| Original Article | ||
Open Vet J. 2023; 13(1): 90-98 Open Veterinary Journal, (2023), Vol. 13(1): 90–98 Original Research Vitamin D ameliorates liver pathology in mice caused by exposure to endocrine disruptor bisphenol AMohamed A. Al-Griw1, Suhila M. Zaed2, Ismail M. Hdud3 and Taher Shaibi2*1Department of Histology and Genetics, Faculty of Medicine, University of Tripoli, Tripoli, Libya 2Zoology Department, Faculty of Science, University of Tripoli, Tripoli, Libya 3Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya Submitted: 13/10/2022 Accepted: 18/12/2022 Published: 15/01/2023 *Corresponding Author: Taher Shaibi. Zoology Department, Faculty of Science, University of Tripoli, Tripoli, Libya. Email: t.shaibi [at] uot.edu.ly © 2023 Open Veterinary Journal
AbstractBackground: Increasing evidence suggests that bisphenol A (BPA) induces liver pathological changes. Further, an association between BPA and circulating vitamin D (VitD) levels were documented. Aim: The role of VitD in BPA-induced liver pathological changes was explored in this study. Methods: Healthy 4.5-week-old male (n=35) and female (n=35) Swiss albino mice were used in this study. The animals were randomly divided into control and treated groups. The control groups were further divided into sham (no treatment) and vehicle (corn oil), whereas the treated groups were also divided into VitD (2195 U/kg), BPA (50 μg/kg), and BPA + VitD (50 μg/kg + 2195 U/kg) groups. For 6 weeks (twice a week), the animals were dosed intraperitoneally. One week later (at 10.5-weeks-old), the animals were sacrificed for biochemical and histological analyses. Results: BPA produced a considerable rise in the body and liver weights in both genders of mice when compared to control mice. BPA also caused significant increases in the liver damage markers alanine transaminase (ALT), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT). It also induced liver histopathological changes, including higher apoptotic indices in both genders. On the other hand, treatment with VitD considerably reduced liver damage and slightly decreased the apoptotic index rate. The ALP, ALT, and GGT levels were also markedly reduced. VitD has been proven to have a protective effect on both genders. Conclusions: According to our findings, VitD protects mice from BPA-induced liver damage, possibly via suppressing liver damage markers. Keywords: Vitamin D, Bisphenol A, Liver, Apoptosis, Mice. IntroductionExposure to environmental factors such as nutritional abnormalities, stress, and toxicants can lead to phenotypic variations (Waterland, 2009; Nilsson et al., 2018). Environmental toxicants have become a serious public health issue (Chen, 2021). They disrupt a variety of metabolic processes and harm biological systems in humans and animals (Humblet et al., 2008). Alterations in appetite, dietary efficacy, and metabolism of lipids, protein, and carbohydrates may occur as a result of this interference. Environmental toxicant exposure during the phase of germ cells, intrauterine, postnatal, and early lives has a significant impact on the phenotypes and vulnerability to diseases later in life (Skinner et al., 2010, 2013; Al-Gubory, 2014; Al-Griw et al., 2017). Endogenous hormonal control is disrupted by synthetic endocrine disruptors such as bisphenol A (BPA) (Elobeid and Allison, 2008). For many years, BPA has been widely utilized in the manufacturing of epoxy resins and plastic products (Willhite et al., 2008; Camarca et al., 2016; Jalal et al., 2018). At all ages, people are inadvertently subjected to BPA in everyday life due to its widespread use in the fabrication of plastic food beverage containers and the coating of food cans (Gassman, 2017; Gear and Belcher, 2017). BPA has been found in the environment, in food, and even in the physiological fluids of humans (Mouneimne et al., 2017). According to epidemiological statistics, more than 90% of people had detectable levels of BPA (Trasande et al., 2013), whereas those exposed to BPA at work had 70 times greater than the general population (Hines et al., 2017). These findings suggest that people are susceptible to BPA, which may be inevitable, as it is hard to find a chemical replacement that is both safe and economical (Warner and Flaws, 2018). BPA can be found in the air at 2–208 ng/m3, in food samples at amounts of 0.2–106 ng/g, and in thermal paper at 54–79 g/cm2. In human blood, BPA concentrations can range from 0.5 to 10 g/l, with 4.8 g/l in the placenta and 59.7 g/l in the urine (Kim and Hong, 2017). The widespread use of BPA and its hazardous potential raises worries about its negative effects on several biological systems (Kim and Hong, 2017). In many investigations, BPA functions as an estrogenic substance (Korkmaz et al., 2010). According to many investigations, certain tissues/organs are particularly prone to the harmful effects of BPA, which can result in diseases (Fenichel et al., 2013; Rochester, 2013; Al-Griw et al., 2021b). BPA impacts the health of humans, therefore, has gotten a lot of attention in public health. Impacts of BPA on the kidneys, lungs, heart, and liver have been demonstrated (Tyl et al., 2008; Al-Griw et al., 2022a). Recent research exhibited that BPA has detrimental effects on testicular cytoarchitecture and male reproductive performance (Al-Griw et al., 2021a, 2022b). The active form of vitamin D (VitD) is essential for human metabolism (Walentowicz-Sadłecka et al., 2013). With anti-apoptotic and anti-inflammatory properties, VitD therapy reduces ischemia-reperfusion damage after myocardial infarction (Bae et al., 2013). VitD deficiency is correlated with an increased oxidative injury (Sinanoglu et al., 2012; Assalin et al., 2013; Seif and Abdelwahed, 2014). In humans and animals, the liver is a crucial organ for detoxification, and malfunction of the liver after environmental toxicant exposures is accompanied by liver pathology (Paradies et al., 2014; Al-Griw et al., 2022a). According to the vest knowledge of the authors, there are no/little studies on the role of VitD in reducing and/ or preventing liver pathology. Therefore, this work aimed to explore the VitD role in BPA-induced liver pathology using a mouse model, as no earlier study had done so. Our findings showed that VitD protects mice from BPA-induced liver damage. Materials and MethodsAnimals and experimental designHealthy 4.5-week-old male (n=35) and female (n=35) Swiss albino mice, weighing 13.5 ± 1.48 g were used in this study. All animals were bred and housed in the animal house at the University of Tripoli, under conventional circumstances of a 12 hours light/ dark cycle and temperature (24°C ± 1°C). The mice were fed with a pelleted diet and water ad libitum. The mice were divided into control and untreated groups, with 14 mice in each (7 males and 7 females). The control groups were further divided into sham (no treatment) and vehicle (corn oil), whereas the treated groups were also divided into VitD (2195 U/kg), BPA (50 μg/kg), and BPA + VitD (50 μg/kg + 2195 U/kg) groups. For 6 weeks (twice a week), the animals were dosed intraperitoneally (Fig. 1). The BPA and VitD were dissolved in sterile corn oil. The BPA and VitD doses were chosen according to previous works (Vom Saal and Hughes, 2005; Tyl et al., 2008; Sadowski et al., 2014; Tesic et al., 2015; Khodayar et al., 2020). One week later (at 10.5-week-old), the animals were killed for biochemical and histopathological analyses. Toxicity assessmentAny abnormal signs and the survival/mortality rate were daily recorded. During the exposure period, mice were observed twice daily for any abnormal clinical signs or behavior that may result from toxicity. Body and organ weightsFor the calculation of body weight alterations, the body weights were measured at the onset and the end of the experiments. At the end of the experiments, the livers were weighted in all experimental groups. Blood and tissue harvestingAt the end of the experiments, approximately 1 ml of blood was drowned from the tail vein of each mouse for biochemical analyses. Sera were obtained by centrifugation at 3,000 rpm for 15 minutes and were stored at−20°C until being used. The animals were killed under anesthesia with 1% ketamine and their livers were rapidly dissected. After washing the livers in sterile normal saline, they were fixed in 10% buffered formalin for histopathological investigations. For determining lipid peroxidation and antioxidant values, pieces of the livers were frozen at−20°C until used. Liver enzyme damage in the seraFor liver damage test, alanine transaminase (ALT), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT) were measured using commercial kits (Biomaghreb, Tunisia) as previously described (Al-Griw et al., 2022a). The enzymatic-based method was used to measure the activities of the enzymes. Histopathological study and microscopyThe histopathological studies were made based on prior published methods (Ginsberg et al., 1981; Al-Griw et al., 2015). Following dissection, 10% neutral buffered formalinfixed tissues were dehydrated in a series of increasing concentrations of ethanol solutions, cleared in xylene, and then embedded in paraffin wax. Paraffin sections were cut at 5 µm thick, deparaffinized, hydrated, stained with H&E, and examined under light microscopy (Leica, Germany). The hepatic tissue architecture was examined and imaged using light microscopy (Leica, Germany). The tissues from each animal were assessed blindly by two pathologists for histopathological changes. Histopathologic investigation of liver sections was performed with particular consideration for inflammatory changes, hepatocellular necrotic changes, biliary ductless changes, fibrosis, architectural changes, and degenerative changes. The frequency of lesions was unbiasedly evaluated to determine a pattern of injury, which was assessed semi-quantitatively according to the severity and distribution of the pathological changes. Note that the only diagnoses that were considered to be accurate based on histopathological changes and data presented in each group were included in the histopathological description and depictions.
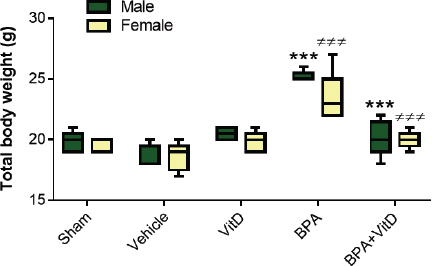
Fig. 1. VitD preserves animal body weights. Male and female mice were subjected to conditions of sham control, vehicle control, VitD, BPA, or BPA + VitD. Measurements of the body weights. The data are represented as mean ± SEM. (***) indicates p < 0.001 in male groups and (≠≠≠) indicates p < 0.001 in female groups. Scoring of cell deathUsing the image-analysis software (ImageJ, National Institutes of Health, MA, http://rsb.info.nih.gov/ij/), the cell death in images of H&E-stained liver sections was measured. Apoptotic cells were characterized morphologically by determining chromatin margination and condensation as well as cell shrinkage (Gujral et al., 2001), while the criteria of cell swelling, karyolysis, karyorrhexis, absence of nucleus, and increased cytoplasmic eosinophilia were signs for hepatocyte necrosis. Statistical analysisGraphPad Prism software (version 7.0) was used to analyze the data. All data represent the mean ± SEM. Normality was assessed using the computerized Kolmogorov–Smirnov test. Normality was assessed using the computerized Kolmogorov–Smirnov test. For data with normal distributions, multiple comparisons were made with a two-way analysis of variance followed by a post-hoc test, Dunnett’s. p values of ≤0.05 were considered statistically significant. Ethical approvalThe Research Ethics Committee of the Biotechnology Research Centre in Tripoli, Libya granted ethical permission for animal work (Reference BEC-BTRC-2020). ResultsVitD preserves the total body weight upon BPA exposureDuring the daily follow-up of the animals, no death was documented in any of the groups, and no symptoms of toxicity were seen. In males and females, there were no marked treatment effects in total body weights compared to the control groups (Fig. 1). No marked gender difference was detected when experimental groups were compared (p > 0.05, Fig. 1). On the contrary, there was a statistically significant difference in the body weights between control and BPA groups in both genders (F=29.3; p < 0.001, F=14.18; p < 0.001, respectively), and VitD treatment preserved the body weights in both males and females, which was similar to that seen in controls (p < 0.001, Fig. 1). VitD preserves liver weights upon BPA exposureIn males and females, there were no significant treatment effects on liver weights compared to the control groups (Fig. 2). On the other hand, there were marked treatment effects in the liver weights between control and BPA groups in both males and females (F=8.22; p=0.0021, F=6.6; p < 0.041, respectively), and that treatment with VitD preserved the liver weights in males but not females (p=0.0019, p=0.0697, respectively, Fig. 2). No marked gender difference was detected when experimental groups were compared (p > 0.05, Fig. 2). VitD alleviates liver damage markers in the sera of mice upon BPA exposureThere were no significant gender effects in the ALP, ALT, and GGT between male and female control groups (Fig. 3A and B). On the other hand, there were significant treatment effects in the ALP, ALT, and GGT levels between individual treated groups (Fig. 3A–C). For males, BPA significantly increased the levels of ALP, ALT, and GGT compared to controls, and that treatment with VitD significantly preserved the levels of ALP and ALT, but not GGT, which were similar to that seen in controls (Fig. 3A and B). For females, BPA significantly increased the levels of ALP and GGT, but not ALT, compared to controls, and that treatment with VitD significantly preserved the levels of ALP, but not ALT and GGT, which was nearly similar to those seen in controls (Fig. 3A–C). The findings also found that there were marked treatment effects in the levels of ALT and GGT, but not ALP between male and female BPA-treated groups (Fig. 3A–C). Specifically, post-hoc tests revealed that the levels of ALT and GGT were markedly higher in males than in females (p < 0.001 and p < 0.01, respectively).
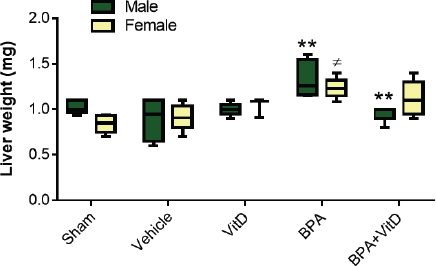
Fig. 2. VitD preserves liver weight. Male and female mice were subjected to conditions of sham control, vehicle control, VitD, BPA, or BPA + VitD. Measurement of liver weights. The data are represented as mean ± SEM. (**) indicates p < 0.01 in male groups and (≠) indicates p < 0.001 in female groups.
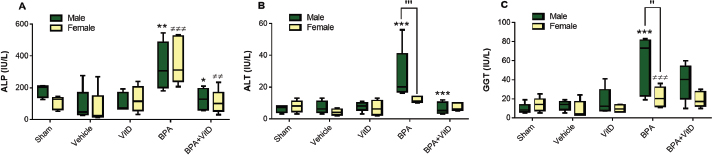
Fig. 3. VitD alleviates liver enzyme damage. Male and female mice were subjected to conditions of sham control, vehicle control, VitD, BPA, or BPA + VitD. (A): ALP levels are measured in micrograms per liter (IU/l). (B): ALT levels (IU/l) are measured. (C): GGT level measurement (IU/l). The data is represented as mean ± SEM. (*) indicates p < 0.05, (**) indicates p < 0.01, and (***) indicates p < 0.001 in male groups and (≠≠) indicates p < 0.01 and (≠≠≠) indicates p < 0.001 in female groups. (′) indicates p < 0.05 and (′′) indicates p < 0.01 males versus females. VitD ameliorates liver pathology upon BPA exposureThe histological examination of this study showed that treatment with VitD was effective against the deteriorating BPA effects on the liver tissues of male and female mice. Specifically, the liver tissues of sham and vehicle control-treated mice of males and females show no/little hepatic architecture changes (Fig. 4A and B). The images showed that the hepatic lobule was composed of a central vein surrounded by radiating cords of the normal hepatocyte. The cords of the hepatocytes were separated by sinusoids. The hepatic lobules were surrounded by the normal portal area comprised of the hepatic artery, hepatic vein, and bile duct. Sections from animals treated with VitD alone did not trigger any alterations in either male or female mice (Fig. 4A and B). In comparison with the liver tissue sections of control-treated mice, tissue sections of BPA-treated mice showed congestion in the central vein and portal area, alteration in hepatocyte architecture, degeneration, and vacuolation in the hepatocytes with pyknotic nuclei. It also showed dilatation in the hepatic sinusoids and central vein with aggregation of lymphocytes (Fig. 4C and D). It also showed infiltration of macrophages in the sinusoids and between hepatocytes. On the other hand, the liver tissues of BPA+VitD-treated mice mitigated the pathological alterations induced by BPA (Fig. 4).
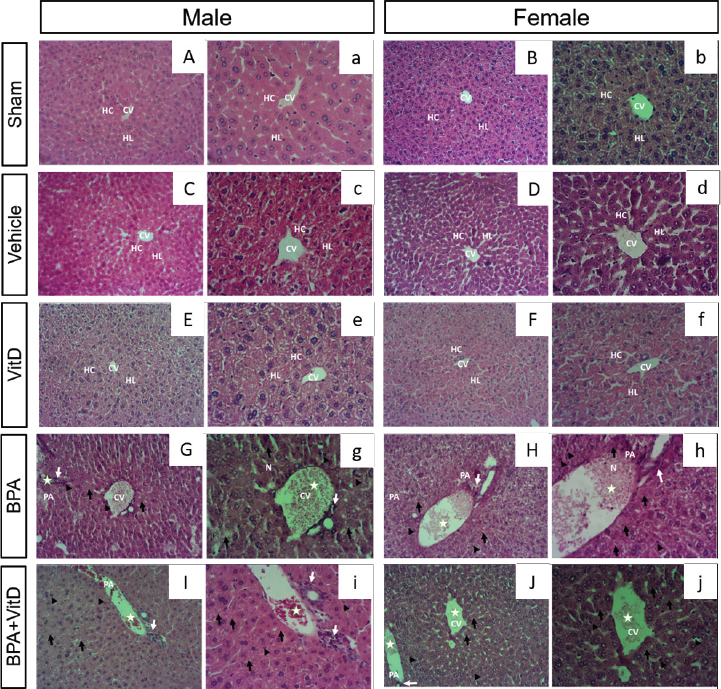
Fig. 4. Representative photomicrographs of H&E-stained male and female liver tissue sections of control (A, a, B, and b), vehicle (C, c, D, and d), Vit D (E, e, F, and f), BPA (G, g, H, and h) and PBA + VitD (I, I, J, and j). The control vehicle and VitD-treated groups of both males (A, B, and C) and females (a, b, and c) exhibited normal hepatocyte architecture, central vein, sinusoids, and portal area components. Liver sections from BPA-exposed animals showed hepatocellular hydropic degeneration (arrows) and pan lobular closed to Glisson’s capsule (open arrows). Hepatocellular coagulative necrosis (arrowhead) with the disintegration of the cytoplasmic membrane, cytoplasmic eosinophilic and nuclear changes including karyopyknosis and karyolysis, is accompanied by lymph-histiocytic infiltration with few neutrophils (white arrows). Sections from BPA + VitD-exposed mice from males (I and i) and females (J and j) revealed slight preservation of liver architecture, including hepatocyte proliferation and repopulation with multifocal hyperemia and small erythrocyte extravasation and minimal lymph-histiocyte infiltration. Scale bars indicate 100 µm, 100× m H&E staining. Hepatocytes (H), central vein (CV). DiscussionThe main findings of this study showed that BPA triggered a marked liver pathological change in later life, as illustrated by biochemical and histopathological analysis. Specifically, BPA significantly increased the total body and liver weights. There was also a significant increase in the levels of liver enzymes (ALP, ALT, and GGT), hepatocellular apoptosis, and hepatocyte injury scores. These noxious treatment impacts were markedly ameliorated by VitD treatment. Our findings also showed significant VitD effects in terms of some biochemical and histopathological findings, although not all findings enhanced markedly in the treated group compared to the untreated group. Even at lower doses, BPA remains a public health issue due to its ability to promote phenotypic variations, as previously discussed (Chan, 2000; USEPA, 2003; Manikkam et al., 2012; Al-Griw et al., 2016; Nilsson et al., 2018). The BPA was found to cause negative impacts on health in early and later life (Anway et al., 2005; Salian et al., 2009; Bruner-Tran and Osteen, 2011; Manikkam et al., 2012; Rogers et al., 2013; Schug et al., 2013; Camarca et al., 2016; Koike et al., 2018; Özaydın et al., 2018; Al-Griw et al., 2021a). The active form of VitD is important in clinical medicine because of its powerful effects on bone metabolism and Ca2+ balance (Piotrowska et al., 2016; Parva et al., 2018). It was found that VitD and VitD receptor activators protect against brain injury (Tokgoz et al., 2018) and kidney damage (Tan et al., 2008). Similarly, it was demonstrated that the VitD receptor activator has a beneficial impact on renal injury after ischemia (Azak et al., 2013). This work aimed to explore the VitD role in BPA-induced liver pathology using a mouse model. Accumulating evidence illustrates BPA-induced hepatotoxicity. BPA exposure is correlated to a raised risk of being obese (Braun, 2017). Exposure to BPA during prenatal and postnatal was linked to increased body weight (Akingbemi et al., 2004; Suttie, 2006; Magliano and Lyons, 2013; Picard and Turnbull, 2013; Gear and Belcher, 2017). Other investigations found a marked decrease in the body weights in the animals that were treated with 0.1 mg/kg BPA (Hassan et al., 2012). In addition, a marked significance and dose-related decrease in liver weights were found when compared to controls with BPA-treated groups (Yamasaki et al., 2002a, 2002b). In this study, we found that BPA significantly increased the total body and liver weights in both mouse males and females when compared to untreated mice. This reduction was attenuated with VitD treatment. It is well known that the liver plays role in protecting the body from chemical substances. Environmental toxicants were found to disrupt normal liver function, causing various diseases (Praveena et al., 2018; Lee et al., 2019). Liver damage tests, including monitoring of AST and ALT enzymes levels, are evaluated in the presence of liver abnormalities. Increased levels of liver damage indices were previously reported considering the BPA toxicity that resulted from 5 mg BPA/kg/day in rats with no effects at ≤5 mg (Korkmaz et al., 2010). It was documented an increase in AST activity in male rats treated with BPA ≥ 200 mg/kg/day and increased ALP activity in rats treated with 600 mg/kg/day (Yamasaki et al., 2002a, 2002b). In this study, we found that BPA (400 mg/kg) markedly increased the serum indices of liver damage enzymes (ALP, ALT, and GGT) compared to controls, which are attributed to liver injury. Interestingly, our findings showed that treatment with VitD upon BPA exposure modulated their levels. This indicates the curative role of VitD against BPA-induced liver pathology. Growing evidence reports that the toxicity of BPA is dependent on its doses and administration routes. Rats subjected to BPA at 125 mg BPA/kg/day orally for 13 weeks were shown to have liver and kidney damage (Tyl et al., 2008; Dong et al., 2013; Yıldız and Barlas, 2013). As a ubiquitous environmental toxicant, BPA damages tissues/organs in humans and animals (Acaroz et al., 2019; Soundararajan et al., 2019). According to Gear and Belcher (2017), BPA has dose- and sex-specific effects on the tissue architecture of the spleens, revealing minor changes in hematopoietic and immunomodulatory functions. When mice were subjected to 5 mg BPA/kg/day in dietary, there were no effects on the liver or kidney, but toxic effects were found at 50 or 600 mg BPA/kg/day (Tyl et al., 2008; Dong et al., 2013). Recently, it was found that BPA promoted heart, kidney, lung, and spleen pathologies in mouse males and females (Al-Griw et al., 2021b; Shaibi et al., 2022). In this study, we found that BPA significantly increased histopathological scores of the hepatocytes, which ultimately led to induced liver pathology. This is likely due to inducing oxidative injury. ConclusionThis study demonstrated that BPA exposure triggered liver pathological changes, as revealed by biochemical and histopathological investigations. Our findings shed light on the possible underlying mechanism(s) for the development of a range of abnormalities induced by BPA. Although some improvement is gained, there are no marked differences in other biochemical and histopathological parameters. We thought that it may be correlated to the long elimination and VitD action time. However, we were not capable of finding the precise mechanism(s) by which VitD exerts its actions. Nevertheless, the findings of this study show that VitD provides effective treatment for BPA-induced liver pathological changes. Further works need to be undertaken for a better understanding of the role of VitD in BPA-induced liver pathological changes. Conflicts of interestAll authors have no conflict of interest to declare. Author contributionsM.A.A. designed the study. M.A.A., S.D.Z., I.M.H., and T.S. contributed to the data analysis and interpretation, and writing the manuscript. ReferencesAcaroz, U., Ince, S., Arslan-Acaroz, D., Gurler, Z., Demirel, H.H., Kucukkurt, I., Eryavuz, A., Kara, R., Varol, N. and Zhu, K. 2019. Bisphenol-A induced oxidative stress, inflammatory gene expression, and metabolic and histopathological changes in male Wistar albino rats: protective role of boron. Toxicol. Res. (Camb). 8(2), 262–269. Akingbemi, B.T., Sottas, C.M., Koulova, A.I., Klinefelter, G.R. and Hardy, M.P. 2004. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology 145(2), 592–603. Al-Griw, A.M., Treesh, S.A., Alghazeer, R.O. and Regeai, S.O. 2017. Environmentally toxicant exposures induced intragenerational transmission of liver abnormalities in mice. Open Vet. J. 7(3), 244–253. Al-Griw, M.A., Al-Ghazeer, R.O., Al-Azreg, S.A. and Bennour, E.M. 2016. Cellular and molecular etiology of hepatocyte injury in a murine model of environmentally induced liver abnormality. Open Vet. J. 6(3), 150–157. Al-Griw, M.A., Alghazeer, R.O., Salama, N.M., Lwaleed, B.A., Eskandrani, A.A., Alansari, W.S., Alnajeebi, A.M., Babteen, N.A., Shamlan, G. and Elnfati, A.H. 2021a. Paternal bisphenol A exposure induces testis and sperm pathologies in mice offspring: possibly due to oxidative stress? Saudi J. Biol. Sci. 28(1), 948–955. Al-Griw, M.A., Alshibani, Z.O., Alghazeer, R., Elhensheri, M., Tabagh, R.M., Eskandrani, A.A., Alansari, W.S., Habibulla, M.M. and Shamlan, G. 2022a. Histone deacetylase 2 inhibitor valproic acid attenuates bisphenol A-induced liver pathology in male mice. Sci. Rep. 12(1), 10. Al-Griw, M.A., Marwan, Z.M., Hdud, I.M. and Shaibi, T. 2021b. Vitamin D mitigates adult onset diseases in male and female mice induced by early-life exposure to endocrine disruptor BPA. Open Vet. J. 11(3), 407–417. Al-Griw, M.A., Salama, N.M., Treesh, S.A. and Elnfati, A.H. 2015. Transgenerational genetic effect of trichloroethane (TCE) on phenotypic variation of acrosomal proteolytic enzyme and male infertility risk. Int. J. Genet. Genom. 3(5), 43–49. Al-Griw, M.A., Shalab, S.M., Alghazeer, R.O., Elnfat, A.H., Treesh, S.A., Benjama, A.E., Shamlan, G., Habibullah, M.M., Eskandrani, A.A., Alnajeebi, A.M., Babteen, N.A. and Alansari, W.S. 2022b. Nigella sativa oil alleviates mouse testis and sperm abnormalities induced by BPA: potentially through redox homeostasis? Comb. Chem. High Throughput Screen. 26(2), 301–312. Al-Gubory, K.H. 2014. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reprod. Biomed. 29(1), 17–31. Anway, M.D., Cupp, A.S., Uzumcu, M. and Skinner, M.K. 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469. Assalin, H.B., Rafacho, B.P., dos-Santos, P.P., Ardisso, L.P., Roscani, M.G., Chiuso-Minicucci, F., Barbisan, L.F., Fernandes, A.A., Azevedo, P.S., Minicucci, M.F., Zornoff, L.A. and de-Paiva, S.A. 2013. Impact of the length of vitamin D deficiency on cardiac remodeling. Circ. Heart Fail. 6(4), 809–816. Azak, A., Huddam, B., Haberal, N., Koçak, G., Ortabozkoyun, L., Şenes, M., Akdoğan, M.F., Denizli, N. and Duranay, M. 2013. Effect of novel vitamin D receptor activator paricalcitol on renal ischaemia/reperfusion injury in rats. Ann. R. Coll. Surg. Engl. 95(7), 489–494. Bae, S., Singh, S.S., Yu, H., Lee, J.Y., Cho, B.R. and Kang, P.M. 2013. Vitamin D signaling pathway plays an important role in the development of heart failure after myocardial infarction. J. Appl. Physiol. 114(8), 979–987. Braun, J M. 2017. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 13(3), 161–173. Bruner-Tran, K.L. and Osteen, K.G. 2011. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod. Toxicol. 31(3), 344–350. Camarca, A., Gianfrani, C., Ariemma, F., Cimmino, I., Bruzzese, D., Scerbo, R., Picascia, S., D’Esposito, V., Beguinot, F., Formisano, P. and Valentino, R. 2016. Human peripheral blood mononuclear cell function and dendritic cell differentiation are affected by bisphenol-A exposure. PLoS One 11(8), e0161122. Chan, C. 2000. NTP technical report on the toxicity studies of 1,1,1-trichloroethane (CAS No. 71-55-6) administered in microcapsules in feed to F344/N rats and B6C3F1 mice. Toxic. Rep. Ser. 41, 1–e20. Chen, L. 2021. Gut microbiota manipulation to mitigate the detrimental effects of environmental pollutants. Toxics 9(6), 127. Dong, Y., Zhai, L., Zhang, L., Jia, L. and Wang, X. 2013. Bisphenol A impairs mitochondrial function in spleens of mice via oxidative stress. Mol. Cell. Toxicol. 9(4), 401–406. Elobeid, M.A. and Allison, D.B. 2008. Putative environmental-endocrine disruptors and obesity: a review. Curr. Opin. Endocrinol. Diabetes Obes. 15(5), 403–408. Fenichel, P., Chevalier, N. and Brucker-Davis, F. 2013. Bisphenol a: an endocrine and metabolic disruptor. Ann. Endocrinol. (Paris) 74, 211–220. Gassman, N.R. 2017. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 58(2), 60–71. Gear, R.B. and Belcher, S.M. 2017. Impacts of bisphenol A and ethinyl estradiol on male and female CD-1 mouse spleen. Sci. Rep. 7(1), 856. Ginsberg, L.C., Johnson, S.C., Salama, N.M. and Ficsor, G. 1981. Acrosomal proteolytic assay for detection of mutagens in mammals. Mutat. Res. 91(4-5), 413–418. Gujral, J.S., Bucci, T.J., Farhood, A. and Jaeschke, H. 2001. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Histology 33, 397–405. Hassan, Z.K., Elobeid, M.A., Virk, P., Omer, S.A., ElAmin, M., Daghestani, M.H. and AlOlayan, E.M. 2012. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid. Med. Cell. Longev. 1, 1–6. Hines, C.J., Jackson, M.V., Deddens, J.A., Clark, J.C., Ye, X., Christianson, A.L., Meadows, J.W. and Calafat, A.M. 2017. Urinary bisphenol A (BPA) concentrations among workers in industries that manufacture and use BPA in the USA. Ann. Work Expo. Health 61(2), 164–182. Humblet, O., Birnbaum, L., Rimm, E., Mittleman, M.A. and Hauser, R. 2008. Dioxins and cardiovascular disease mortality. Environ. Health Perspect. 116(11), 1443–1448. Jalal, N., Surendranath, AR., Pathak, JL., Yu S. and Chung CY. 2018. Bisphenol A (BPA) the mighty and the mutagenic. Toxicol. Rep. 5, 76–84. Khodayar, M.J., Kalantari, H., Mahdavinia, M., Khorsandi, L., Alboghobeish, S., Samimi, A., Alizadeh, S. and Zeidooni, L. 2020. Protective effect of naringin against BPA-induced cardiotoxicity through prevention of oxidative stress in male Wistar rats. Drug Chem. Toxicol. 43(1), 85–95. Kim, J.H. and Hong, Y. 2017. Increase of urinary malondialdehyde level by bisphenol A exposure: a longitudinal panel study. Environ. Health 16(1), 8. Koike, E., Yanagisawa, R., Win-Shwe, T.T. and Takano, H. 2018. Exposure to low-dose bisphenol A during the juvenile period of development disrupts the immune system and aggravates allergic airway inflammation in mice. Int. J. Immunopathol. Pharmacol. 32, 2058738418774897. Korkmaz, A., Ahbab, M.A., Kolankaya, D. and Barlas, N. 2010. Influence of vitamin C on bisphenol A, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food Chem. Toxicol. 48(10), 2865–2871. Lee, J., Hong, S., Sun, J.H., Moon, J.K., Boo, K.H., Lee, S.M. and Lee, J.W. 2019. Toxicity of dietary selenomethionine in juvenile steelhead trout, Oncorhynchus mykiss: tissue burden, growth performance, body composition, hematological parameters, and liver histopathology. Chemosphere 226, 755–765. Magliano, D.J. and Lyons, J.G. 2013. Bisphenol A and diabetes, insulin resistance, cardiovascular disease and obesity: Controversy in a(plastic) cup? J. Clin. Endocrinol. Metab. 98(2), 502–504. Manikkam, M., Guerrero-Bosagna, C., Tracey, R., Haque, M.M. and Skinner, M.K. 2012. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One 7(2), e31901. Mouneimne, Y., Nasrallah, M., Khoueiry-Zgheib, N., Nasreddine, L., Nakhoul, N., Ismail, H., Abiad, M., Koleilat, L. and Tamim, H. 2017. Bisphenol A urinary level, its correlates, and association with cardiometabolic risks in Lebanese urban adults. Environ. Monit. Assess. 189(10), 517–529. Nilsson, E.E., Sadler-Riggleman, I. and Skinner, M.K. 2018. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenet. 4(2), dvy016. Özaydın, T., Öznurlu, Y, Sur, E., Çelik İ. and Uluışık, D. 2018. The effects of bisphenol A on some plasma cytokine levels and distribution of CD8+ and CD4+ T lymphocytes in spleen, ileal Peyer’s patch and bronchus associated lymphoid tissue in rats. Acta Histochem. 120(8), 728–733. Paradies, G., Paradies, V., Ruggiero, F.M. and Petrosillo, G. 2014. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 20(39), 14205–14218. Parva, N.R., Tadepalli, T., Singh, P., Qian, A., Joshi, R., Kandala, H., Nookala, V.K. and Cheriyath, P. 2018. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus 10(6), e2741. Picard, M. and Turnbull, D.M. 2013. Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging. Diabetes 62(3), 672–678. Piotrowska, A., Wierzbicka, J., Ślebioda, T., Woźniak, M., Tuckey, R.C., Slominski, A.T. and Żmijewski, M.A. 2016. Vitamin D derivatives enhance cytotoxic effects of H2O2 or cisplatin on human keratinocytes. Steroids 110, 49–61. Praveena, S.M., Teh, S.W., Rajendran, R.K., Kannan, N., Lin, C.C., Abdullah, R. and Kumar, S. 2018. Recent updates on phthalate exposure and human health: a special focus on liver toxicity and stem cell regeneration. Environ. Sci. Pollut. Res. Int. 25(12), 11333–11342. Rochester, J.R. 2013. Bisphenol A and human health: a review of the literature. Reprod. Toxicol. 42, 132–155. Rogers, J.A., Metz, L. and Yong, V.W. 2013. Review: endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol. Immunol. 53(4), 421–430. Sadowski, R.N., Wise, L.M., Park, P.Y., Schantz, S.L. and Juraska, J.M. 2014. Early exposure to bisphenol A alters neuron and glia number in the rat prefrontal cortex of adult males, but not females. Neuroscience 279, 122–131. Salian, S., Doshi, T. and Vanage, G. 2009. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to Bisphenol A. Life Sci. 85, 11–18. Schug, T.T., Heindel, J.J., Camacho, L., Delclos, K.B., Howard, P., Johnson, A.F., Aungst, J., Keefe, D., Newbold, R., Walker, N.J., Thomas Zoeller, R. and Bucher, J.R. 2013. A new approach to synergize academic and guideline-compliant research: the CLARITY-BPA research program. Reprod. Toxicol. 40, 35–40. Seif, A.A. and Abdelwahed, D.M. 2014. Vitamin D ameliorates hepatic ischemic/reperfusion injury in rats. J. Physiol. Biochem. 70, 659–666. Shaibi, T., Balug, H.N., Ben-Othman, M.E., Benjama, A.E., Elhensheri, M., Lwaleed, B.A. and Al-Griw, M.A. 2022. Exposure to low-dose bisphenol A induces spleen damage in a murine model: potentially through oxidative stress? Open Vet. J. 12(1), 23–32. Sinanoglu, O., Sezgin, G., Ozturk, G., Tuncdemir, M., Guney, S., Aksungar, F.B. and Yener, N. 2012. Melatonin with 1,25-dihydroxyvitamin D3 protects against apoptotic ischemia-reperfusion injury in the rat kidney. Ren. Fail. 34(8), 1021–1026. Skinner, M.K., Manikkam, M. and Guerrero-Bosagna, C. 2010. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 21(4), 214–222. Skinner, M.K., Manikkam, M., Tracey, R., Guerrero-Bosagna, C., Haque, M. and Nilsson, E.E. 2013. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 11(1), 228–243. Soundararajan, A., Prabu, P., Mohan, V., Gibert, Y. and Balasubramanyam, M. 2019. Novel insights of elevated systemic levels of bisphenol-A (BPA) linked to poor glycemic control, accelerated cellular senescence and insulin resistance in patients with type 2 diabetes. Mol. Cell. Biochem. 458(1–2), 171–183. Suttie, A.W. 2006. Histopathology of the spleen. Toxicol. Pathol. 34(5), 466–503. Tan, X., Wen, X. and Liu, Y. 2008. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor–mediated sequestration of Nf-Κb signaling. J. Am. Soc. Nephrol. 19(9), 1741–1752. Tesic, D., Hawes, J.E., Zosky, G.R. and Wyrwoll, C.S. 2015. Vitamin D deficiency in BALB/c mouse pregnancy increases placental transfer of glucocorticoids. Endocrinology 156(10), 3673–3679. Tokgoz, V.Y., Sipahi, M., Keskin, O., Guvendi, G.F. and Takir, S. 2018. Protective effects of vitamin D on ischemia-reperfusion injury of the ovary in a rat model. Iran J. Basic Med. Sci. 21(6), 593–599. Trasande, L., Attina, T.M. and Trachtman, H. 2013. Bisphenol A exposure is associated with low-grade urinary albumin excretion in children of the United States. Kidney Int. 83(4), 741–748. Tyl, R.W., Myers, C.B., Marr, M.C., Sloan, C.S., Castillo, N.P., Veselica, M.M., Seely, J.C., Dimond, S.S., Van Miller, J.P., Shiotsuka, R.N., Beyer, D., Hentges, S.G. and WaechterJ.M.Jr. 2008. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci. 104(2), 362–384. USEPA. (2003). Interpretation of body weight data. H. E. DH. toxicology science advisory council, office of pesticide programs (OPP). USEPA. Vom Saal, F.S. and Hughes, C. 2005. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 113(8), 926–933. Walentowicz-Sadłecka, M., Sadłecki, P., Walentowicz, P. and Grabiec, M. 2013. The role of vitamin D in the carcinogenesis of breast and ovarian cancer. Ginekol. Pol. 84(4), 305–308. Warner, G.R. and Flaws, J.A. 2018. Bisphenol a and phthalates: how environmental chemicals are reshaping toxicology. Toxicol. Sci. 166(2), 246–249. Waterland, R.A. 2009. Is epigenetics an important link between early life events and adult disease? Horm. Res. 71(Suppl. 1), 13–16. Willhite, C.C., Ball, L. and McLellan, C.J. 2008. Derivation of a bisphenol A oral reference dose (RfD) and drinking-water equivalent concentration. J. Toxicol. Environ. Health 11(2), 69–146. Yamasaki, K., Sawaki, M., Noda, S., Imatanaka, N. and Takatsuki, M. 2002a. Subacute oral toxicity study of ethynylestradiol and bisphenol a, based on the draft protocol for the "enhanced OECD test guideline no. 407." Arch. Toxicol. 76(2), 65–74. Yamasaki, K., Takeyoshi, M., Noda, S. and Takatsuki, M. 2002b. Changes of serum alpha2u-globulin in the subacute oral toxicity study of ethynyl estradiol and bisphenol A based on the draft protocol for the "Enhanced OECD Test Guideline no. 407." Toxicol. 176(1). 101–112. Yıldız, N. and Barlas, N. 2013. Hepatic and renal functions in growing male rats after bisphenol A and octylphenol exposure. Hum. Exp. Toxicol. 32(7), 675–686. | ||
| How to Cite this Article |
| Pubmed Style Al-griw MA, Zaed SM, Hdud IM, Shaibi T. Vitamin D ameliorates liver pathology in mice caused by exposure to endocrine disruptor bisphenol A. Open Vet J. 2023; 13(1): 90-98. doi:10.5455/OVJ.2023.v13.i1.9 Web Style Al-griw MA, Zaed SM, Hdud IM, Shaibi T. Vitamin D ameliorates liver pathology in mice caused by exposure to endocrine disruptor bisphenol A. https://www.openveterinaryjournal.com/?mno=118892 [Access: July 01, 2025]. doi:10.5455/OVJ.2023.v13.i1.9 AMA (American Medical Association) Style Al-griw MA, Zaed SM, Hdud IM, Shaibi T. Vitamin D ameliorates liver pathology in mice caused by exposure to endocrine disruptor bisphenol A. Open Vet J. 2023; 13(1): 90-98. doi:10.5455/OVJ.2023.v13.i1.9 Vancouver/ICMJE Style Al-griw MA, Zaed SM, Hdud IM, Shaibi T. Vitamin D ameliorates liver pathology in mice caused by exposure to endocrine disruptor bisphenol A. Open Vet J. (2023), [cited July 01, 2025]; 13(1): 90-98. doi:10.5455/OVJ.2023.v13.i1.9 Harvard Style Al-griw, M. A., Zaed, . S. M., Hdud, . I. M. & Shaibi, . T. (2023) Vitamin D ameliorates liver pathology in mice caused by exposure to endocrine disruptor bisphenol A. Open Vet J, 13 (1), 90-98. doi:10.5455/OVJ.2023.v13.i1.9 Turabian Style Al-griw, Mohamed A., Suhila M. Zaed, Ismail M. Hdud, and Taher Shaibi. 2023. Vitamin D ameliorates liver pathology in mice caused by exposure to endocrine disruptor bisphenol A. Open Veterinary Journal, 13 (1), 90-98. doi:10.5455/OVJ.2023.v13.i1.9 Chicago Style Al-griw, Mohamed A., Suhila M. Zaed, Ismail M. Hdud, and Taher Shaibi. "Vitamin D ameliorates liver pathology in mice caused by exposure to endocrine disruptor bisphenol A." Open Veterinary Journal 13 (2023), 90-98. doi:10.5455/OVJ.2023.v13.i1.9 MLA (The Modern Language Association) Style Al-griw, Mohamed A., Suhila M. Zaed, Ismail M. Hdud, and Taher Shaibi. "Vitamin D ameliorates liver pathology in mice caused by exposure to endocrine disruptor bisphenol A." Open Veterinary Journal 13.1 (2023), 90-98. Print. doi:10.5455/OVJ.2023.v13.i1.9 APA (American Psychological Association) Style Al-griw, M. A., Zaed, . S. M., Hdud, . I. M. & Shaibi, . T. (2023) Vitamin D ameliorates liver pathology in mice caused by exposure to endocrine disruptor bisphenol A. Open Veterinary Journal, 13 (1), 90-98. doi:10.5455/OVJ.2023.v13.i1.9 |