| Original Article | ||
Open Vet J. 2023; 13(1): 11-19 Open Veterinary Journal, (2023), Vol. 13(1): 11–19 Original Research Curcuma longa supplementation reduces MDA, TNF-α, and IL-6 levels in a rat model exposed to soot particulatesMuhammad Aminuddin1, Djanggan Sargowo2, Teguh Wahju Sardjono3 and Widjiati Widjiati4*1Postgraduate Program Students, Faculty of Medicine, Brawijaya University, Malang, Indonesia 2Department of Internal Disease, Faculty of Medicine, Brawijaya University, Malang, Indonesia 3Department of Parasitology, Faculty of Medicine, Brawijaya University, Malang, Indonesia 4Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia Submitted: 13/08/2022 Accepted: 06/12/2022 Published: 05/01/2023 *Corresponding Author: Widjiati Widjiati. Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: widjiati [at] fkh.unair.ac.id © 2023 Open Veterinary Journal
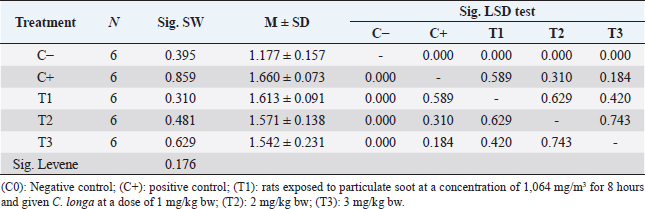
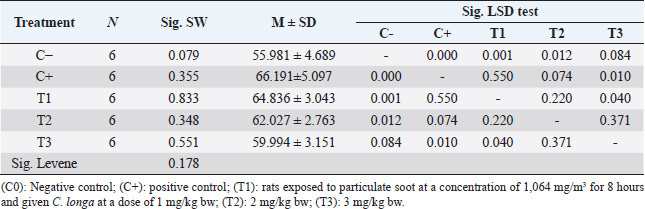
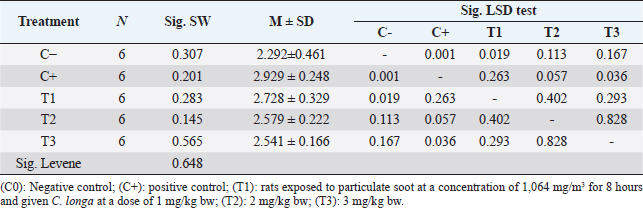
AbstractBackground: Particulate matter (PM) is one of the important components in air pollution that can cause endothelial vascular dysfunction through exacerbation of atherosclerosis and inflammation of the respiratory system. Increased levels of malondialdehyde (MDA) in blood plasma can be an indicator of oxidative stress. Then, macrophages can secrete proinflammatory cytokines that will stimulate immune cells and vascular endothelial cells to release inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrotic factor-α (TNF-α). Curcuma longa works by scavenging the active free radicals involved in the peroxidation process. Aims: This study aims to prove that the administration of C. longa can reduce MDA, TNF-α, and IL-6 levels in Rattus norvegicus exposed to soot particulates. Methods: The subjects of this study were 30 male rats which were divided into 5 treatment groups with the following: (C0): negative control; (C+): positive control; (T1): Treatment group 2, rats exposed to particulate soot at a concentration of 1,064 mg/m3 for 8 hours and given C. longa at a dose of 1 mg/kg bw; (T2): Treatment group 3 was rats exposed to soot particulates at a concentration of 1,064 mg/m3 for 8 hours and given C. longa at a dose of 2 mg/kg bw; (T3): Treatment group 4 was rats exposed to soot particulates at a concentration of 1,064 mg/m3 for 8 hours and given C. longa at a dose of 3 mg/kg bw. Giving the C. longa extract orally with a probe every day for 30 days after treatment of exposure to soot. Examination of MDA, TNF-, and IL-6 levels with the ELISA method. Results: The administration of C. longa can reduce MDA while the lowest MDA levels were obtained in the T3 treatment with an average of 1.542 ± 0.231. The results of the description of the lowest levels of TNF-α were obtained in the C-treatment with an average of 55.981 ± 4.689. Then, the lowest levels of IL-6 were obtained in the C-treatment with an average of 2.292 ± 0.461. Conclusion: The results stated that the administration of C. longa could reduce MDA levels, TNF-α, and IL-6 levels. Curcuma longa as an anti-inflammatory and anti-oxidant play an effective role in inhibiting inflammation by decreasing IL-6 cytokine and TNF-α. Curcuma longa can inhibit lipid peroxidation initiated by free radicals and then reduce MDA levels. Keywords: Curcuma longa, Health care, IL-6-MDA, Soot, TNF-α. IntroductionAir pollution significantly increases morbidity and mortality due to endothelial dysfunction, but the mechanism of this process is unclear. Particulate matter (PM) is one of the important components in air pollution that can cause endothelial vascular dysfunction through exacerbation of atherosclerosis and inflammation of the respiratory system. The evidence shows that air pollution contributes to the incidence of acute cardiovascular disease that begins with the occurrence of vascular endothelial dysfunction through various pathways, including systemic inflammation, activity of homeostatic pathways, accelerated atherosclerosis, plaque instability, changes in autonomic control, and cardiac arrhythmias and direct inhalation, causes vasoconstriction, hypertension, and platelet aggregation. The Global Burden Disease reports that air pollution caused by PM causes 4.2 million deaths due to cardiovascular disease during the last 5 years with 33% resulting in ischemic vascular dysfunction. Zhang et al. (2018) reported exposure to particulates at risk for pregnant women and children, especially in third-trimester pregnant women and children aged 3–9 years. Halonen et al. (2009) in Simoni et al. (2015) also reported that parents >65 years of age are at risk for pneumonia and asthma/chronic obstructive pulmonary disease (COPD). Romieu et al. (2012) also reported that parents aged >65 years had a risk of mortality due to particulate exposure greater than 0.16% compared to normal mortality (Simoni et al., 2015; Finch and Conklin, 2016; Munzel et al., 2018). PM can cause oxidative stress through the direct formation of reactive oxygen species (ROS) through the physico-chemical properties of the surface of the particulate material. One mechanism that is thought to play a role is the occurrence of oxidative stress which will then increase ROS in the body, namely hydrogen peroxide (H2O2), and hydroxyl radicals (–OH). Free radicals, especially hydroxyl radicals (–OH), can cause cell damage through the so-called lipid peroxidation with the final result in the form of compounds that are damaging to cells, one of which is malondialdehyde (MDA). Increased levels of MDA in blood plasma can be an indicator of oxidative stress. This oxidative stress then induces cell degeneration and causes endothelial cell dysfunction (Lin et al., 2015; Kozlov et al., 2016). When soot particulates enter the body, the body will carry out a defense mechanism in the form of increased production of proinflammatory cytokines by inflammatory cells, especially macrophages. Macrophages can secrete proinflammatory cytokines. These cytokines activate nuclear factor kappa B (NF-кB) in cells that respond to inflammation. Activation of NF-кB will trigger the release of proinflammatory cytokines, such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrotic factor-α (TNF-α) (Erta et al., 2012; Salomon et al., 2018; Lewis and Elks, 2019; Nabarawy et al., 2019; Silva et al., 2019). These cytokines will stimulate immune cells and vascular endothelial cells to release inflammatory cytokines. Excessive production of inflammatory cytokines and ROS will result in tissue damage, this situation can lead to impaired vascular cell dysfunction. Curcuma longa or turmeric (turmeric) is a plant that is a genus of the family Zingiberaceae. Curcuma longa, also known as turmeric, is one of the native herbs/spice plants from Southeast Asia, spread from India, Malaysia, and Indonesia. Many studies report the polyphenol content of curcumin in C. longa and its pharmacological effects, including as an antioxidant, anti-inflammatory, anti-microbial, and anti-cancer alternative (Raju et al., 2006; Sadeghi et al., 2018; Song et al., 2019). The antioxidants contained in C. longa have phenol and diketone groups. Several studies have shown that C. longa has the ability to protect biomembranes against peroxidative damage. Curcuma longa works by scavenging the active free radicals involved in the peroxidation process (Sarvalkar et al., 2011). Curcuma longa or curcumin can reduce MDA levels by donating a hydrogen atom (H) from the phenolic hydroxyl (OH) group when reacting with free radicals. This reaction will produce a phenoxy curcumin radical or flavonoid which is less reactive because the curcumin phenoxyl radical can undergo a resonance structure change by redistributing unpaired electrons in the conjugated double bond structure in the aromatic ring. The curcumin or flavonoid phenoxyl radicals will react further to form unreactive compounds, possibly through termination reactions. Curcuma longa’s natural anti-inflammatory activity is comparable to that of steroidal drugs and non-steroidal drugs such as indomethacin and phenylbutazone, which have dangerous side effects. The anti-inflammatory properties of curcumin appear to be mediated by inhibition of the induction of COX-2, Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), inducible nitric oxide synthase (iNOS), and the production of cytokines such as IFN-γ and TNF and the activation of transcription factors such as NF-κB, and activator protein (AP-1). Several other studies also reported the role of C. longa in effectively inhibiting inflammation by decreasing the cytokines IL-1β and IL-6 in cases of psoriasis-like inflammation given imiquimod (Darwadi et al., 2013; Jun et al., 2013; Shakeri et al., 2017; Pourbagher-Shahri et al., 2021). This study aims to prove that the administration of C. longa can reduce MDA, TNF-α, and IL-6 levels in Rattus norvegicus exposed to soot particulates. Based on these facts, researchers are encouraged to conduct further studies on the mechanism of the effect of exposure to particulate air pollutants, especially soot on vascular dysfunction and the effect of C. longa as an anti-inflammatory and anti-oxidant using laboratory experimental methods and rats as experimental animals. Materials and MethodsThe subjects of this study were male white rats (R. norvegicus) in laboratory animal units. The criteria for the research subjects used were healthy male white rats, aged 5 months with body weight ranging from 170 to 200 grams with the characteristics of fine fur, shining eyes, no defects, no scars, and had never been used for other studies. The subjects of this study were 30 male rats which were divided into 5 treatment groups with the following details: C- : The group was rats that were not exposed to particulate soot and not treated with C. longa as a negative control. C+ : Treatment group + is rats exposed to soot particulates at a concentration of 1,064 mg/m3 for 8 hours as a positive control (Ramírez-Tortosa et al., 1999; Quiles et al., 2002; Aulia, 2017; Hendrawan et al., 2018; Juliprihanto et al., 2019). T1 : Treatment group 1, namely rats exposed to particulate soot at a concentration of 1,064 mg/m3 for 8 hours and given C. longa at a dose of 1 mg/kg bw (Ramírez-Tortosa et al., 1999; Quiles et al., 2002; Aulia, 2017; Hendrawan et al., 2018; Juliprihanto et al., 2019). T2 : Treatment group 2 was rats exposed to soot particulates at a concentration of 1,064 mg/m3 for 8 hours and given C. longa at a dose of 2 mg/kg bw (Ramírez-Tortosa et al., 1999; Quiles et al., 2002; Aulia, 2017; Hendrawan et al., 2018; Juliprihanto et al., 2019). T3 : Treatment group 3 was rats exposed to soot particulates at a concentration of 1,064 mg/m3 for 8 hours and given C. longa at a dose of 3 mg/kg bw (Ramírez-Tortosa et al., 1999; Quiles et al., 2002; Aulia, 2017; Hendrawan et al., 2018; Juliprihanto et al., 2019). The dose of exposure to soot particulates comes from the effective dose according to previous research reports, namely a concentration of 1,064 mg/m3 for 8 hours (Hougaard et al., 2008; Dachlan et al., 2011; Hendrawan et al., 2018; Juliprihanto et al., 2019). While the oral dose of C. longa is the effective dose in experimental rabbits that have been converted to rats at 1, 2, and 3 mg/kg bw (Ramírez-Tortosa et al., 1999; Quiles et al., 2002; Aulia, 2017). The variables measured were MDA levels, TNF-α expression, and IL-6 levels with the ELISA method. The size of the exposure box is determined based on the calculation of the rat’s need for oxygen 2.68 ml/g/hour, the weight of the rat is 150 g, the number of rats in one box is six, the duration of exposure is 8 hours, and the percentage of oxygen in the air is 21%. Then the minimum box volume needed is: Minimum exposure box size is: ~ 0.5 m × 0.5 m × 0.5 m (length × width × height)/6 mice Procedure for administration of powdered soot particulate exposureExperimental animals of all groups were placed in rearing cages until treatment started. Prior to the experiment, acclimatization was carried out in the exposure box. Experimental animals in groups T1, T2, and T3 were treated with exposure to particulate soot at a concentration of 1,064 mg/m3 for 8 hours every day in an exposure box for treatment for 30 days. Experimental animals in group C− (negative control) remained in the maintenance cage because they were not given any treatment (Hougaard et al., 2008; Dachlan et al., 2011; Hendrawan et al., 2018; Juliprihanto et al., 2019). The particulate soot powder (carbon, mesoporous < 100 µm) that will be used for exposure before the treatment begins has been prepared in advance by being calculated and weighed on a micro scale, the dosage needs according to the area per cage exposure per day until a concentration of 1,064 mg/m3 is reached. After the experimental animals were placed in each exposure box per group, the particulate soot powder used as the exposure was sprayed in the exposure box air through a pipe that was blown by a fan continuously for 8 hours. During the treatment, the air temperature, the flow velocity of 5–7.5 km/hour (breeze), and local humidity with a pressure of one atmosphere were monitored by inhalation. During the exposure in the box, the experimental animals were still given food and drinking water ad libitum. After the treatment was completed for 8 hours, the experimental animals were returned to the rearing cage according to their group. Procedure for administration of C. longa extractCurcuma longa extracts are given in the form of C. longa extract powder [Curcumin, (C. longa) Turmeric, C1385-Sigma-Aldrich]. Curcuma longa extract solution was made per treatment group. Group T1 was given extract at a dose of 1 mg/kg bw per animal, group T2 was given extract at a dose of 2 mg/kg bw per animal, and group T3 was given extract at a dose of 3 mg/kg bw per animal. The extract was given orally with a probe every day for 30 days after treatment of exposure to soot (Ramírez-Tortosa et al., 1999; Quiles et al., 2002; Aulia, 2017). Examination of MDA, TNF-α, and IL-6 levels with the ELISA methodMeasurement of MDA, TNF-α, and IL-6 levels was carried out with thiobarbituric acid (TBA) and read with a spectrophotometric device to determine the amount of MDA compound produced from the lipid peroxidation process and inflammation level. This examination used the MDA ELISA Kit (MDA Assay Kit competitive ELISA, Abcam- ab238537), TNF-α ELISA Kit (Rat Tumor Necrosis Factor Alpha ELISA Kit, Cat. No. E0764Ra, Bioassay Technology Lab.), IL-6 ELISA Kit (Rat Interleukin 6 ELISA Kit, Cat. No. E0135Ra, Bioassay Technology Lab.), and read using the ELISA Reader at a wavelength of 450 nm. Blood serum was added with 9 ml of cold phosphate-buffered saline (PBS) solution. Then centrifuged for 15 minutes at 3,000 rpm. About 4 ml of supernatant was taken and dissolved in 1 ml of 15% trichloroacetic acid (TCA) solution and 1 ml of 0.3% TBA solution was added in 0.25 N HCl. This mixture was then heated in an 800°C water bath for 15 minutes, then cooled at room temperature, and centrifuged at 3,000 rpm for 15 minutes. The absorbance of the supernatant was observed by spectrophotometry at a wavelength of 450 nm. Data analysisThe measurement data obtained from the levels of MDA, TNF-α, and IL-6, were compiled and tabulated in the form of distribution and frequency tables for each variable. Data were analyzed using one-way analysis of variance (ANOVA) analysis. The analysis was carried out with the assumption that the tested population was normally distributed and homogeneous, and that the samples were not related to each other. If the data are not normally distributed, the arc sin transformation will be carried out. If there are differences between treatments, further tests are carried out using the Tukey test. To determine the relationship between the research parameters, path analysis is carried out. Ethical approvalThis research has conducted an ethical feasibility test at the Faculty of Veterinary Medicine, Universitas Airlangga, and has received an ethics certificate no 3.KE.043.03.2021. ResultsThe study was conducted by measuring three parameters, namely MDA levels, TNF-α levels, and IL-6 levels. In this study, there were five treatment groups used with each treatment consisting of six replications, namely treatment C− (negative control), treatment C+ (positive control), treatment T1 (treatment 1), treatment T2 (treatment 2), and T3 (treatment 3). The analysis was carried out using the normality test and the homogeneity of variance test first, then continued with the ANOVA test and the correlation test. The normality test was conducted first to determine the distribution of the data to be tested. A normality test was carried out using the Shapiro–Wilk test on each variable in each treatment group. If the obtained significance value or p-value is more than 0.05 in each group, it indicates the data distribution is normal, whereas if the obtained significance value or p-value is less than 0.05, it indicates the data distribution is not normal. A homogeneity of variance test was conducted to determine the similarity of variance between treatment groups. The homogeneity of variance test was carried out using Levene’s test on each variable. The normality test and the homogeneity of variance test are requirements for using the ANOVA test which aims to test whether there are differences in the various treatment groups. If a significance value or p-value is less than 0.05, it indicates a significant difference, so it can be continued using the least significant difference (LSD) test if the homogeneity test of variance is met and the Dunnett T3 test if the homogeneity test of variance is not met to test the differences between each treatment group. Effect of treatment on MDA levelsThe results of the normality test showed that the significance value of each treatment was more than 0.05 (sig. > 0.05), so it could be stated that each treatment had data that were normally distributed. The results of the homogeneity of variance test showed a significance value of more than 0.05 (sig. > 0.05), so it could be stated that the variance between treatments was homogeneous. ANOVA test results obtained a calculated F value of 10.062 with a significance value (p-value) of 0.000. For comparison, the F table value is 2.759 at db1=4, db2=25, and 5% alpha. These results show the calculated F value is more than the F table value (F hit > F table) and the significance value is less than 0.05 (p < 0.05), so that it is stated that there are significant differences between the five treatment groups on MDA levels (Table 1). The results of the description of the highest MDA levels were obtained in the C+ treatment with an average of 1.660 ± 0.073, while the lowest MDA levels were obtained in the T3 treatment with an average of 1.542 ± 0.231 (Table 1). The results of the hypothesis test which stated that the administration of C. longa could reduce MDA levels showed that the hypothesis was proven correct. Effect of treatment on TNF-α levelsThe results of the normality test showed that the significance value of each treatment was more than 0.05 (sig. > 0.05), so it could be stated that each treatment had data that were normally distributed. The results of the homogeneity of variance test showed a significance value of more than 0.05 (sig. > 0.05), so it could be stated that the variance between treatments was homogeneous. The results of the ANOVA test obtained a calculated F value of 6.585 with a significance value (p-value) of 0.001. For comparison, the F table value is 2.759 at db1=4, db2=25, and 5% alpha. These results show the calculated F value is more than the F table value (F hit > F table) and the significance value is less than 0.05 (p < 0.05), so that it is stated that there are significant differences between the five treatment groups on TNF-α levels (Table 2). The results of the description of the highest levels of TNF-α were obtained in the C+ treatment with an average of 66.191 ± 5.097, while the lowest levels of TNF-α were obtained in the C-treatment with an average of 55.981 ± 4.689 (Table 2). The results of the hypothesis test which stated that the administration of C. longa could reduce TNF-α levels showed that the hypothesis was proven correct. Effect of treatment on IL-6 levelsThe results of the normality test showed that the significance value of each treatment was more than 0.05 (sig. > 0.05), so it could be stated that each treatment had data that were normally distributed. The results of the homogeneity of variance test showed a significance value of more than 0.05 (sig. > 0.05), so it could be stated that the variance between treatments was homogeneous. ANOVA test results obtained a calculated F value of 3.637 with a significance value (p-value) of 0.018. For comparison, the F table value is 2.759 at db1=4, db2=25, and 5% alpha. These results show the calculated F value is more than the F table value (F hit > F table) and the significance value is less than 0.05 (p < 0.05), so that it is stated that there are significant differences between the five treatment groups on IL-6 levels (Table 3). The results of the description of the highest levels of IL-6 were obtained in the C+ treatment with an average of 2.929 ± 0.248, while the lowest levels of IL-6 were obtained in the C-treatment with an average of 2.292 ± 0.461 (Table 3). The results of the hypothesis test which stated that the administration of C. longa could reduce IL-6 levels showed that the hypothesis was proven correct. Table 1. Results of data analysis on MDA levels.
Table 2. Results of data analysis on TNF-α levels.
Table 3. Results of data analysis on IL-6 levels.
DiscussionExposure to soot can increase MDA levels. The highest MDA levels were obtained in the C+ treatment with an average of 1.660 ± 0.073. The administration of curcumin can reduce MDA levels due to exposure to soot in the treatment group. The administration of curcumin reduced the lowest MDA levels obtained in the T3 treatment with an average of 1.542 ± 0.231 (p < 0.05). Damage is caused by soot particulates through various biological mechanisms, including inflammation, endotoxin effects, autonomic activity, pro-coagulant effects, and ROS production, which causes oxidative stress (Li et al., 2008). Exposure to soot particulates has a significant risk to the cardiovascular system (De Prins, 2014). Inhalation of soot particulates that penetrate into the alveolar epithelium, can increase the inflammatory response by increasing oxidative stress. In addition, there is also an increase in protein and fat oxidation (Franklin et al., 2007). One of the mechanisms thought to play a role is the occurrence of oxidative stress, which in turn will increase the amount of ROS in the body, namely hydrogen peroxide (H2O2) and hydroxyl radicals (−OH) (De Prins, 2014). MDA is a compound formed due to oxidative stress. MDA is a metabolite compound resulting from lipid peroxidation caused by ROS, where MDA can describe free radical activity in cells, so that it is used as a marker of oxidative stress. Increased levels of MDA in blood plasma, can be used as an indicator of oxidative stress that occurs due to increased levels of blood soot particulates. This oxidative stress then induces changes in vascular dysfunction (Kozlov et al., 2016). Increased levels of free radicals in the body due to exposure to soot will cause a decrease in plasma MDA. According to Alaiya et al. (2015), a decrease in MDA levels will trigger an increase in superoxide dismutase as opposed to a decrease in MDA levels in the body. Therefore, the body needs natural antioxidants that are able to capture free radicals stably and are able to reduce their negative effects. MDA is one of the most frequently used indicators as an indication of fat peroxidation. C. longa has the ability to protect the biomembrane against peroxidative damage. Lipid peroxidation is a chain reaction mediated by free radicals, which causes cell membrane damage, and C. longa works by scavenging active free radicals involved in the peroxidation process (Sarvalkar et al., 2011). A study that intends to test C. longa in a rat model of asthma, showed that this substance can reduce MDA levels significantly. MDA level is an indicator of lipid peroxidation. Administration of C. longa or curcumin therapy can reduce MDA levels, namely by means of curcumin donating a hydrogen atom (H) from the phenolic hydroxyl (OH) group when reacting with free radicals. This reaction will produce a phenoxy curcumin radical or a less reactive flavonoid because the curcumin phenoxyl radical can undergo a resonance structure change by redistributing unpaired electrons in the conjugated double bond structure in the aromatic ring. The curcumin or flavonoid radicals will react further to form unreactive compounds, possibly via a termination reaction. Through this reaction, curcumin can inhibit lipid peroxidation initiated by free radicals (Lee et al., 2010). The decrease in MDA levels shows the benefits of C. longa as an anti-oxidant. Oxidative stress is usually always associated with an increase in MDA and protein carbohydrates (Shakeri et al., 2017). Exposure to soot increased TNF-α levels and the highest TNF-α levels in the K+ treatment with an average of 66.191 ± 5.097 (p < 0.05). The administration of curcumin was able to reduce the TNF-α inflammatory factor. In the treatment group with 3 mg/kg bw of curcumin, TNF-α expression decreased the lowest compared to the other two treatment groups. Exposure to soot particulates causes Inflammatory processes due to exposure to particulate soot can increase free radicals. Endotoxins present in soot particulates stimulate the production of proinflammatory cytokines (Pekkanen et al., 2002; Lee, 2009). Oxidative stress causes the activation of inflammatory signaling pathways, such as NF-κB, which is a transcription factor of mediators such as the proinflammatory cytokine IL-1, TNF-α, and IL-6 (Donaldson, 2003). The soot particulates can translocate into the blood vessels. When soot particulates enter the body, the body will carry out a defense mechanism in the form of increased production of proinflammatory cytokines by inflammatory cells, especially macrophages. Macrophages can secrete proinflammatory cytokines such as IL-1, IL-6, IL-8, and TNF-α (Tang et al., 2020). In cells affected by exposure to particulate soot, the appropriate receptor for TNF-α is TNF-R1, and for IL-1 is IL-1R, the receptor will cause apoptosis extrinsically (death receptor-initiated). Increased production of TNF-α due to the stimulation of soot particulates indicates that this substance is consistently responsible for the release of inflammatory mediators (Pozzi et al., 2003; Tang et al., 2020). Exposure to soot particulates increased IL-6 levels and the highest was in the C+ treatment with an average of 2.929 ± 0.248 (p < 0.05). The administration of curcumin was able to reduce IL-6 levels and the lowest was T3 treatment with a dose of curcumin 3 mg/kg bw. The soot particulates that caused cardiovascular disturbances can either be through direct effects on the cardiovascular system or indirectly through pulmonary oxidative stress and inflammatory responses. IL-6 is a B cell differentiation factor cytokine (BSF2) that functions in B cell maturation. IL-6 is a mediator of acute inflammatory processes and an important regulator of cell immune responses. The role of mediator or regulator of the IL-6 inflammatory system is very potential because it can inhibit or cause inflammatory reactions. The mechanism of this IL-6 cytokine extracellularly is through a membrane receptor consisting of two subunits, namely the gp130 subunit and the IL-6 receptor (IL-6R) (Nazariah et al., 2013). In the process of exposure to soot, increased oxidative stress reactions in cells will induce signals and will be captured by IL-6Rs. Activation of these receptors, such as Toll-like receptors, which respond to PM exposure produces cytokines and chemokines that react to cellular homeostatic and immune processes at the site of injury. Exposure to particulate soot caused the oxidant chain to increase, not only from the increasing number of oxygen atoms but also the percentage of –COOH, C=O, and CO groups. The collection of these oxidant groups in the lungs then stimulates the release of macrophages and leukocytes. Systemically, proinflammatory cytokines such as IL-6, TNF-α, and IL-1βare produced by macrophages and leukocytes. Increased IL-6 will stimulate inflammatory responses such as an increase in leukocytes and platelets, the production of fibrinogen by the liver, and the synthesis of adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule (VCAM), and E-selectin (Tsai et al., 2012; Pope et al., 2016). In the study of Totlandsdal et al. (2010), a concentration of 100 g/ml carbon exposure increased mRNA and protein expression of IL-1β and IL-6 in primary lung epithelial cells. Al Housseiny et al. (2020) also said that IL-6 expression was increased in the bronchial tree during PM exposure (Totlandsdal et al., 2010). Curcuma longa was significantly effective on the expression at the protein and gene levels of the IL-6 cytokine. Curcumin is able to influence the signaling interference mechanism that mediates the expression of the IL-6 gene in vascular muscle cells. The decrease in IL-6 expression was also in line with standard resveratrol levels. In addition, it was also reported that curcumin at concentrations of 5, 10, and 15 M was known. Several other studies also reported the role of C. longa on IL-6 cytokines. Curcuma longa has been reported to play an effective role in inhibiting inflammation by decreasing IL-1β and IL-6 cytokines in psoriasis-like inflammation cases treated with imiquimod (Jun et al., 2013). Gulcubuk et al. (2013) reported that in mice induced by pancreatitis, curcumin (C. longa) has the potential to reduce inflammation by decreasing the activation of NF-κB and AP-1 which also inhibits mRNA induction of iNOS, TNF-α, and IL. -6 in the pancreas. In a study in aortic tissue, Parody et al. (2006) showed that C. longa relative decreased the activation of DNA binding of AP-1 and NF-кB proteins and decreased concentrations of cytokines IL-1β, IL-6, MCP-1, and MMP- 9. In line with this study, Lee et al. (2014) reported in vitro study that curcumin compounds, namely curcumin-like diarylpentanoid [2,6-bis(2,5-dimethoxybenzylidene) cyclohexanone], were molecular targets in rheumatoid arthritis cases of nuclear translocation. p65 NF-κB is able to bind to NF-κB fibroblast synovial DNA through inhibition of cyclooxygenase-2 (COX-2), IL-6. ConclusionThe results stated that the administration of C. longa could reduce MDA levels, TNF-α, and IL-6 levels. Curcuma longa as an anti-inflammatory and anti-oxidant play an effective role in inhibiting inflammation by decreasing IL-6 cytokine and TNF-α. Curcuma longa can inhibit lipid peroxidation initiated by free radicals and then reduce MDA levels. Authors’ contributionMA, JS, TWS, and WW designed this research. MA, JS, TWS, and WW conducted a survey and took samples at the sample fields. All authors examined samples in the research laboratories. All authors compiled, read, revised, and approved the final manuscript. Conflict of interestAll authors declare that there is no conflict of interest. FundingThe authors received no financial support for the research, authorship, and/or publication of this article. ReferencesAlaiya, S., Athiroh, A.S. and Santoso, H. 2015. Role of Centella asiatica (Centella asiatica) juice on superoxide dismutase (SOD) in rats. Bioscience 1(1), 34–45. Al Housseiny, H., Singh, S., Emile, M., Nicoleau, R.L.V. and Wal, P.S. 2020. Identification of toxicity parameters associated with combustion produced soot surface chemistry and particle structure by in vitro assays. Biomedicine 8(9), 345–351. Aulia, D.A.R.E. 2017. Side effects of curcumin-msn on the histopathological picture of male kidney (Rattus norvegicus) as a special toxicity test. Undergraduate Thesis, Widya Mandala Catholic University, Surabaya, Indonesia. Darwadi, R.P., Aulanni’am, and Mahdi, C. 2013. Effect of curcumin therapy on malondialdehyde (MDA) levels isolated from parotid and protein profiles of white rats exposed to lipopolysaccharide (LPS). Chem. Student J. 1(1), 133-139. Dachlan, E.G., Widjiati, and Santoso, B. 2011. The Effect of exposure to soot particulates on increased lipid peroxidase, placental apoptosis and pregnancy outcomes in the molecular mechanisms of pregnancy disorders in rats (Rattus novergicus). Thesis. Universitas Airlangga. De Prins, S. 2014. Airway oxidative stress and inflammation markers in exhaled breath from children are linked with exposure to black carbon. Environ. Int.73, 440–446. Erta, M,. Quintana, A. and Hidalgo, J. 2012. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 8(9), 1254–1266 Finch, J. and Conklin, D.J. 2016. Air pollution-induced vascular dysfunction: potential role of endothelin-1 (ET-1) system. Cardiovasc. Toxicol. 16(3), 260–275. Gulcubuk, A., D. Haktanir, A., Cakiris, A., Ustek, D., Guzel, O. Erturk, M. Karabagli, M., Akyazi, I., Cicekci, H. and Altunatmaz, K. 2013. Effects of curcumin on proinflammatory cytokines and tissue injury in the early and late phases of experimental acute pancreatitis. Pancreatology 13, 347–354. Halonen, J.I., Lanki, T., Yli-Tuomi, T., Tiittanen, P., Kulmala M. and Pekkanen, J. 2009. Particulate air pollution and acute cardiorespiratory hospital admissions and mortality among the elderly. Epidemiology 20(1), 143–153. Hendrawan, V.F., Wulansari, D., Oktanella, Y. and Widjiati, W. 2018. Effectiveness of chlorogenic acid supPLEMENTAL on VEGF serum and placental MAP kinase expression in carbon black-exposed pregnant Rattus norvegicus. Res. J. Pharm. Tech. 11(5), 1830–1834. Hougaard, K.S., Jansen, K.A., Nordly, P., Taxvig, C., Vogel, U., Saber, A.T. and Wallin, H. 2008. Effects of prenatal exposure to diesel exhaust particles on postnatal development, behavior, genotoxicity and inflammation in mice. Part. Fibre Toxicol. 5(3), 1-15. Juliprihanto, A., Hendrawan, V. F., Wulansari, D., Oktanella, Y. and Widjiati, W. 2019. Placental cells number and caspase-9 expression in white rat (Rattus norvegicus) apoptosis exposed with carbon black. Res. J. Pharm. Tech. 12(4), 1935–1942. Jun, S., Yi, Z. and Jinhong, H. 2013. Curcumin Inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS One 8, e67078. Kozlov, V.S., Shmargunov, V.P. and Panchenko, M.V. 2016. Modified aethalometer for monitoring of black carbon concentration in atmospheric aerosol and technique for correction of the spot loading effect. In 22nd International Symposium on Atmospheric and Ocean Optics: Atmospheric Physics. Lee, H.S. 2009. Suppression effect of Curcuma Longa rhizome-derived components against nitric oxide synthase. J. Appl. Biol. Chem. 52, 212–215. Lee, H.S., Lee, M.J., Kim, H., Choi, S.K., Kim, J.E., Moon, H.I. and Park, W.H. 2010. Curcumin inhibits TNFα-induced lectin-like oxidized LDL receptor-1 (LOX-1) expression and suppresses the inflammatory response in human umbilical vein endothelial cells (HUVECs) by an antioxidant mechanism. J. Enzyme Inhib. Med. Chem. 25(5), 720–729. Lee, H.S., Lee, M.J., Kim, H., Choi, S.K., Kim, J.E., Moon, H.I. and Park, W.H. 2010. Curcumin inhibits TNFα-induced lectin-like oxidised LDL receptor-1 (LOX-1) expression and suppresses the inflammatory response in human umbilical vein endothelial cells (HUVECs) by an antioxidant mechanism. J. Enzyme Inhib. Med. Chem. 25(5), 720-729. Lewis, A. and Elks, P.M. 2019. Hypoxia induces macrophage tnfa expression via cyclooxygenase and prostaglandin E2 in vivo. Front. Immunol. 10(2321), 1–14. Lin, W., Zhu, T., Xue, T., Peng, W., Brunekreef, B., Gehring, U., Huang, W., Hu, M., Zhang, Y. and Tang, X. 2015. Association between changes in exposure to air pollution and biomarkers of oxidative stress in children before and during the Beijing Olympics. Am. J. Epidemiol. 181(8), 575–583. Munzel, T., Tommaso, G., Al-Kindi, S., John, D., Andreas, D. and Rajagopalan, J.L.S. 2018. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart J. 39, 3543–3550. Nabarawy, N.E., Gouda, A. and Shalaby, E. 2019. therapeutic intervention of curcumin on interleukin-6 and oxidative stress induced by Paraquat toxicity of lung and liver in rats. Biomed. Pharmacol. J. 12(4), 1737–1748. Nazariah, S.S., Juliana, J. and Abdah, M.A. 2013. Interleukin-6 via sputum induction as a biomarker of inflammation for indoor particulate matter among primary school children in Klang Valley, Malaysia. Glob. J. Health Sci. 5, 93–105. Parody, F.E.D., Mao, T.L., Ennis, M.B. and Pagano, R.W. 2006. Oral administration of diferuloylmethane (curcumin) suppresses proinflammatory cytokines and destructive connective tissue remodeling in experimental abdominal aortic aneurysms. Ann. Vasc. Surg. 20, 360–368. Pourbagher-Shahri, A.M., Farkhondeh, T., Ashrafizadeh, M., Talebi, M. and Samargahndian, S. 2021. Curcumin and cardiovascular diseases: Focus on cellular targets and cascades. Biomed. Pharmacother. 136, 111214. Pozzi, R., Barbara de B., Luigi, P., and Cecilia, G. 2003. Inflammatory mediators induced by coarse (PM2.5-10) and fine (PM2.5) urban air particles in RAW 264.7 cells Toxicology 183(1–3), 243–254. Quiles, J.L., Mesa, M.D., César, L., Concepción R.T., Aguilera, M., Battino, M. and Ramírez-Tortosa, M.C. 2002. Curcuma Longa extract supplementation reduces oxidative stress and attenuates aortic fatty streak development in rabbits. Arterioscler. Thromb. Vasc. Biol. 22, 1225–1231. Ramírez-Tortosa, M.C., Mesa, M.D., Aguilera, M.C., Quiles, J.L., Baro, L., Ramires-Tortosa, C.L., Martines-Victoria, E. and Gil, A. 1999. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. J. Atherosclerosis 147(2), 371–378. Romieu, I., Gouveia, L.A., Cifuentes, A.P., de Leon, W., Junger, J., Vera, V., Strappa, M., Hurtado-Díaz, V., Miranda-Soberanis, V., Rojas-Bracho, L. Carbajal-Arroyo, L. and Tzintzun-Cervantes, G. 2012. HEI Health Review Committee. Multicity study of air pollution and mortality in Latin America (the ESCALA study). Res. Rep. Health Eff. Inst. 171, 5–86. Shakeri, M., Oskoueian, E., Le, H. and Shakeri, M. 2020. Strategies to combat heat stress in broiler chickens: unveiling the roles of selenium, vitamin E and vitamin C. Vet Sci. 7(2), 1-9. Tang, J., Cheng, W., Gao, J., Li, Y., Yao, R., Rothman, N., Lan, Q., Campen, M.J., Zheng, Y. and Leng, S. 2020. Occupational exposure to carbon black nanoparticles increases inflammatory vascular disease risk: an implication of an ex vivo biosensor assay. Toxicol. Fiber Parts. 17, 47. Raju, V.S., Reddy C.S. and Ragan, A. 2006. Curcuma L. (Zingiberaceae) in Andhra Pradesh: a preliminary study. J. Econ. Taxon. Bot. 30(4), 773–775. Sadeghi, A., Rostamirad, A., Seyyedebrahimi, S. and Meshhani, R. 2018. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NFkB pathway and ROS production. Inflammopharmacology 26(5), 1265–1272. Sarvalkar, P.P., Walvekar, M.V. and Bhopale, L.P. 2011. Antioxidative effect of curcumin (Curcuma Longa) on lipid peroxidation and lipofuscinogenesis in submandibular gland of D-galactose-induced aging male mice. J. Med. Plant Res. 5(20), 5191–5193. Salomon, B.L., Leclerc, M., Tosello, J., Ronin, E., Piaggio, E. and Cohen, J.L. 2018.Tumor necrosis factor and regulatory T cells in oncoimmunology. Front. Immunol. 9(444), 1–12. Silva, L.B., Alexandrino, P.S.N., Sandra, M.A.S.M., Carolina, S.G., Illiana, L.Q., Alessandra, A.A.T.C., Severino, A.J. and Jair, C.L. 2019. The role of TNF-α as a proinflammatory cytokine in pathological processes: review articles. Open Dent. J. 13, 332–338. Simoni, M., Baldacci, S., Maio, S., Cerrai, G., Sarno, G. and Viegi, G. 2015. Adverse effects of outdoor pollution in the elderly. J. Thorac. Dis. 7(1), 34–45. Song, X., Zhang, M., Dai, E. and Luo, Y. 2019. Molecular targets of curcumin in breast cancer. Mol. Med. Rep. 19(1), 23–29. Totlandsdal, A.I., Refsnes, M. and Lg, M. 2010. Mechanisms involved in ultrafine carbon black-induced release of IL-6 from primary rat epithelial lung cells. Toxicol. In Vitro 24, 10–20. Tsai, D.H., Amyai, N., Marques-Vidal, P., Wang, J.L., Riediker, M., Mooser, V., Paccaud, F., Waeber, G., Vollenweider, P. and Bochud, M. 2012. Effects of particulate matter on inflammatory markers in the general adult population. Toxicol. Fiber Parts 9, 24–33. Zhang, M., Mueller, N.T., Wang, H., Hong, V., Appel, L.J. and Wang, X. 2018. Maternal Exposure to Ambient Particulate Matter ≤2.5 µm During Pregnancy and the Risk for High Blood Pressure in Childhood. Hypertension 72(1), 194-201. | ||
| How to Cite this Article |
| Pubmed Style Aminuddin M, Sargowo D, Sardjono TW, Widjiati W. Curcuma longa supplementation reduce MDA, TNF-α, and IL-6 levels in a rat model exposed to soot particulates. Open Vet J. 2023; 13(1): 11-19. doi:10.5455/OVJ.2023.v13.i1.2 Web Style Aminuddin M, Sargowo D, Sardjono TW, Widjiati W. Curcuma longa supplementation reduce MDA, TNF-α, and IL-6 levels in a rat model exposed to soot particulates. https://www.openveterinaryjournal.com/?mno=104184 [Access: July 05, 2025]. doi:10.5455/OVJ.2023.v13.i1.2 AMA (American Medical Association) Style Aminuddin M, Sargowo D, Sardjono TW, Widjiati W. Curcuma longa supplementation reduce MDA, TNF-α, and IL-6 levels in a rat model exposed to soot particulates. Open Vet J. 2023; 13(1): 11-19. doi:10.5455/OVJ.2023.v13.i1.2 Vancouver/ICMJE Style Aminuddin M, Sargowo D, Sardjono TW, Widjiati W. Curcuma longa supplementation reduce MDA, TNF-α, and IL-6 levels in a rat model exposed to soot particulates. Open Vet J. (2023), [cited July 05, 2025]; 13(1): 11-19. doi:10.5455/OVJ.2023.v13.i1.2 Harvard Style Aminuddin, M., Sargowo, . D., Sardjono, . T. W. & Widjiati, . W. (2023) Curcuma longa supplementation reduce MDA, TNF-α, and IL-6 levels in a rat model exposed to soot particulates. Open Vet J, 13 (1), 11-19. doi:10.5455/OVJ.2023.v13.i1.2 Turabian Style Aminuddin, Muhammad, Djanggan Sargowo, Teguh Wahju Sardjono, and Widjiati Widjiati. 2023. Curcuma longa supplementation reduce MDA, TNF-α, and IL-6 levels in a rat model exposed to soot particulates. Open Veterinary Journal, 13 (1), 11-19. doi:10.5455/OVJ.2023.v13.i1.2 Chicago Style Aminuddin, Muhammad, Djanggan Sargowo, Teguh Wahju Sardjono, and Widjiati Widjiati. "Curcuma longa supplementation reduce MDA, TNF-α, and IL-6 levels in a rat model exposed to soot particulates." Open Veterinary Journal 13 (2023), 11-19. doi:10.5455/OVJ.2023.v13.i1.2 MLA (The Modern Language Association) Style Aminuddin, Muhammad, Djanggan Sargowo, Teguh Wahju Sardjono, and Widjiati Widjiati. "Curcuma longa supplementation reduce MDA, TNF-α, and IL-6 levels in a rat model exposed to soot particulates." Open Veterinary Journal 13.1 (2023), 11-19. Print. doi:10.5455/OVJ.2023.v13.i1.2 APA (American Psychological Association) Style Aminuddin, M., Sargowo, . D., Sardjono, . T. W. & Widjiati, . W. (2023) Curcuma longa supplementation reduce MDA, TNF-α, and IL-6 levels in a rat model exposed to soot particulates. Open Veterinary Journal, 13 (1), 11-19. doi:10.5455/OVJ.2023.v13.i1.2 |