| Original Article | ||
Open Vet. J.. 2023; 13(3): 322-326 Open Veterinary Journal, (2023), Vol. 13(3): 322–326 Original Research Effect of site of sample collection on blood glucose concentrations measured with a portable blood glucose meter in healthy and diabetic dogsSina Jahan1*, Samin Jahan1, Shahram Jamshidi1, Guido Linari2, Federico Fracassi2 and Hesameddin Akbarein11Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran 2Department of Veterinary Medical Sciences, University of Bologna, Bologna, Italy *Corresponding Author: Sina Jahan. Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran. Email: sjahan58 [at] yahoo.com. Submitted: 30/07/2022 Accepted: 22/02/2023 Published: 12/03/2023 © 2023 Open Veterinary Journal
AbstractBackground: Blood glucose (BG) monitoring with portable blood glucose meters (PBGMs) is a critical aspect of managing canine diabetes mellitus. Some dogs best tolerate sampling from the ear, others from the lip, and others from other body sites. Therefore, it is relevant to know if the choice of the sampling site affects the glucose concentration. Aim: To compare different sampling sites for BG measurement in diabetic and non-diabetic dogs using veterinary PBGM. Moreover, determining the possible impact of body condition score (BCS) on BG concentration. Methods: Thirty-seven healthy and 12 diabetic dogs were included. A veterinary PBGM was used to measure BG concentrations in a total of 196 blood samples collected from the marginal ear vein (MEV), carpal pad, saphenous vein, and cephalic vein. The results obtained from the different sampling sites were compared. Results: The carpal pad, MEV, cephalic vein, and saphenous vein BG values were not significantly different at the different blood collection sites. There was no significant difference between higher and lower BCS in BG measurements in the different sampling sites. Conclusion: Different sampling sites, likewise utilizing either a venous or capillary sample, had no significant effect on BG measurement using veterinary PBGMs. The BCS seems to have no relevant influence on dog BG measurement. Keywords: Glycemia, Canine, PBGMs, Sampling site, Body condition score. IntroductionDiabetes mellitus is one of the most common canine endocrine disorders that is primarily identified by long-term hyperglycemia and glycosuria (Fracassi, 2017). The treatment of diabetes mellitus is based on blood glucose control and, therefore, repeated glucose measurements are mandatory for therapeutic management. Portable blood glucometers (PBGMs) are specifically designed to measure capillary blood glucose concentration in human medicine. In recent years, PBGMs specifically for veterinary use have also been developed. AlphaTRACK2®, Zoetis, USA is a PBGM produced for dogs and cats, and when compared with other PBGMs, it seems the most accurate and, therefore, the recommended device for these species (Cohen et al., 2009; Zini et al., 2009). The PBGMs are initially calibrated for the capillary blood samples; however, several studies did evaluate the analytical performances using venous blood samples, and their result were clinically acceptable (Cohn et al., 2000; Kang et al., 2010). One of the main practical problems in using the glucometer in dogs is the blood collection site. While people usually prick their fingertips to get a drop of blood, deferral sampling sites are often used in dogs. Most of the dogs best tolerate sampling from the ear, others from the lip, and some of them from another body site. Marginal ear vein (MEV) is a feasible sampling site (Thompson et al., 2017); there are alternative sampling sites such as carpal and metacarpal pads and buccal mucosa that can be used as a sampling site for PBGMs in dogs (Zeugswetter et al., 2010; Borin et al., 2012). There are conflicting data on the concordance of blood glucose measurements from different sampling sites using PBGMs. Few studies have evaluated the performance of the PBGMs in various locations of the body; however, no study compares dogs to the results obtained from the blood collected on carpal pads, MEV, saphenous and cephalic veins (Borin et al., 2012; Guevera et al., 2019). The body condition score (BCS) (Laflamme, 1997) might influence the blood glucose measurement in different sampling sites. Footpads mainly consist of adipose tissue and capillaries. Hence, there is a scale in the proportion of fat and other tissues of footpads to BCS (Chi et al., 2010); a higher BCS score indicates more adipose tissue (Miao et al., 2017), and this could potentially have an influence in glucose measurement in the carpal pad. Therefore, we aimed to investigate whether BCS influenced blood glucose measured by PBGMs. Thus, this study first aims to compare different sampling sites for blood glucose determination in diabetic and non-diabetic dogs using veterinary PBGM. The second aim was to determine the impact of BCS on blood glucose concentration in dogs with low and high BCS by comparing the difference between blood glucose measurements from the carpal pad and MEV. Materials and MethodsStudy design and populationThis cross-sectional study was performed at a teaching hospital from June 1, 2019, to August 20, 2019. The protocol was approved by the local ethics and welfare committee. (R.UT.VETMED.REC.1401.007) Before collecting the samples from every dog, pet owners obtained written consent. Forty-nine clients owned dogs (17 mixed reeds, 8 German Shepherds, 4 Pomeranians, 4 Shi-Tzu, 3 Shi-Tzu Terrier, 3 Siberian Husky, 2 Labrador Retriever, 2 Pekingese, 1 Finnish Spitz, 1 Miniature Poodle, 1 Standard Poodle, 1 English Setter, 1 Boxer, 1 Chihuahua) which admitted for routine checkups or diabetes mellitus monitoring were included in this study. The study population consisted of 26 females (17 spayed) and 23 males (17 castrated), the median age of the dogs was 2 years (range, 1–14 years), and the median body weight was 15.31 kg (range, 2–39 kg). Thirty-seven were healthy dogs, and 12 had diabetes mellitus. The need for blood glucose measurement was the only inclusion criterion. The sampling could be done by fasting or post-prandial. All the participants were evaluated for BCS using a 9-point scoring system (Laflamme, 1997), which is listed here: BCS 2/9 (1), BCS 3/9 (7), BCS 4/9 (15), BCS 5/9 (11), BCS 6/9 (9), BCS 7/9 (5), BCS 8/9 (1). Dogs were divided into normal/fat (BCS ≥ 5/9) and thin groups (BCS < 5/9). Blood sampling and the principle of blood glucose measurementDogs were gently restrained, and whole fresh blood was drawn from a cephalic and saphenous vein with a 23-gauge syringe. Afterward, the glucose was immediately measured with a veterinary PBGM (AlphaTRACK2®, Zoetis, USA) (Fig. 1C). With a standard lancing device and via the MEV Nick technique (Thompson et al., 2002), a drop of blood from the MEV (Fig. 1A) and the carpal pad (Fig. 1B) was obtained to be measured with PBGM. The order of blood collection from sampling sites was determined randomly by a statistical program (pickatrandom.com). All the procedures took place within 15 minutes to prevent the dampening effect of time on blood glucose concentration. One of the trained authors performed all the sampling procedures to eliminate the sampling discrepancies and minimize personal errors. Description of the portable blood glucose meterAlphaTRAK2® PBGMs measure blood glucose concentration with a colorimetric method utilizing a flavin-adenine dinucleotide-glucose dehydrogenase-catalyzed reaction. This device requires 0.3 µl of whole blood, and the linear range is 20–720 mg/dl. The PBGM was calibrated based on manufacturer recommendations. Data analysisThe data were analyzed using commercial statistical software packages (GraphPad Prism 7® and R Core Team, 2019). Data distribution was evaluated using the D'Agostino and Pearson test, and parametric or non-parametric tests were used accordingly. The Kruskal–Wallis test with Dunn’s post-test was used to compare blood glucose among sampling sites (MEV, carpal pad, saphenous vein, cephalic vein). The differences between the glucose concentrations measured from the carpal pad and MEV were compared in dogs with BCS ≥ 5/9 and dogs with BCS < 5 using the Mann-Whitney U test. The differences between the glucose concentrations measured from the carpal pad and MEV were correlated to the BCS using the Spearman test. The statistical evaluations mentioned above were also carried out by separately analyzing the group of diabetic dogs and non-diabetic dogs. p < 0.05 was considered significant.
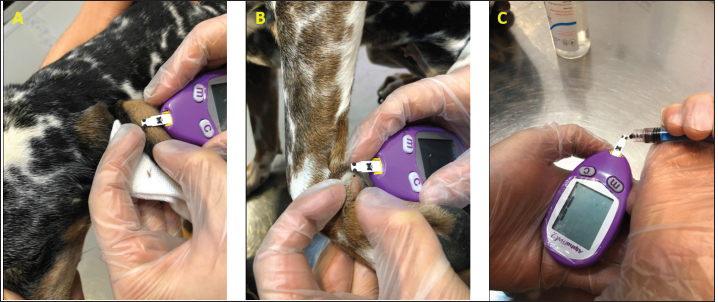
Fig. 1. (A) Pinna puncturing and blood glucose measurement via MEV Nick technique. (B) Carpal pad glucose measurement after a drop of blood was well up at the site. (C) Venous blood glucose measurement with AlphaTRAK2® PBGM using a 23-gauge syringe. ResultsA total of 196 blood samples were obtained from 49 dogs. The glucose concentration of all 49 participants of each sampling site is displayed in Figure 2. Mean ± SD blood glucose measured from MEV was 134.47 ± 68.96. Mean ± SD blood glucose measurement from the carpal pad was 134.47 ± 70.14. Mean ± SD blood glucose measurement from the saphenous vein was 141.51 ± 67.94, and mean ± SD blood glucose measurement from the cephalic vein was 139.45 ± 69.25. The carpal pad, MEV, cephalic vein, and saphenous vein blood glucose values did not differ significantly (p=0.33). The median difference between the glucose concentrations measured from the carpal pad and MEV was −3.0 (min −73, max 27) in dogs with BCS < 5 and 2.0 (min −38 max 130) in dogs with BCS ≥ 5/9. This difference was not significant (p=0.31) (Fig. 3). No correlation was found between the difference between the glucose concentrations measured from the carpal pad and MEV and the BCS (r=0.11, p=0.43). Analyzing the group of diabetic dogs and non-diabetic dogs separately, no significant differences were observed regarding all the statistical evaluation listed above. DiscussionAccurate and rapid measurement of blood glucose is crucial for managing many metabolic and endocrine disorders such as diabetes mellitus (Cohen et al., 2009). PBMS has facilitated blood glucose measurement in various veterinary settings from multiple sampling sites. Hence, data on the differences in blood glucose measured from known carpal and venous sampling sites have been conflicting (Cohen et al., 2009; Borin et al., 2012). Based on the literature, AlphaTRAK2 is presented as a valid veterinary glucometer and provides clinically acceptable results (Cohen et al., 2009); and is one of the most commonly used PBMs in veterinary medicine; for this reason, it was chosen for the present study.
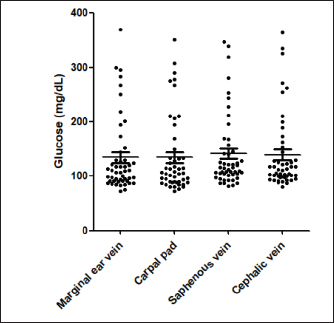
Fig. 2. Blood glucose concentrations measured from the MEV (median 110 mg/dl, minimum 73 mg/dl, maximum 370 mg/dl), carpal pad (median 106 mg/dl, minimum 72 mg/dl, maximum 351 mg/dl), saphenous veins (median 115 mg/dl, minimum 82 mg/dl, maximum 347 mg/dl), cephalic (median 112 mg/dl, minimum 81, maximum 364 mg/dl) and, of 49 dogs. The Kruskal–Wallis test with Dunn’s post-test was used to compare the different blood sampling sites. No significant difference between the different sampling sites was observed.
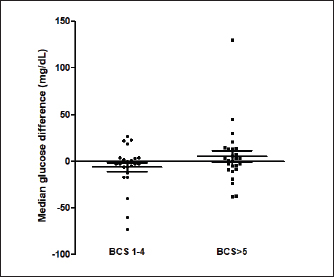
Fig. 3. The median difference between the glucose concentrations measured from the carpal pad and the MEV in dogs with BCS < 5 and in dogs with BCS ≥ 5/9. The differences between the two groups was investigated using the Mann-Whitney U test. This difference was not significant (p=0.31). The primary purpose of this study was to evaluate different sampling sites for measuring the diversity of blood glucose values measured via PBGM. The results suggest that various sampling sites and utilizing either venous or capillary blood samples had no significant effect on blood glucose measured by PBGM. A previous study also investigated different sampling sites to measure glucose in dogs (Borin et al., 2012). It revealed no significant difference between blood glucose values calculated from the auricular capillary, carpal pad, and venous samples. In the above-mentioned study, the authors evaluated only diabetic dogs, while our study included a wide range of different glycemic values containing diabetic and non-diabetic dogs. Another survey on cats aimed at assessing the accuracy of metatarsal/metacarpal pads to measure glucose using a PBGM showed no difference in respect of blood glucose measurement regarding the pinna site and the sites mentioned (Zeugswetter et al., 2010). Therefore, both in our study and in the studies cited above, it is observed that the sampling site has no significant influence on the glycemic measurement. The results of our study are not in agreement with the findings of a previous study conducted by Guevera et al. (2019) on 12 healthy dogs, intending to evaluate the impact of the prandial state and sampling site on blood glucose concentrations. The authors claimed that the blood glucose collecting area is a prominent factor in glucose measurement as values obtained from capillary sites of the pinna reflect a more accurate and closer value to blood glucose measurement from the venous site than blood samples obtained from oral mucosa or carpal pad (Guevera et al., 2019). This latter study is the only one that shows significant differences with the sampling site. It is difficult to identify the reasons for these differing results. Only healthy dogs were included in the study of Guevera et al. (2019), while in our study both healthy and diabetic dogs were included, but this difference alone hardly justifies the different results. The carpal pad is considered a valid and feasible sampling site, as pinna puncturing showed difficulties in obtaining a drop of blood in some patients. Moreover, some considerations must be noted regarding the feasibility of MEV, such as some deformities or condemned cosmetic surgeries of the ear, which leads to the identification of alternative sampling sites. The result of our study was compatible with a previous study (Borin et al., 2012) that compared the glycemia of 30 diabetic dogs on the carpal pad, auricular capillary, and venous glycemia. This study observed that all the sampling sites mentioned were viable. Another factor investigated in this study is the correlation between the BCS and its impact on the glucose measured in the carpal pad site compared with MEV. Chi and Roth explained that the structural properties of digitigrades are scaled in proportion to their BCS (Chi et al., 2010). We had a variety of BCSs in our study, from BCS 2/9 to BCS 8/9. We did not observe a statistical difference between the blood glucose measured from each site. A limitation of this study is that blood samples from fasted and non-fasted animals were included and this variable was not recorded or analyzed. It was decided not to take into account the "fasting" variable because such condition reflects what happens in a normal population of diabetic dogs monitored with blood glucose curves. In diabetic dogs, some glycemic values are evaluated in the fasted state (e.g., pre-prandial glucose) and most of the blood glucose measurements are assessed post-prandial. Future studies are needed to evaluate whether the fasting state may have an influence on the sampling site. The results of this study show no significant difference among the MEV, carpal pad, cephalic, and saphenous sampling sites in terms of glucose measurement, suggesting that any of these sites can be utilized as has been reported in previous studies (Zeugswetter et al., 2010; Borin et al., 2012). There is no significant influence between higher and lower BCS in blood glucose measurement. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsAuthors contribution conformation: Study design: Sina Jahan, Shahram Jamshidi, Federico Fracassi. Data collection: Sina Jahan, Samin Jahan, Guido Linari. Analysis performer: Hesameddin Akbarein, Sina Jahan, Samin Jahan. Interpretation of the data: Hesameddin Akbarein, Sina Jahan, Federico Fracassi. Draft manuscript preparation: Sina Jahan, Samin Jahan, Guido Linari. ReferencesBorin, S., Crivelenti, L.Z., Rondelli, M.C.H. and Tinucci-Costa, M. 2012. Capillary blood glucose and venous blood glucose measured with portable digital glucometer in diabetic dogs. Braz. J. Vet. Pathol. 5(2), 42–46. Borin, S., Crivellenti, L.Z. and Tinucci-Costa, M. 2012. The carpal pad as an alternative sampling site for blood glucose testing in dogs. J. Small Anim. Pract. 53(12), 684–686. Chi, K.J. and Louise Roth, V. 2010. Scaling and mechanics of carnivoran footpads reveal the principles of footpad design. J. R. Soc. Interface 7(49), 1145–1155. Cohen, T.A., Nelson, R.W., Kass, P.H., Christopher, M.M. and Feldman, E.C. 2009. Evaluation of six portable blood glucose meters for measuring blood glucose concentration in dogs. J. Am. Vet. Med. Assoc. 235(3), 276–280. Cohn, L.A., McCaw, D.L., Tate, D.J. and Johnson, J.C. 2000. Assessment of five portable blood glucose meters, a point-of-care analyzer, and color test strips for measuring blood glucose concentration in dogs. J. Am. Vet. Med. Assoc. 216(2), 198–202. Costa, P.B., Ricarti, M.L., Augustavo, C.A.A., Santos, L.S., Crivellenti, L.Z. and Borin, S. 2021. Transoperative glycemia in pets: validating old ones, and presenting lip mucosa as new sampling site. Domest. Anim. Endocrinol. 74, 106540. Guevara, J.L., Tobias, K.M., Stokes, J.E., Zhu, X. and Smith, R.A. 2019. Effect of site of sample collection and prandial state on blood glucose concentrations measured with a portable blood glucose meter in healthy dogs. Am. J. Vet. Res. 80(11), 995–1000. Kang, M.H., Kim, D.H., Jeong, I.S., Choi, G.C. and Park, H.M. 2016. Evaluation of four portable blood glucose meters in diabetic and non-diabetic dogs and cats. Vet. Q. 36(1), 2–9. Laflamme, D.R.P.C. 1997. Development and validation of a body condition score system for dogs. Canine Pract. 22(1), 10–15. Miao, H., Fu, J., Qian, Z., Ren, L. and Ren, L. 2017. How does the canine paw pad attenuate ground impacts? A multi-layer cushion system. Biol. Open 6(12), 1889–1896. Morbeg, E., Hagstro-Toft, E., Arner, P. and Bolinder, J. 1997. Protracted glucose fall in subcutaneous adipose tissue and skeletal muscle compared with blood during insulin-induced hypoglycemia. Diabetologia 40, 1320–1326. Thompson, M.D., Taylor, S.M., Adams, V.J., Waldner, C.L. and Feldman, E.C. 2002. Comparison of glucose concentrations in blood samples obtained with a marginal ear vein Nick technique versus from a peripheral vein in healthy cats and cats with diabetes mellitus. J. Am. Vet. Med. Assoc. 221(3), 389–392. Zeugswetter, F.K., Rebuzzi, L. and Karlovits, S. 2010. Alternative sampling site for blood glucose testing in cats: giving the ears a rest. J. Feline Med. Surg. 12(9), 710–713. Zini, E., Moretti, S., Tschuor, F. and Reusch, E. 2009. Evaluation of a new portable glucose meter designed for the use in cats. Schweiz. Arch. Tierheilkd. 151(9), 448–451. | ||
| How to Cite this Article |
| Pubmed Style Jahan S, Jahan S, Jamshidi S, Inari G, Fracassi F, Akbarein H. Effect of site of sample collection on blood glucose concentrations measured with a portable blood glucose meter in healthy and diabetic dogs. Open Vet. J.. 2023; 13(3): 322-326. doi:10.5455/OVJ.2023.v13.i3.8 Web Style Jahan S, Jahan S, Jamshidi S, Inari G, Fracassi F, Akbarein H. Effect of site of sample collection on blood glucose concentrations measured with a portable blood glucose meter in healthy and diabetic dogs. https://www.openveterinaryjournal.com/?mno=70532 [Access: January 23, 2026]. doi:10.5455/OVJ.2023.v13.i3.8 AMA (American Medical Association) Style Jahan S, Jahan S, Jamshidi S, Inari G, Fracassi F, Akbarein H. Effect of site of sample collection on blood glucose concentrations measured with a portable blood glucose meter in healthy and diabetic dogs. Open Vet. J.. 2023; 13(3): 322-326. doi:10.5455/OVJ.2023.v13.i3.8 Vancouver/ICMJE Style Jahan S, Jahan S, Jamshidi S, Inari G, Fracassi F, Akbarein H. Effect of site of sample collection on blood glucose concentrations measured with a portable blood glucose meter in healthy and diabetic dogs. Open Vet. J.. (2023), [cited January 23, 2026]; 13(3): 322-326. doi:10.5455/OVJ.2023.v13.i3.8 Harvard Style Jahan, S., Jahan, . S., Jamshidi, . S., Inari, . G., Fracassi, . F. & Akbarein, . H. (2023) Effect of site of sample collection on blood glucose concentrations measured with a portable blood glucose meter in healthy and diabetic dogs. Open Vet. J., 13 (3), 322-326. doi:10.5455/OVJ.2023.v13.i3.8 Turabian Style Jahan, Sina, Samin Jahan, Shahram Jamshidi, Guido Inari, Federico Fracassi, and Hesameddin Akbarein. 2023. Effect of site of sample collection on blood glucose concentrations measured with a portable blood glucose meter in healthy and diabetic dogs. Open Veterinary Journal, 13 (3), 322-326. doi:10.5455/OVJ.2023.v13.i3.8 Chicago Style Jahan, Sina, Samin Jahan, Shahram Jamshidi, Guido Inari, Federico Fracassi, and Hesameddin Akbarein. "Effect of site of sample collection on blood glucose concentrations measured with a portable blood glucose meter in healthy and diabetic dogs." Open Veterinary Journal 13 (2023), 322-326. doi:10.5455/OVJ.2023.v13.i3.8 MLA (The Modern Language Association) Style Jahan, Sina, Samin Jahan, Shahram Jamshidi, Guido Inari, Federico Fracassi, and Hesameddin Akbarein. "Effect of site of sample collection on blood glucose concentrations measured with a portable blood glucose meter in healthy and diabetic dogs." Open Veterinary Journal 13.3 (2023), 322-326. Print. doi:10.5455/OVJ.2023.v13.i3.8 APA (American Psychological Association) Style Jahan, S., Jahan, . S., Jamshidi, . S., Inari, . G., Fracassi, . F. & Akbarein, . H. (2023) Effect of site of sample collection on blood glucose concentrations measured with a portable blood glucose meter in healthy and diabetic dogs. Open Veterinary Journal, 13 (3), 322-326. doi:10.5455/OVJ.2023.v13.i3.8 |