| Original Article | ||
Open Vet. J.. 2021; 11(4): 530-534 Open Veterinary Journal, (2021), Vol. 11(4): 530–534 Original Research Caseous lymphadenitis outbreak in a small ruminant herdAngela Burmayan and Cord M. Brundage*California State Polytechnic University, 3801 West Temple Avenue, Pomona, CA 91768, USA *Corresponding Author: Cord M. Brundage. California State Polytechnic University, 3801 West Temple Avenue, Pomona, CA 91768, USA. Email: cmbrundage [at] cpp.edu Submitted: 04/05/2021 Accepted: 23/09/2021 Published: 03/10/2021 © 2021 Open Veterinary Journal
AbstractBackground: Caseous lymphadenitis (CLA) is a chronic disease caused by the bacterium Corynebacterium pseudotuberculosis that affects small ruminants worldwide. Aim: The objective of this case report is to describe an outbreak of CLA that occurred at the California State Polytechnic University, Pomona, in the summer of 2019 and the management strategies employed therein to contain the spread. Methods: After careful physical screening, blood serum samples from the entire herd (n=218 sheep, n=32 goats) were tested using the synergistic hemolysis inhibition test to reveal antibodies present. Results: Animals with titer counts above 1:64 and/or containing CLA lesions were isolated and culled (n=33 sheep,n=4 goats) within 2 weeks of testing. Female sheep (n=160) had higher titer counts and were culled at a much higher rate than male sheep (n=58) (20% vs. 1.72%), whereas male goats (n=9) more often had high titer counts and were culled as opposed to female goats (n=23) (33.33% vs. 4.35%). Conclusion: Vaccines were administered to the remainder of the herd following culling. Additional management strategies were employed, the outcome of which was a zero recurrence through August 2021. Keywords: Caseous lymphadenitis, Small ruminants, Sheep, Goats, Corynebacterium pseudotuberculosis. IntroductionCaseous lymphadenitis (CLA) is a chronic and contagious bacterial disease caused by Corynebacterium pseudotuberculosis. CLA results in weight loss, decreased wool and milk production, a decrease in reproductive performance, and economic losses due to culling of infected animal with cases being reported worldwide (Dorella et al., 2006). The prevalence of infection in small ruminants is dependent on a number of environmental factors such as poor hygiene, herd size, housing arrangements, and skin wounds of the animals (de La Fuente et al., 2011). A challenge farmers and producers face today is managing long-term areas where CLA is endemic (Colom-Cadena et al., 2014). In wildlife, C. pseudotuberculosis has been described as isolated cases in pronghorns (Clark et al., 1972), elk (Kelly et al., 2012), and Arabian oryx (Tarello and Theneyan, 2008). Horses, cattle, camels, swine, and buffaloes develop a disease commonly known as “pigeon fever” by a different strain of the same bacterium (Peel et al., 1997; Selim, 2001; Williamson, 2001; Yeruham et al., 2004). This strain causes intramuscular abscesses almost exclusively in horses in arid regions of the US and Brazil. Complications from the disease include weight loss, abortion, recurrent infection, and occasionally death (Bucknam, 2009), similar to CLA in small ruminants. CLA is also a zoonotic disease with human incidences not as uncommon as previously thought. Human illnesses caused by C. pseudotuberculosis are characterized by its chronicity rather than its severity (Blackwell et al., 1974). CLA is spread from animal to animal primarily through direct contact of bacteria excreted from the abscesses caused by C. pseudotuberculosis and through contamination of the environment (Underwood et al., 2015). Corynebacterium pseudotuberculosis is a Gram-positive rod-shaped, intracellular, facultative aerobic bacterium with worldwide distribution (Love and Mair, 2012). The bacterium can survive several months within the environment and the soil, making the containment of the disease difficult to control and highly contagious (Guimaraes et al., 2011). The primary mode of infection is direct contact with secretions from abscesses containing C. pseudotuberculosis or by ingestion or inhalation. The bacterium will multiply and spread throughout the blood and subsequently infect lymph nodes and internal organs (Batey, 1986). In small ruminants, CLA causes abscesses of peripheral and/or internal lymph nodes and organs, as shown in Figure 1. Abscesses can form as soon as 2 weeks to several months after initial exposure to the bacterium (Kuria et al., 2001). Although there are recommendations on strategies to control for CLA, such as isolating and culling infected animals, there are a limited number of case reports discussing the efficacy (Baird and Malone, 2010). The objective of this case report is to describe an outbreak of CLA that occurred at the sheep unit at the California State Polytechnic University, Pomona in the summer of 2019.
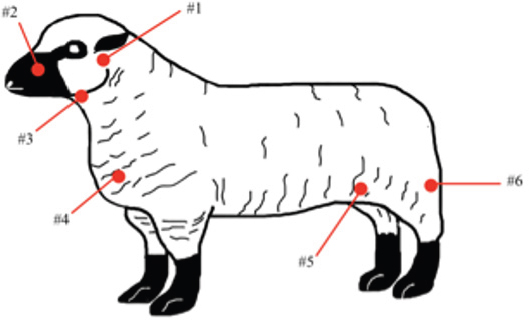
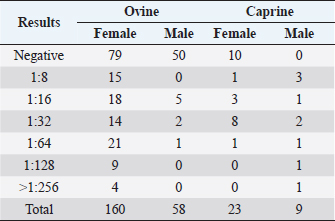
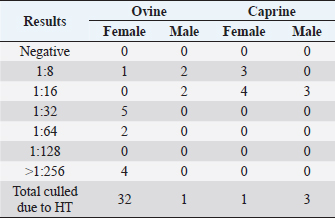
Fig. 1. CLA in superficial lymph nodes. (1) Parotid; (2) retropharyngeal; (3) submandibular; (4) prescapular; (5) prefemoral; and (6) popliteal. Materials and MethodsClinical presentationIn August 2019, an outbreak of CLA was presumptively reported at the sheep unit at the California State Polytechnic University, Pomona. The university’s on-campus animals were being utilized at the Los Angeles County Fair petting farm, where a veterinarian at the fair first noticed the abscess affecting the closed mandibular lymph nodes on a goat and reported their findings to the university. The suspected goat was isolated for up to 2 weeks for further diagnostic testing. Swab samples were taken from the abscessed region and were cultured in blood agar at 37°C for 48 hours. PCR products of growth regions were identified as C. pseudotuberculosis as previously described (Cetinkaya et al., 2002). The remainder of the herd was then physically screened for the following signs: enlargement of lymph nodes and abscesses on lymph nodes. Once identified, suspected animals with lymph node abscesses were kept in an isolation pen with a 25-feet buffer on either side of the pen to avoid further spread. Diagnostic testingAfter careful physical screening, blood samples were taken from the jugular vein from 218 sheep and 32 goats with ages ranging from 1 to 9 years old. Serum samples were then tested using synergistic hemolysis inhibition (SHI) titer test at the University of California, Davis, to confirm whether antibodies were present. The SHI test is a serological assay which measures antibodies present due to the exotoxin of C. pseudotuberculosis and has shown promising results when applied to small ruminants with CLA in an experimental setting (Knight, 1978; Brown et al., 1985; Brown et al., 1987). Sixteen sheep and 10 goats were retested for SHI titer counts to ensure that nursing and petting zoo animals were free of infection prior to taking prophylactic measures and culling of infected animals due to the high level of human handling and foot traffic at the university. Of those animals retested, six sheep were culled due to high titer (HT) counts. A total of 33 sheep and 4 goats were culled based on diagnostic testing. Prior to culling, animals with HT counts (1:64 and above) were placed in isolation pens with a 25-feet buffer on either side of the pen for up to 2 weeks or date of culling. TreatmentImmunization has shown to reduce the spread of the infection and the gradual decline of prevalence of the disease (Fontaine and Baird, 2008). Following physical screenings, serological testing and culling of infected animals, vaccinations were administered to the remainder of the herd. Sheep were administered the Bacterin-Toxoid Case-Bac vaccine manufactured by the Colorado Serum Company. Case-Bac has shown to prevent internal and external abscesses in sheep and significantly reduce disease severity ( p < 0.001) (Piontkowski and Shivvers, 1998). Vaccines were administered subcutaneously in the left axillary region, followed by a booster shot 1 month later on the right axillary region. Goats were administered the C. pseudotuberculosis Bacterin vaccine manufactured by Texas Vet Lab Inc. Goat vaccines were administered subcutaneously on the left side of the neck, followed by a booster shot 2 weeks later on the right side of the neck. Annual vaccines will be administered beginning January 2021. Other prophylactic measures taken at the university for the containment of the disease following vaccinations included implementing foot baths between units to avoid cross contamination of pathogens, cleaning pens more frequently, and digging up the first six inches of dirt in each pen to ensure eradication of the bacterium within the soil. Hydrated lime powder was also used and tossed over the dirt in each pen to restore soil health. Lime powder is known to change pH and affect growth of microorganisms in the environment. However, optimal usage of lime powder is still unknown (Mori et al., 2019). Ethical approvalNo ethical approval was needed for this study. ResultsTiters may indicate active infection or historic exposure. SHI titer results <1:8 to 1:32 were not considered significant. Animals with HT counts of 1:64 and above were immediately culled. In small ruminants, C. pseudotuberculosis titers of 1:8 occur with similar frequency in animals with and without active infection and may represent early infection or historic exposure. Titers 1:16–1:32 occur four times more often in animals with active infection versus those without, and titers 1:64 to >1:256 occur 8.5 times more often in animals with active infection versus those without (Knight, 1978; Brown et al., 1987; Brown et al., 1986). Results of SHI tests are shown in Table 1 and the retest results for nursing and petting farm animals are shown in Table 2. Of the 218 sheep serum samples tested, 33 were culled due to titer counts testing at 1:64 and above, 6 of which had been retested prior to culling because they were either nursing at the time of the first SHI test or were petting farm animals, as shown in Table 2. Of those 33 sheep culled, 4 had superficial abscesses present. Out of the 32 goat serum samples tested and 10 samples retested, 4 goats were also culled due to HT counts, none of which had present abscesses. Female sheep were culled at a higher rate (20%) as opposed to male sheep (1.72%), whereas male goats were culled at a higher rate (33.33%) than female goats (4.35%). Serological assays and bacterial culture have been commonly used for the detection of C. pseudotuberculosis; however, these methods are unreliable and cannot detect early cases of CLA (Shigidi, 1979). Table 1. SHI titer results categorized by species and sex with ages ranging from 1 to 9 years old.
Table 2. SHI titer results of the “retest” group categorized by species and sex with ages ranging from 1 to 9 years old.
DiscussionCLA is responsible for substantial economic losses in goat herds and sheep flocks (Williamson, 2001). Losses range from condemnation of skins and carcasses due to abscesses to losses due to reproductive inefficiency. According to the Food and Agricultural Organization, United Nations, a significant proportion of the world’s goat population is found in the countries defined by FAO as net food-importing countries (44%), low income food-deficit countries (86%), and the least developed countries (31%) (FAOSTAT, 2011). CLA is widely distributed within these countries, which causes farmers to suffer significant financial losses due to loss of fertility, decreased milk and meat production, hide and wool loss, culling of infected animals, and carcass condemnation. CLA affects industries worldwide and proves to be a hindrance to animal health and disease prevention. The prevalence rate for all forms of CLA in sheep and goats in the western United States was 42.41% (Stoops et al., 1984). CLA may become endemic within herds or flocks due to its resistance to certain antibiotics, such as ampicillin, clindamycin, and doxycycline HCl (Abebe and Tessema, 2015); its constant increasing ability to survive in harsh environments for extended periods of time; and the limitations associated with detecting subclinically infected animals (Williamson, 2001). In most cases, treatment of the external forms of CLA involves identification of infected animals prior to abscess rupturing to prevent contamination of the environment and soil. Once identified, isolation of the affected animals is required to avoid further spread of the disease. These methods were consistent with our study and have shown to be successful in preventing further spread of infection. However, our findings show that female sheep had higher titer counts and were culled at a much higher rate than male sheep (20% vs. 1.72%). The opposite trend was found in goats, where male goats more often had higher titer counts and were culled as opposed to female goats (33.33% vs. 4.35%). This could be due to the animals’ housing arrangements and it is possible that female sheep were housed in close proximity to male goats at the time of outbreak, allowing for the infection to not only spread, but to disproportionally infect the herd. CLA is a non-sex linked disease, indicating that the occurrence of the disease is dependent on immunity, which can be altered by gestation, lactation, immunosuppressive disease, nutrition, and other management factors which both sexes are subject to (Zaitoun and Ali, 1999; Oreiby et al., 2013) A striking finding in this study was the low prevalence of abscesses in sheep testing with HTs. Only 4 ewes had abscesses present, while a total of 32 female sheep tested with HT counts were culled. This raises questions about diagnostic testing and whether physical screening for lesions is sufficient enough when looking for CLA in small ruminants. LimitationsThe scientific basis for certain decisions and strategies used was not always clear. It is difficult to interpret how effective each management strategy is because of the multimodality of the strategies used. Future researchDue to the worldwide distribution of CLA, further research should be carried out on CLA diagnostics and the efficacy of vaccines currently available. Current treatment strategies for CLA include drainage of abscesses, followed by cleaning and chemical cauterization (Nozaki et al., 2000); however, these techniques are not always effective and may become expensive. The best strategy for preventing the disease is immunization (Paton et al., 2003), but not all vaccines available for sheep have the same efficacy in goats (Guimaraes et al., 2011). It would be beneficial to further study currently available vaccines and the efficacies in both sheep and goats (Dorella et al., 2009). This case report shows the importance of monitoring and documentation during the time of an outbreak to better control for the spread. ConclusionThere has been a zero recurrence of CLA at CPP through August 2021. The multimodal strategies used to contain the spread were successful thus far. The discrepancy in titer counts between female and male sheep and goats should be further researched. Individual prophylactic measures should also be further studied to determine the efficacy of each management strategy. Although the university has been successful in managing the spread thus far, it is difficult to determine what role individual measures played in the success of containing the spread. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAbebe, D. and Tessema, T.S. 2015. Determination of Corynebacterium pseudotuberculosis prevalence and antimicrobial susceptibility pattern of isolates from lymph nodes of sheep and goats at an organic export abattoir, Modjo, Ethiopia. Lett. Appl. Microbiol. 61, 479–476. Baird, G.J. and Malone, F.E. 2010. Control of caseous lymphadenitis in six sheep flocks using clinical examination and regular ELISA testing. Vet. Rec. 166, 358–362. Batey, R.G. 1986. Pathogenesis of caseous lymphadenitis in sheep and goats. Aust. Vet. J. 63, 269–272. Blackwell, J.B., Smith, F.H. and Joyce, P.R. 1974. Granulomatous lymphadenitis caused by Corynebacterium ovis. Pathology 6, 243–249. Brown, C.C., Olander, H.J. and Alves, S.F. 1987. Synergistic hemolysis-inhibition titers associated with caseous lymphadenitis in a slaughterhouse survey of goats and sheep in Northeastern Brazil. Can. J. Vet. Res. 51, 41–49. Brown, C.C., Olander, H.J., Biberstein, E.L. and Moreno, D. 1985. Serologic response and lesions in goats experimentally infected with Corynebacterium pseudotuberculosis of caprine and equine origins. Am. J. Vet. Res. 46, 2322–2326. Brown, C.C., Olander, H.J., Zometa, C. and Alves, S.F. 1986. Serodiagnosis of inapparent caseous lymphadenitis in goats and sheet, using the synergistic hemolysis inhibition test. Am. J. Vet. Res. 47, 1461–1463. Bucknam, A.L. 2009. Corynebacterium pseudotu-berculosis in California horses. Master’s Thesis, California State Polytechnic University, San Luis Obispo, CA. Cetinkaya B., Karahan M., Atil E., Kalin R., Baere T.D. and Vaneechoutte M. 2002. Identification of Corynebacterium pseudotuberculosis isolates from sheep and goats by PCR. Vet. Microbiol. 88, 75–83. Clark, K.A., Robinson, R.M., Weishuhn, L.L., Litton, G.W. and Marburguer, R.G. 1972. Caseous lymphadenitis in pronghorns (Antilocapra Americana). J. Wildl. Dis. 8(1), 67–71. Colom-Cadena, A., Velarde, R., Salinas, J., Borge, C., Garcia-Bocanegra, I., Serrano, E., Gassó, D., Bach, E., Casas-Díaz, E., López-Olvera, J. R., Lavín, S., León-Vizacaíno, L. and Mentaberre, G. 2014. Management of a caseous lymphadenitis outbreak in a new Iberian ibex (Capra pyrenaica) stock reservoir. Acta. Vet. Scand. 56(1), 83. de La Fuente, R., Vid, R., Sanz, R. and Quiteria, R.S. 1997. An outbreak of abscess disease associated with shearing. Small. Ruminant. Res. 26, 283–286. Dorella, F., Pacheco, L.G.C., Oliveira, S.C., Miyoshi, A. and Azevedo, V. 2006. Corynebacterium pseudotuberculosis: microbiology, biochemical properties, pathogenesis and molecular studies of virulence. Vet. Res. 37, 201–218. Dorella, F.A., Pacheco, L.G.C., Seyffert, N., Portela, R.W., Meyer, R., Miyoshi, A. and Azevedo, A. 2009. Antigens of Corynebacterium pseudotuberculosis and prospects for vaccine development. Expert. Rev. 2, 205–213. FAOSTAT. 2011. Food and Agricultural Organization, United Nations. Fontaine, M.C. and Baird, G.J. 2008. Caseous lymphadenitis. Small. Ruminant. Res. 76(1-2), 42–48. Guimaraes, A., Carmo, F.B., Pauletti, R.B., Seyffert, N., Ribeiro, D., Lage, A.P., Heinemann, M.B., Miyoshi, A., Azevedo, V. and Gouveia, A.M.G. 2011. Caseous lymphadenitis: epidemiology, diagnosis, and control. IIOAB. J. 2, 33–43. Kelly, E.J., Rood, K.A. and Skirpstunas, R. 2012. Abscesses in captive elk associated with Corynebacterium pseudotuberculosis, Utah USA. J. Wildl. Dis. 48, 803–805. Knight, M.D. 1978. A serologic method for the detection of Corynebacterium pseudotuberculosis infections in horses. Cornell. Vet. 68, 220–237. Kuria, J., Mbuthia, P., Kang’etht, E. and Wahome, R.G. 2001. Caseous lymphadenitis in goats: the pathogenesis, incubation period and serological response after experimental infection. Vet. Res. Commun. 25, 89–97. Love, S. and Mair, T.S. 2012. Infectious diseases and parasitology. In Equine medicine, surgery and reproduction, pp: 399–422. Mori, M., Yoshikazu, S., Hamazaki, Y. and Jojima, T. 2019. Evaluation of the influence of sprinkling powdered slaked lime on microorganisms for the prevention of domestic animal infectious diseases. Environ. Technol. 40(23), 3094–3104. Nozaki, C.N., Faria, M.A.R. and Machado, T.M.M. 2000. Surgical removal of abscesses of the Caseous lymphadenitis in goats. Arq. Inst. 67(2), 187–189. Oreiby, A.F., Osman, S.A., Hegazy, Y.M., Ghanem, Y.A. and Al-Gaabary, M.H. 2013. Caseous lymphadenitis in small ruminants: descriptive, epidemiological and clinical studies. Kafrelsheikh. Vet. Med. J. 11(1), 41–61. Paton, M.W., Walker, S.B., Rose, I.R. and Watt, G.F. 2003. Prevalence of caseous lymphadenitis and usage of caseous lymphadenitis vaccines in sheep blocks. Aust. Vet. J. 81, 91–95. Peel, M.M., Palmer, G.G., Stacpoole, A.M. and Kerr, T.G. 1997. Human lymphadenitis due to Corynebacterium pseudotuberculosis: report of ten cases from Australia and review. Clin. Infect. Dis. 24(2), 185–191. Piontkowski, M.D. and Shivvers, D.W. 1988. Evaluation of a commercially available vaccine against Corynebacterium pseudotuberculosis for use in sheep. JAVMA. 212, 1765–1768. Selim, A.S. 2001. Oedematous skin disease of buffalo in Egypt. J. Vet. Med. B. 48, 241–258. Shigidi, M.T.A. 1979. A comparison of five serological tests for the diagnosis of experimental Corynebacterium ovis infection in sheep. Br. Vet. J. 135, 172–177. Stoops, S.G., Renshaw, H.W. and Thilsted, J.P. 1984. Ovine caseous lymphadenitis diseases prevalence, lesion distribution, and thoracic manifestations in a population of mature culled sheep form western United States. Am. J. Vet. Res. 45, 557–561. Tarello, W. and Theneyan, M. 2008. Corynebacterium pseudotuberculosis and Corynebacterium regale isolated from two Arabian Oryx (Oryx leucoryx). Vet. Rec. 162(26), 862–863. Underwood, W.J., Blauwiekel, R., Delano, M.L., Gillesby, R., Mischler, S.A. and Schoell, A. 2015. Biology and diseases of ruminants (sheep, goats, and cattle), 3rd ed. In Laboratory Animal Medicine, pp: 623–694. Williamson, L.H. 2001. Caseous lymphadenitis in small ruminants. Vet. Clin. North. Am. Food Anim. 17, 359–371. Yeruham, I., Friedman, S., Perl, S., Elad, D., Berkovich, Y. and Kalgardm Y. 2004. A herd level analysis of Corynebacterium pseudotuberculosis outbreak in a dairy cattle herd. Vet. Dermatol. 15, 315–320. Zaitoun, A.M. and Ali, H.S. 1999. Clinical and experimental pseudotuberculosis on a multiple ages sheep and goats flock with control trials via treatment and BCG vaccination. Assuit. Vet. Med. J. 42, 239–259. | ||
| How to Cite this Article |
| Pubmed Style Brundage CM, Burmayan A. Caseous lymphadenitis outbreak in a small ruminant herd. Open Vet. J.. 2021; 11(4): 530-534. doi:10.5455/OVJ.2021.v11.i4.2 Web Style Brundage CM, Burmayan A. Caseous lymphadenitis outbreak in a small ruminant herd. https://www.openveterinaryjournal.com/?mno=78919 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.2 AMA (American Medical Association) Style Brundage CM, Burmayan A. Caseous lymphadenitis outbreak in a small ruminant herd. Open Vet. J.. 2021; 11(4): 530-534. doi:10.5455/OVJ.2021.v11.i4.2 Vancouver/ICMJE Style Brundage CM, Burmayan A. Caseous lymphadenitis outbreak in a small ruminant herd. Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 530-534. doi:10.5455/OVJ.2021.v11.i4.2 Harvard Style Brundage, C. M. & Burmayan, . A. (2021) Caseous lymphadenitis outbreak in a small ruminant herd. Open Vet. J., 11 (4), 530-534. doi:10.5455/OVJ.2021.v11.i4.2 Turabian Style Brundage, Cord M, and Angela Burmayan. 2021. Caseous lymphadenitis outbreak in a small ruminant herd. Open Veterinary Journal, 11 (4), 530-534. doi:10.5455/OVJ.2021.v11.i4.2 Chicago Style Brundage, Cord M, and Angela Burmayan. "Caseous lymphadenitis outbreak in a small ruminant herd." Open Veterinary Journal 11 (2021), 530-534. doi:10.5455/OVJ.2021.v11.i4.2 MLA (The Modern Language Association) Style Brundage, Cord M, and Angela Burmayan. "Caseous lymphadenitis outbreak in a small ruminant herd." Open Veterinary Journal 11.4 (2021), 530-534. Print. doi:10.5455/OVJ.2021.v11.i4.2 APA (American Psychological Association) Style Brundage, C. M. & Burmayan, . A. (2021) Caseous lymphadenitis outbreak in a small ruminant herd. Open Veterinary Journal, 11 (4), 530-534. doi:10.5455/OVJ.2021.v11.i4.2 |