| Short Communication | ||
Open Vet. J.. 2020; 10(1): 39-43 doi: 10.4314/ovj.v10i1.7 Open Veterinary Journal, (2020), Vol. 10(1): 39–43 Short Communication DOI: http://dx.doi.org/10.4314/ovj.v10i1.7 First molecular detection of Plasmodium relictum in Anopheles sinensis and Armigeres subalbatusJilei Zhang1,2, Guangwu Lu1, Patrick Kelly3, Jing Li1, Min Li1, Jiawei Wang1, Ke Huang1, Haixiang Qiu1, Jinfeng You1, Rong Zhang1, Yaoyao Wang1, Yuanyuan Zhang1 and Chengming Wang1,4*1College of Veterinary Medicine, Yangzhou University, Yangzhou 225009, China 2Division of Gastroenterology and Hepatology, Department of Medicine, University of Illinois at Chicago, Chicago, IL 60607, USA 3Ross University School of Veterinary Medicine, Basseterre, St. Kitts & Nevis, West Indies 4College of Veterinary Medicine, Auburn University, Auburn, AL 36849, USA *Corresponding Author: Chengming Wang. College of Veterinary Medicine, Auburn University, Auburn, AL 60607, USA. Email: wangche [at] auburn.edu Submitted: 07/12/2019 Accepted: 04/02/2020 Published: 22/02/2020 © 2020 Open Veterinary Journal
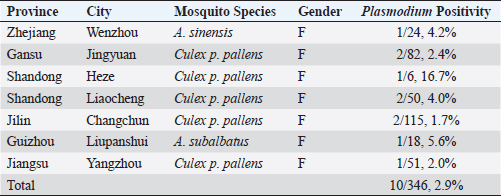
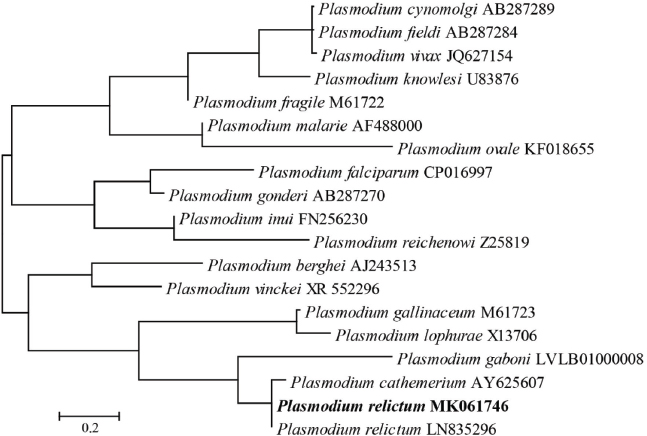
AbstractBackground: Plasmodium relictum is one of the most important avian malaria species, which is mainly seen in wild birds, with infections reported in more than 70 different species and at high prevalence. Aim: The aim of this study was to determine the molecular prevalence of Plasmodium spp. in mosquitoes collected in China. Method: A Plasmodium -specific fluorescence resonance energy transfer (FRET) polymerase chain reaction (PCR) was established in this study to analyze five species of mosquitoes (1,620 Culex pipiens pallens, 806 Aedes albopictus, 377 Armigeres subalbatus, 168 Anopheles sinensis, and 80 Culex tritaeniorhynchus) collected in hand nets from homes in 25 provinces of China. Results: Only females originated from six provinces were determined to be positive (0.6%, 10/1,809). Plasmodium species were detected in three mosquito species, such as C. pipiens pallens (0.5%, 8/1,620), A. sinensis (0.6%, 1/168), and A. subalbatus (0.3%, 1/377). Of the three mosquito species positive for P. relictum, only C. pipiens pallens is known to feed on birds and is recognized as the natural vector of P. relictum. Conclusion: This is the first time that P. relictum has been detected in A. sinensis and A. subalbatus. P. relictum, the agent of avian malaria, was present in mosquitoes in China, including mosquito species not previously thought to be the vectors. Keywords: Anopheles sinensis, Armigeres subalbatus, China, Mosquito, Plasmodium relictum. IntroductionPlasmodium, the mosquito-borne agent of malaria, belongs to the phylum Apicomplexa which is a taxonomic group of single-celled parasites with characteristic secretory organelles (de Koning-Ward et al., 2016). The genus Plasmodium contains over 200 species which can be divided into 14 subgenera based on morphology and host range (Martinsen and Perkins, 2013; Perkins, 2014). Plasmodium parasites are the most common in tropical areas, such as India, Australia, and Southeast Asia (Schoener et al., 2017), and have been described in a broad array of vertebrate hosts. In particular, over 150 Plasmodium species can be found infecting a variety of birds (Sylvie et al., 2008) with Plasmodium relictum, Plasmodium elongatum, Plasmodium vaughani, and Plasmodium sp. lineage LINN1 being the most common in birds and mosquitoes in Europe (Schoener et al., 2017). With its size and multiple geographies, China has a wide diversity of mosquito species (Guo et al., 2018), many of which have been reported to transmit Plasmodium species elsewhere in the world. Four human malarial species have been reported in China, such as Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale (Li et al., 2016), but the cases of human malaria have decreased dramatically recently, from the more than 30 million cases a year reported in the 1940s to 1,380 cases in 2018 (Zhang et al., 2018; Zhou et al., 2008). Mosquito control programs have resulted in near elimination of the historically most important mosquito vectors of malaria in people in China, Anopheles lesteri (synonym: An. anthropophagus) and Anopheles dirus s.l. This, however, has led to other species such as Anopheles sinensis now becoming important vectors (Zhang et al., 2017b). There are only limited data on bird malaria in China with infection rates of up to 8% in wild species (Zhang et al., 2014b), and a variety of species, such as P. relictum and Plasmodium homonucleophilum, and their lineages were described in wild and captive birds (Huang et al., 2015; Jia et al., 2018). To the best of our knowledge, there are no data on the mosquitoes carrying Plasmodium species infecting birds in China. In this report, we describe the development of a Plasmodium-specific FRET-qPCR and its use in detecting Plasmodium species in mosquitoes collected from across China. Materials and MethodsTo establish the Plasmodium-specific FRET-qPCR, we obtained 18S rRNA sequences for representative Plasmodium species from GenBank: P. falciparum (M19172, CP016997, JQ627152, U07367), P. malariae (AF487999, AF488000, M54897), Plasmodium inui (FN256230, FN430725, XR_606809), Plasmodium cynomolgi (L08241, AB287289), P. ovale (KF018655, L48987), P. vivax (X13926, JQ627154), Plasmodium knowlesi (U83876, DQ350263), Plasmodium berghei (AJ243513), Plasmodium vinckei (XR_552296), Plasmodium cathemerium (AY625607), Plasmodium gallinaceum (M61723), Plasmodium lophurae (X13706), Plasmodium juxtanucleare (AF460507), Plasmodium reichenowi (Z25819), Plasmodium gaboni (LVLB01000008), Plasmodium brasilianum (AF130735), Plasmodium gonderi (AB287270), Plasmodium fieldi (AB287284), and Plasmodium fragile (M61722). The Clustal multiple alignment program was used to identify a conserved region of the 18S rRNA common to all the species. Primers and probes were selected to amplify a 234-242 bp target (forward: 5′-TAAGGATAACTACGGAAAAGCTGTA-3′; reverse: 5′-CGTTACCCGTCATAGCCATGT-3′; FAM-probe: 5′-TAGGCCAATACCCTAACATCAAAAG-6-FAM-3′; and LCRed640-probe: 5′-LCred640-TGATAGGTCAGAAACTCGATTGATACAC-phos-3′) and synthesized by Integrated DNA Technologies (Coralville, IA). The Plasmodium-specific FRET-qPCR reaction and high-resolution melting curve analysis were performed on the LightCycler 480II Real-time PCR platform (Roche, Basal, Switzerland) under previously described conditions (Zhang et al., 2013), except that the hybridization temperature was 53°C. The specificity and sensitivity of the FRET-qPCR were determined using DNA of four plasmids manufactured with the pUC57 cloning vector (GenScript, Nanjing, Jiangsu, China) containing an appropriate portion of the 18S rRNA gene of P. falciparum, P. malariae, P. ovale, and P. vivax. Specificity was also tested, with DNA of Babesia canis, Hepatozoon americanum, Theileria equi, Hepatocystis kochi, and Dirofilaria immitis obtained as described before (Zhang et al., 2014a, 2015). The Plasmodium-specific FRET-qPCR proved to be highly sensitive detecting two copies of the Plasmodium 18S rRNA per 20 µl reaction system. Further, it was highly specific, not detecting the closely related organisms which were included in the study. The validated Plasmodium-specific FRET-qPCR was used to analyze five species of mosquitoes [Culex pipiens pallens (n=1,620), Aedes albopictus (806), Armigeres subalbatus (377), A. sinensis (168), and Culex tritaeniorhynchus (80)] which were collected in hand nets from homes in 25 provinces and identified as described previously (Zhang et al., 2019). Briefly, between July and September 2014, student volunteers from Yangzhou University used hand nets to collect convenience samples of mosquitoes in their primary homes located in 26 cities in 25 provinces or municipalities in China. Mosquitoes were placed individually in sterile tubes containing 400-µl DNA/RNA Stabilization Reagent (Roche Molecular Biochemicals, Indianapolis, IN, USA). Then, the samples were transported to the Yangzhou University College of Veterinary Medicine at room temperature. Results and DiscussionOnly females were positive (0.6%, 10/1,809) for Plasmodium, and these originated from six provinces, such as Zhejiang (4.2%, 1/24), Gansu (2.4%, 2/82), Shandong (5.4%, 3/56), Jilin (1.7%, 2/115), Guizhou (5.6%, 1/18), and Jiangsu (2.0%, 1/51). Plasmodium species were only detected in three mosquito species, such as C. pipiens pallens (0.5%, 8/1,620), A. sinensis (0.6%, 1/168), and A. subalbatus (0.3%, 1/377) (Table 1). The 18S rRNA sequences of all the positive samples were identical to one another (GenBank accession number: MK061746) and to that of a reference sequence of P. relictum (LN835296) from GenBank (Fig. 1). As far as we know, this is the first time that P. relictum has been detected in A. sinensis and A. subalbatus. Table 1. Data on mosquitoes positive for P. relictum identified with a Plasmodium-specific FRET-qPCR.
Of the three mosquito species we found positive for P. relictum, only C. pipiens pallens is known to feed on birds (Wang et al., 2012) and recognized as the natural vector of P. relictum (Zele et al., 2014). A. sinensis and A. subalbatus are found widely in China and other countries in Southeast Asia (Chaves et al., 2015; Guo et al., 2018) and feed mainly on cattle (Ramesh et al., 2015; Zhang et al., 2017a). From the data showing very low infection rates with P. relictum, it seems that they might also infrequently feed on birds. It is of note that all three species which are positive for P. relictum feed on people raising the possibility that humans are exposed to infection. Although it has been reported that host shifts appear to have a common occurrence in the evolution of the genus Plasmodium among avian and reptilian malaria parasites (Rich and Ayala, 2003), what would now be impermissible and unethical experiments have shown potential infections are unlikely in human being (McLendon, 1943).
Fig. 1. Phylogenetic analysis of Plasmodium spp. detected in this study. Distances and groupings of Plasmodium detected from the mosquitoes (bold font) were determined by applying the neighbor-joining method to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach with MEGA version 6 software based on 18S rRNA gene (242 bp). Scale bar indicates a genetic distance of 0.2-nt substitution per position. P. relictum is one of the most important avian malaria species, which is mainly seen in wild birds, with infections reported in more than 70 different species (Garcia-Longoria et al., 2014) and at high prevalence. The organism has been reported in birds in China previously, such as Beijing (Jia et al., 2018) and Gansu Province (Jia et al., 2018; Zehtindjiev et al., 2013), and we now report its presence in mosquitoes in five further provinces, indicating that the organism is present in China, consistent with the findings in Europe (Schoener et al., 2017). To date, avian Plasmodium parasites have been found in Aedes vexans, C. pipiens complex, Culex modestus, Culex hortensis, Culiseta annulata, Ochlerotatus caspius, and A. albopictus in Central Europe and Culex torrentium in Australia (Schoener et al., 2017). The findings of P. relictum in A. sinensis and A. subalbatus (Table 1) expand the possible vector range for the parasite. ConclusionIn conclusion, this study describes the establishment of a sensitive and specific Plasmodium-specific FRET-qPCR. With this validated PCR, we found that P. relictum, the agent of avian malaria, was present in mosquitoes in China, including species not previously thought to be the vectors. AcknowledgmentsThis work was supported in part by the National Natural Science Foundation of China under Grant 31472225 and in part by the Alabama Agricultural Experimental Station and the USDA National Institute of Food and Agriculture, Hatch project under Grant ALA052-1-17026. Conflicts of interestThe authors have no conflicts of interest to declare. Author’s contributionJilei Zhang and Chengming Wang designed this study. Jilei Zhang, Guangwu Lu, Jing Li, Min Li, Jiawei Wang, Ke Huang, Haixiang Qiu, Jinfeng You, Yaoyao Wang, and Yuanyuan Zhang collected the mosquitoes and performed DNA extraction and PCR. Jilei Zhang, Patrick Kelly, and Chengming Wang wrote the manuscript. ReferencesChaves, L.F., Imanishi, N. and Hoshi, T. 2015. Population dynamics of Armigeres subalbatus (Diptera: Culicidae) across a temperate altitudinal gradient. Bull. Entomol. Res. 105, 589–597. de Koning-Ward, T.F., Dixon, M.W., Tilley, L. and Gilson, P.R. 2016. Plasmodium species: master renovators of their host cells. Nat. Rev. Microbiol. 14, 494–507. Garcia-Longoria, L., Hellgren, O. and Bensch, S. 2014. Molecular identification of the chitinase genes in Plasmodium relictum. Malar. J. 13, 239. Guo, Y., Song, Z., Luo, L., Wang, Q., Zhou, G., Yang, D., Zhong, D. and Zheng, X. 2018. Molecular evidence for new sympatric cryptic species of Aedes albopictus (Diptera: Culicidae) in China: a new threat from Aedes albopictus subgroup? Parasit. Vectors. 11, 228. Huang, X., Dong, L., Zhang, C. and Zhang, Y. 2015. Genetic diversity, temporal dynamics, and host specificity in blood parasites of passerines in north China. Parasitol. Res. 114, 4513–4520. Jia, T., Huang, X., Valkiunas, G., Yang, M., Zheng, C., Pu, T., Zhang, Y., Dong, L., Suo, X. and Zhang, C. 2018. Malaria parasites and related haemosporidians cause mortality in cranes: a study on the parasites diversity, prevalence and distribution in Beijing Zoo. Malar. J. 17, 234. Li, P., Zhao, Z., Xing, H., Li, W., Zhu, X., Cao, Y., Yang, Z., Sattabongkot, J., Yan, G., Fan, Q. and Cui, L. 2016. Plasmodium malariae and Plasmodium ovale infections in the China-Myanmar border area. Malar. J. 15, 557. Martinsen, E.S. and Perkins, S.L. 2013. The diversity of Plasmodium and other haemosporidians: the intersection of taxonomy, phylogenetics and genomics. In: Malaria parasites: comparative genomics, evolution and molecular biology. Caister Academic Press, Norfolk, UK, pp. 1–15. McLendon, S.B. 1943. Experimental attempts to infect man with avian malaria. Am. J. Hyg. 37, 19–20. Perkins, S.L. 2014. Malaria’s many mates: past, present, and future of the systematics of the order Haemosporida. J. Parasitol. 100, 11–25. Ramesh, D., Muniaraj, M., Samuel, P.P., Thenmozhi, V., Venkatesh, A. and Tyagi, B. 2015. Blood feeding behaviour of mosquitoes in Japanese encephalitis endemic and non-endemic areas. J. Vector Borne Dis. 52, 108–109. Rich, S.M. and Ayala, F.J. 2003. Progress in malaria research: the case for phylogenetics. Adv. Parasitol. 54, 255–280. Schoener, E., Uebleis, S.S., Butter, J., Nawratil, M., Cuk, C., Flechl, E., Kothmayer, M., Obwaller, A.G., Zechmeister, T., Rubel, F., Lebl, K., Zittra, C. and Fuehrer, H.P. 2017. Avian Plasmodium in Eastern Austrian mosquitoes. Malar. J. 16, 389. Sylvie, M., Pierre, C. and Jean, M. 2008. Biodiversity of Malaria in the World. John Libbey Eurotext. Bât. A, France. Wang, G., Li, C., Guo, X., Xing, D., Dong, Y., Wang, Z., Zhang, Y., Liu, M., Zheng, Z., Zhang, H., Zhu, X., Wu, Z. and Zhao, T. 2012. Identifying the main mosquito species in China based on DNA barcoding. PLoS One. 7, e47051. Zehtindjiev, P., Ivanova, K., Mariaux, J. and Georgiev, B.B. 2013. First data on the genetic diversity of avian haemosporidians in China: cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Gansu Province. Parasitol. Res. 112, 3509–3515. Zele, F., Vezilier, J., L’Ambert, G., Nicot, A., Gandon, S., Rivero, A. and Duron, O. 2014. Dynamics of prevalence and diversity of avian malaria infections in wild Culex pipiens mosquitoes: the effects of Wolbachia, filarial nematodes and insecticide resistance. Parasit. Vectors. 7, 437. Zhang, C., Shi, G., Cheng, P., Liu, L. and Gong, M., 2017a. Host preferences and feeding patterns of Anopheles sinensis Wiedemann in three sites of Shandong province, China. J. Vector Borne Dis. 54, 328–333. Zhang, J., Kelly, P., Guo, W., Xu, C., Wei, L., Jongejan, F., Loftis, A. and Wang, C. 2015. Development of a generic Ehrlichia FRET-qPCR and investigation of ehrlichioses in domestic ruminants on five Caribbean islands. Parasit. Vectors. 8, 506. Zhang, J., Lu, G., Kelly, P., Zhang, Z., Wei, L., Yu, D., Kayizha, S. and Wang, C. 2014a. First report of Rickettsia felis in China. BMC Infect. Dis. 14, 682. Zhang, J., Wei, L., Kelly, P., Freeman, M., Jaegerson, K., Gong, J., Xu, B., Pan, Z., Xu, C. and Wang, C. 2013. Detection of Salmonella spp. using a generic and differential FRET-PCR. PLoS One. 8, e76053. Zhang, J.L.G., Li, J., Kelly, P., Li, M., Wang, J., Huang, K., Qiu, H., You, J., Zhang, R., Wang, Y., Zhang, Y. and Wang, C. 2019. Molecular detection of Rickettsia felis and R. bellii in mosquitoes. Vector Borne Zoonotic Dis. 19, 802–809. Zhang, S., Guo, S., Feng, X., Afelt, A., Frutos, R., Zhou, S. and Manguin, S. 2017b. Anopheles vectors in mainland China while approaching malaria elimination. Trends Parasitol. 33, 889–900. Zhang, S., Zhang, L., Feng, J., Yin, J., Feng, X., Xia, Z., Frutos, R., Manguin, S. and Zhou, S. 2018. Malaria elimination in the People’s Republic of China: current progress, challenges, and prospects, Towards Malaria Elimination-A Leap Forward. IntechOpen. Doi:10.5772/intechopen.77282. Available via https://www.intechopen.com/books/towards-malaria-elimination-a-leap-forward/malaria-elimination-in-the-people-s-republic-of-china-current-progress-challenges-and-prospects Zhang, Y., Wu, Y., Zhang, Q., Su, D. and Zou, F. 2014b. Prevalence patterns of avian Plasmodium and Haemoproteus parasites and the influence of host relative abundance in southern China. PLoS One. 9, e99501. Zhou, S.S., Wang, Y., Fang, W. and Tang, L.H. 2008. Malaria situation in the People’s Republic of China in 2007. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 26(6), 401-403. | ||
| How to Cite this Article |
| Pubmed Style Zhang J, Lu G, Kelly P, Li J, Li M, Wang J, Huang K, Qiu H, You J, Zhang R, Wang Y, Zhang Y, Wang C. First molecular detection of Plasmodium relictum in Anopheles sinensis and Armigeres subalbatus. Open Vet. J.. 2020; 10(1): 39-43. doi:10.4314/ovj.v10i1.7 Web Style Zhang J, Lu G, Kelly P, Li J, Li M, Wang J, Huang K, Qiu H, You J, Zhang R, Wang Y, Zhang Y, Wang C. First molecular detection of Plasmodium relictum in Anopheles sinensis and Armigeres subalbatus. https://www.openveterinaryjournal.com/?mno=77058 [Access: January 25, 2026]. doi:10.4314/ovj.v10i1.7 AMA (American Medical Association) Style Zhang J, Lu G, Kelly P, Li J, Li M, Wang J, Huang K, Qiu H, You J, Zhang R, Wang Y, Zhang Y, Wang C. First molecular detection of Plasmodium relictum in Anopheles sinensis and Armigeres subalbatus. Open Vet. J.. 2020; 10(1): 39-43. doi:10.4314/ovj.v10i1.7 Vancouver/ICMJE Style Zhang J, Lu G, Kelly P, Li J, Li M, Wang J, Huang K, Qiu H, You J, Zhang R, Wang Y, Zhang Y, Wang C. First molecular detection of Plasmodium relictum in Anopheles sinensis and Armigeres subalbatus. Open Vet. J.. (2020), [cited January 25, 2026]; 10(1): 39-43. doi:10.4314/ovj.v10i1.7 Harvard Style Zhang, J., Lu, . G., Kelly, . P., Li, . J., Li, . M., Wang, . J., Huang, . K., Qiu, . H., You, . J., Zhang, . R., Wang, . Y., Zhang, . Y. & Wang, . C. (2020) First molecular detection of Plasmodium relictum in Anopheles sinensis and Armigeres subalbatus. Open Vet. J., 10 (1), 39-43. doi:10.4314/ovj.v10i1.7 Turabian Style Zhang, Jilei, Guangwu Lu, Patrick Kelly, Jing Li, Min Li, Jiawei Wang, Ke Huang, Haixiang Qiu, Jinfeng You, Rong Zhang, Yaoyao Wang, Yuanyuan Zhang, and Chengming Wang. 2020. First molecular detection of Plasmodium relictum in Anopheles sinensis and Armigeres subalbatus. Open Veterinary Journal, 10 (1), 39-43. doi:10.4314/ovj.v10i1.7 Chicago Style Zhang, Jilei, Guangwu Lu, Patrick Kelly, Jing Li, Min Li, Jiawei Wang, Ke Huang, Haixiang Qiu, Jinfeng You, Rong Zhang, Yaoyao Wang, Yuanyuan Zhang, and Chengming Wang. "First molecular detection of Plasmodium relictum in Anopheles sinensis and Armigeres subalbatus." Open Veterinary Journal 10 (2020), 39-43. doi:10.4314/ovj.v10i1.7 MLA (The Modern Language Association) Style Zhang, Jilei, Guangwu Lu, Patrick Kelly, Jing Li, Min Li, Jiawei Wang, Ke Huang, Haixiang Qiu, Jinfeng You, Rong Zhang, Yaoyao Wang, Yuanyuan Zhang, and Chengming Wang. "First molecular detection of Plasmodium relictum in Anopheles sinensis and Armigeres subalbatus." Open Veterinary Journal 10.1 (2020), 39-43. Print. doi:10.4314/ovj.v10i1.7 APA (American Psychological Association) Style Zhang, J., Lu, . G., Kelly, . P., Li, . J., Li, . M., Wang, . J., Huang, . K., Qiu, . H., You, . J., Zhang, . R., Wang, . Y., Zhang, . Y. & Wang, . C. (2020) First molecular detection of Plasmodium relictum in Anopheles sinensis and Armigeres subalbatus. Open Veterinary Journal, 10 (1), 39-43. doi:10.4314/ovj.v10i1.7 |