similarly, also the human knee laxity is considered highly specific for each case and its clinical evaluation biased by the examiners’ skills and experience (Mouton et al., 2012). To this regard, literature affirms that has been found discrepancy among different ACL deficient knees in the magnitude of translation despite the same degree of internal injury (Dawson et al., 2013). In addition, the clinical evidence of CCL injury remains a complex issue depending from different factors as partial or complete rupture, acute or chronic damage, weight, age, development of muscle mass as well as the clinical experience, and ability of the examiner (Cook et al., 2010; Griffon, 2010). The proposed method has been proved to be useful for the biomechanical analysis of joint kinematics and laxity. However, the obtained results could suggest its use also in routine clinical practice during CCL reconstruction surgery, following the similar application to ACL reconstructions in human knee. Clearly further analysis and validation are required in order to optimize the procedure and to reduce the morbidity for the dogs. In fact, the methodology would aim to ensure that preparation and surgical techniques will not be affected by the use of the computer-assisted tracking system. It is worth noting that even if the presented procedure aims to be a minimally invasive method, it could affect the dog’s tissue (i.e., additional trauma) and thus any clinical use needs to be verified before the in vivo application. Indeed the proposed methodology would however allow a precise quantification of the joint laxity both before and after the surgery making possible a quantification of the surgery outcome for each individual stifle. We would underline that the methodology is neither a diagnostic nor a prognostic tool and provides only a possible quantification of stifle laxity, whereas the choice of the clinical correctness of the reconstruction remain a matter of the surgeon. In part, the high value of ICC can be facilitated by the fact that for within-subject evaluation the tools required by the tracking system for the anatomical localization are fixed to the limb and they do not require to be re-positioned between one acquisition and the following as it happens with other tools used in human knee join laxity evaluation. Even during the CCL reconstruction, the tools of the system are not removed from the limb reducing the margin of error in the comparison of pre-to-postoperative laxity. The only source of errors in kinematic decomposition could be due to the anatomical registration phase; this variability was contained in this study by involving the same expert surgeon who performed all the registration phases. Moreover, because of the bone-fixed trackers, the data of the tacking system are not affected by the soft tissue artifacts that represent a source of possible error. As with any in vitro study, there are some limitations to the current study. Both the kinematic and anatomical acquisitions as well as the CCL lesion were performed by only one veterinary surgeon and were not validated by a second or third surgeon. The inter-tester variability will be matter of a future study. Second, the study was based on manual clinical examination and accordingly provided a lack of consistent stress loading among the specimens. However, according also to studies performed on human knees (Martelli et al., 2007), the high degree of repeatability noted on the test–retest suggests that this lack of objective force measurement did not significantly affect our results recommending the analyzed method as a valid approach for the clinical evaluation of CCL laxity. Moreover, even if computer-assisted tracking system allows the surgeon to obtain a real-time feedback of surgery outcome giving the possibility to improve them and reduce intraoperative errors as well as soft tissues dissection, there are also some critically that need to be considered for in-vivo computer-assisted analysis (Gøthesen et al., 2011; Mavrogenis et al., 2013). Overall disadvantages associate to tracking system for orthopedic surgery can be summarized as: increased surgery time, risk of fracture, superficial infection in markers insertion place, need of learning curve, quadriceps recovery delayed (Stulberg et al., 2002; Li et al., 2008; Bae and Song, 2011; Gøthesen et al., 2011). The risk of fracture in human surgery has been reduced passing from 5 to 3 mm pins. Given the small size of canine bone should be evaluated to further reduce the diameter of the pins for in-vivo canine computer-assisted surgery still ensuring their stability, maybe changing the design as well. While the increase in the rate of infection has been proved to be not statistically significant (Gøthesen et al., 2011). For reducing the muscles damage due to the pins insertion an optimal position should be evaluated, considering the kind of provided surgery. This investigation reported on ICC and percentage error for laxity parameters both before and after CCL resection. In quantify all these factors, a very high intra-class correlation as well as precision in intra-class laxity analysis were found. This analysis allows considering the proposed method exploitable not only for biomechanical studies but also for clinical applications in veterinary surgery. The possible intra-operative setup, which will allow to assess the laxity of the joint at time-zero, should be however optimized for reduced invasiveness and morbidity. Conflict of interestThe Authors declares that there is no conflict of interest. Authors’ contributionAll authors contributed to conception of study, study design, acquisition of data, and data analysis and interpretation. All authors drafted and revised and approved the submitted manuscript. ReferencesArnoczky, S.P., Tarvin, G.B., Marshall, J.L. and Saltzman, B. 1979. The over-thetop procedure: a technique for anterior cruciate ligament substitution in the dog. J. Am. Anim. Hosp. Assoc. 15, 283–290. Bae, D.K. and Song, S.J. 2011. Computer assisted navigation in knee arthroplasty. Clin. Orthop. Surg. 3(4), 259–267. Chailleux, N., Lussier, B., De Guise, J., Chevlier, Y. and Hagemeister, N. 2007. In vitro 3-dimensional kinematic evaluation of 2 corrective operations for cruciate ligament-deficient stifle. Can. J. Vet. Surg. 71(3), 175–180. Cinti, F., Signorelli, C., Lopomo, N., Baracchi, M., Del Magno, S., Foglia, A., Zaffagnini, S. and Pisoni, L. 2015. Two different approaches for novel extracapsular cranial cruciate ligament recostruction: an in vitro Kinematic study. J. Small Anim. Pract. 56, 398–406. Cook, J.L., Luther, J.K., Beetem, J., Karmes, J. and Cook, C.R. 2010. Clinical comparison of a novel extracapsular stabilization procedure and tibial plateau leveling osteotomy for treatment of cranial cruciate ligament deficiency in dogs. Vet. Surg. 39, 315–323. D’Amico, L.L., Lanz, O.I., Aulakh, K.S., Butler, J.R., McLaughlin, R.M., Harper, T.A. and Werre, S.R. 2013. The effects of a novel lateral extracaosular suture system on the kinematics of the cranial cruciate deficient canine stifle. Vet. Comp. Orthop. Traumatol. 26, 271–279. Dawson, C.K., Suero, E.M. and Pearle, A.D. 2013. Variability in knee laxity in anterior cruciate ligament deficiency using a mechanized model. Knee Surg. Sports Traumatol. Arthrosc. 21(4), 784–788. De Rooster, H., De Bruin, T. and Van Bree, H. 2006. Morphology and function features of the canine cruciate ligaments. Vet. Surg. 35, 769–780. Di Gioia, A.M., Davidson, D. and Jaramaz, B. What can go wrong in CAOS. In Proceedings of the Fifth Annual Meeting of CAOS-International, Helsinki, Finland, 2005, pp 90–92. Duval, J.M., Budsberg, S.C., Flo, G.L. and Sammarco, J.L. 1999. Breed, sex, and body weight as risk factors for rupture of the cranial cruciate ligament in young dogs. J. Am. Vet. Med. Assoc. 215, 811–814. Flo, G.L. 1975. Modification of the lateral retinacular imbrications technique for stabilizing cruciate ligament injuries. J. Am. Vet. Med. Assoc. 11, 570–576. Gøthesen, O., Espehaug, B., Havelin, L., Petursson, G. and Furnes, O. 2011. Short-term outcome of 1.465 computer-navigated primary total knee replacements 2005-2008. Acta Orthop. 82(3), 293–300. Griffon, D.J. 2010. A review of the pathogenesis of canine cranial cruciate ligament disease as a basis for future preventive strategies. Vet. Surg. 39, 399–409. Grood, E.S. and Suntay, W.J. 1983. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J. Biomech. Eng. 105(2), 136–144. Harasen, G.L.G. 1995. A retrospective study of 165 cases of rupture of the canine cranial cruciate ligament. Can. Vet. J. 36, 250–251. Li, C.H., Chen, T.H., Su, Y.P., Shao, P.C., Lee, K.S. and Chen, W.M. 2008. Periprosthetic femoral supracondylar fracture after total knee arthroplasty with navigation system. J. Arthroplasty 52(2), 304–307. Li, L. and Nawar, S. 2007. Reliability analysis: Calculate and Compare Intra-class Correlation Coefficients (ICC) in SAS. In NorthEast SAS Users Group (NESUG) 2007 Annual Conference Proceedings, pp: 89–91. Lopomo, N., Sun, L., Zaffagnini, S., Giordano, G. and Safran, M.R. 2010a. Evaluation of formal methods in hip joint center assessment: an in vitro analysis. Clin. Biomech. (Bristol, Avon) 25(3), 206–212. Lopomo, N., Zaffagnini, S., Bignozzi, S., Visani, A. and Marcacci, M. 2010b. Pivot-shift test: analysis and quantification of knee laxity parameters using a navigation system. J. Orthop. Res. 28(2), 164–169. Martelli, S., Lopomo, N. and Bignozzi, S. 2007. Validation of a new protocol for navigated intraoperative assessment of knee kinematics. Comput. Biol. Med. 37(6), 872–878. Mavrogenis, A.F., Savvidou, O.D., Mimidis, G., Papanastasiou, J., Koulalis, D., Demertzis, N. and Papagelopoulos, P.J. 2013. Computer-assisted navigation in orthopedic surgery. Orthopedics 36(8), 631–642. McGraw, K.O. and Wong, S.P. 1996. Forming inferences about some intraclass correlation coefficients. Psychol. Methods 1(1), 30–46. Montavon, P.M., Damur, D.M. and Tepic, S. 2002. Advancement of the tibial tuberosity for the treatment of cranial cruciate deficient canine stifle. In ESVOT – VOS first world orthopaedic veterinary congress. Munich, Germany 2002, pp: 152. Mouton, C., Theisen, D., Pape, D., Nuhrenborger, C. and Seli, R. 2012. Static rotational knee laxity in anterior cruciate ligament injuries. Knee Surg Sports Traumatol. Arthrosc. 20, 652–662. Muir, P. 2010. History and clinical signs of cruciate ligament rupture. In Advances in the canine cranial cruciate ligament, 1st ed. Eds., Peter. M. Hoboken, NJ: Wiley-Blackwell, pp: 101–104. Mushal, V., Burkart, A., Debski, RE., Van Scyoc, A., Fu, F.H. and Woo, S.L.Y. 2003. Anterior cruciate ligament tunnel placement: comparison of insertion site anatomy with the guidelines of a computer-assisted surgical system. Arthroscopy 19, 152–160. Sati, M., Staubli, H., Bourquin, Y., Kunz, M. and Nolte, L.P. 2002. Realtime computerized in situ guidance system for ACL graft placement. Comput. Aided. Surg. 7, 25–40. Slocum, B. and Devine, T. 1984. Cranial tibial wedge osteotomy: a technique for eliminating cranial tibial thrust in cranial cruciate ligament repair. J. Am. Vet. Med. Assoc. 184, 564–569. Stulberg, S.D., Loan, P. and Sarin, V. 2002. Computer-assisted navigation in total knee replacement: results of an initial experience in thirty-five patients. J. Bone Joint Surg. Am. 84, 90–98. Tonks, C.A., Lewis, D. and Pozzi, A. 2011. A review of extra-articular prosthetic stabilization of the cranial cruciate ligament-deficient stifle. Vet. Comp. Orthop. Traumatol. 24, 167–177. Tonks, C.A., Pozzi, A., Ling, H.Y. and Lewis, D.D. 2010. The effect of extra-articular suture tension on contact mechanics of the lateral compartment of cadaveric stifle treated with the TightRope CCL or lateral suture technique. Vet. Surg. 39(3), 343–349. Vasseur, P.B., Pool, R.R., Arnoczky, S.P. and Lau, R.E. 1985. Correlative biomechanical and histologic study of the cranial cruciate ligament in dogs. Am. J. Vet. Res. 46, 1842–1854. Wiles, A.D., Thompson, D.G. and Frantz, D.D. 2004. Accuracy assessment and interpretation for optical tracking systems light-emitting diodes: research manufacturing and applications VIII. In Proceedings of the SPIE. Eds. Stockman, S.A., Yao, H.W. and Schubert, E.F. Bellingham (WA), USA, pp: 421–432. Wu, G., Siegler, S., Allard, P., Kirtley, C., Leardini, A., Rosenbaum, D., Whittle, M., D’Lima, D.D., Cristofolini, L., Witte, H., Schmid, O. and Stokes, I. 2002. ISB recommendation on definition of joint coordinate system of various joints for the reporting of human joint motion—part I: ankle, hip, and spine. J. Biomech. 35(4), 543–548. Zaffagnini, S., Bignozzi, S., Martelli, S., Imakiire, N., Lopomo, N. and Marcacci, M. 2006. New intraoperative protocol for kinematic evaluation of ACL reconstruction: preliminary results. Knee Surg. Sports Traumatol. Arthrosc. 14(9), 811–816. | ||
| How to Cite this Article |
| Pubmed Style Signorelli C, Cinti F, Zaffagnini S, Lopomo NF. Validation of an optical, computer-assisted technique for intraoperative tracking of 3-dimensional canine stifle joint motion. Open Vet. J.. 2020; 10(1): 86-93. doi:10.4314ovj.v10i1.14 Web Style Signorelli C, Cinti F, Zaffagnini S, Lopomo NF. Validation of an optical, computer-assisted technique for intraoperative tracking of 3-dimensional canine stifle joint motion. https://www.openveterinaryjournal.com/?mno=59644 [Access: January 25, 2026]. doi:10.4314ovj.v10i1.14 AMA (American Medical Association) Style Signorelli C, Cinti F, Zaffagnini S, Lopomo NF. Validation of an optical, computer-assisted technique for intraoperative tracking of 3-dimensional canine stifle joint motion. Open Vet. J.. 2020; 10(1): 86-93. doi:10.4314ovj.v10i1.14 Vancouver/ICMJE Style Signorelli C, Cinti F, Zaffagnini S, Lopomo NF. Validation of an optical, computer-assisted technique for intraoperative tracking of 3-dimensional canine stifle joint motion. Open Vet. J.. (2020), [cited January 25, 2026]; 10(1): 86-93. doi:10.4314ovj.v10i1.14 Harvard Style Signorelli, C., Cinti, . F., Zaffagnini, . S. & Lopomo, . N. F. (2020) Validation of an optical, computer-assisted technique for intraoperative tracking of 3-dimensional canine stifle joint motion. Open Vet. J., 10 (1), 86-93. doi:10.4314ovj.v10i1.14 Turabian Style Signorelli, Cecilia, Filippo Cinti, Stefano Zaffagnini, and Nicola Francesco Lopomo. 2020. Validation of an optical, computer-assisted technique for intraoperative tracking of 3-dimensional canine stifle joint motion. Open Veterinary Journal, 10 (1), 86-93. doi:10.4314ovj.v10i1.14 Chicago Style Signorelli, Cecilia, Filippo Cinti, Stefano Zaffagnini, and Nicola Francesco Lopomo. "Validation of an optical, computer-assisted technique for intraoperative tracking of 3-dimensional canine stifle joint motion." Open Veterinary Journal 10 (2020), 86-93. doi:10.4314ovj.v10i1.14 MLA (The Modern Language Association) Style Signorelli, Cecilia, Filippo Cinti, Stefano Zaffagnini, and Nicola Francesco Lopomo. "Validation of an optical, computer-assisted technique for intraoperative tracking of 3-dimensional canine stifle joint motion." Open Veterinary Journal 10.1 (2020), 86-93. Print. doi:10.4314ovj.v10i1.14 APA (American Psychological Association) Style Signorelli, C., Cinti, . F., Zaffagnini, . S. & Lopomo, . N. F. (2020) Validation of an optical, computer-assisted technique for intraoperative tracking of 3-dimensional canine stifle joint motion. Open Veterinary Journal, 10 (1), 86-93. doi:10.4314ovj.v10i1.14 |