| Original Article | ||
Open Vet J. 2023; 13(1): 99-107 Open Veterinary Journal, (2023), Vol. 13(1): 99–107 Original Research Effect of ethanol extract from Chrysanthemum cinerariifolium leaves on Ki-67 proliferation and dysplasia severity in a rat model of oral squamous cell carcinomaAnik Listiyana1*, Risma Aprinda Kristanti1, Al Mazida Fauzil Aishaqeena1, Anggun Putri Maulana Ahmad1, Lina Fitria Astari1, Christyaji Indradmojo1 and Fidia Rizkiah Inayatilah21Department of Medical Education, Faculty of Medicine and Health Sciences, Universitas Islam Negeri Maulana Malik Ibrahim Malang, Malang, Indonesia 2Department of Pharmacy, Faculty of Medical and Health Sciences, Universitas Islam Negeri Maulana Malik Ibrahim Malang, Malang, Indonesia Submitted: 08/11/2022 Accepted: 20/12/2022 Published: 17/01/2023 *Corresponding Author: Anik Listiyana. Department of Medical Education, Faculty of Medicine and Health Sciences, Universitas Islam Negeri Maulana Malik Ibrahim Malang, Malang, Indonesia. Email: anik.listiyana.biomed [at] gmail.com © 2023 Open Veterinary Journal
AbstractBackground: Oral squamous cell carcinoma (OSCC) is a malignant tumor that can rapidly infiltrate the oral epithelial tissue and cause high mortality worldwide because the available therapies are less effective. Chrysanthemum cinerariifolium leaf contains secondary metabolites as anti-inflammatory, antioxidant, anticancer, and antimutagenic. Aims: The study aimed to analyze the ethanolic extract of C. cinerariifolium leaf in reducing proliferation (Ki-67) and the degree of dysplasia in OSCC rats. Methods: This study used male Sprague Dawley induced by 7,12-dimethylbenz(a)anthracene (DMBA) 0.5% and divided into five treatment groups, namely positive control/C+ (sick), negative control/C- (healthy), and treatment group induced with DMBA and given extract C. cinerariifolium leaf with successive doses of T1, T2, and T3 (50, 100, and 200 mg/kg bw). The oral epithelium was stained with hematoxylin and eosin and immunohistochemically stained with a Ki-67 monoclonal antibody. The statistical analysis utilizes the one-way analysis of variance test. Results: The results showed that T1 at a dose of 200 mg/kg bw could significantly reduce Ki-67 expression and the degree of oral epithelial dysplasia (OED; p < 0.05) close to healthy controls. Conclusion: The conclusion shows that C. cinerariifolium leaf extract can be a therapy against OSCC by decreasing cell proliferation and the degree of OED. Keywords: Carcinoma, C. cinerariifolium, Inflammation, Proliferation, DMBA. IntroductionCancer is characterized by abnormal and uncontrolled cell growth through developmental stages such as invasion, metastasis, and pre-cancerous lesions (Singh et al., 2021). Oral squamous cell carcinoma (OSCC) is one of the most common cancers in the world, both in developed and developing countries (Suresh et al., 2019), especially in Southeast Asia (Gracia et al., 2018). OSCC is the most prevalent kind of head and neck cancer, accounting for approximately 90% of all oral cavity malignancies (Singh et al., 2021), with a death rate of 500,000 per year and is predicted to increase to 62% by 2035 (Tołoczko-Iwaniuk et al., 2020). The 5-year survival rate only occurs around 60%–80% if OSCC disease can be detected in the early stages of the disease (Yakob et al., 2014). OSCC often affects cats, with an incidence of about 70%–80% of oral neoplasms. OSCC cases were 594, including 526 mixed breeds (90%), and 61 purebred cats (10%) (Zaccone et al., 2022). OSCC originates from epithelial tissue that can infiltrate through the bloodstream and lymphatics, then spread throughout the body (Warshawsky and Killblane, 2005). OSCC locations are on the tongue (ventral and lateral), lips, the floor of the mouth, buccal mucosa, and retromolar (King and Robins, 2006). The development of OSCC occurs due to changes in gene expression that are influenced by specific genetics (Khan et al., 2016), chronic inflammation, viral infections, sun exposure, radiation, phenols, malnutrition, and anemia, as well as tobacco and alcohol consumption (Suresh et al., 2019). The risk factors for OSCC in cats and dogs are the same as in humans: exposure to cigarette smoke and the papilloma virus (Rathore et al., 2014). In cats, also a result of wearing flea collars and vulnerability to oral carcinogens during treatment and consumption of canned cat food (Zaccone et al., 2022). Diagnosis of OSCC disease can use Ki-67. Ki-67 is a nuclear protein that indicates active cell proliferation (Takkem et al., 2018) in the G1, S, G2, and M cycles (Yulianti and Hernowo, 2015). OSCC therapy will be effective if the disease is detected in the early phase (Chen et al., 2020). OSCC treatment uses a combination of surgery and chemotherapy (Magnano et al., 2021); however, OSCC has a low response to chemotherapy and most anticancer drugs. Chemotherapy not only destroys cancer cells but also attacks non-cancerous cells. It has several side effects, including hair loss, nausea and vomiting, canker sores, sleep disturbances, and weight gain (Hamdy and Halim, 2019). Therefore, to overcome this problem, it is necessary to develop new strategies to treat OSCC, such as using traditional plants, considered safer because of common side effects, easy to obtain, and inexpensive (Ningsih, 2016). One of the plants that have medicinal properties is Chrysanthemum cinerariifolium (Setiawati, 2019). This plant has antibacterial, anti-inflammatory, allergic, anticancer, antigenotoxic, and antimutagenic effects (Shahrajabian et al., 2019). Chrysanthemum cinerariifolium contains quercetin (Pires et al., 2013) which has the potential as an anticancer through the induction of the P21 gene with the mechanism of inhibiting the cell cycle and reducing the Rb gene (tumor Suppressor gene), which inhibits the cell cycle in the G1/S phase through E2F inhibition (gene transcription factor) (Scheff et al., 2017). Chrysanthemum cinerariifolium leaf extract contains flavonoids that induce apoptosis through irreversible DNA damage (Lour and Meiyanto, 2007). This study used the protein marker Ki-67 and histopathology of oral epithelium. Increased Ki-67 Expression is followed by the severity of oral epithelial dysplasia (OED) (Takkem et al., 2018). Therefore, this study aimed to analyze the effect of ethanol extract from C. cinerariifolium leaves on KI-67 expression and degree of dysplasia in the OSCC rat model. Material and MethodsExperimental designThis research is quantitative with a true experimental post-test-only control group design. This study used male Sprague Dawley rats weighing 150–180 g and 2–3 months old. This study was divided into five groups, namely positive control [7,12-dimethylbenz(a)anthracene (DMBA) induced but not given therapy], negative control (healthy, not induced by DMBA and C. cinerariifolium extract), and treatment groups T1, T2, and T3, namely the group with DMBA induction and were given C. cinerariifolium leaf extract at doses of 50, 100, and 200 mg/kg bw. Making of OSCC model animalRats were acclimatized for seven days at a temperature of 28°C–32°C. Rats were further induced by DMBA (0.5%) (Sigma-Aldrich®, St. Louis, MO) which was dissolved in acetone, and injected 0.1 ml/100-g bw on the lateral side of the tongue for 5 weeks (days 1–35) and observed for pre-malignant signs or clinical symptoms of OSCC (Zhang et al., 2017). The rats were then orally given C. cinerariifolium leaf extract for 2 weeks (14 days). Rats were euthanized on the 50th day by cervical dislocation, and the tongue was taken for histopathological preparations and immunohistochemical testing. The tongue and the buccal mucosa are the most common sites for OSCC (Pires et al., 2013). Preparation of extract of C. cinerariifolium leavesChrysanthemum cinerariifolium (Trev.) Vis leaves were collected at Punten Village, Batu city, East Java district, Indonesia. Plant determination was carried out at UPT Materia Medica, Batu City. According to statement number 074/093A/102.7/2020, the white chrysanthemum used in this study was C. cinerariifolium (Trevir.) Vis. Chrysanthemum cinerariifolium leaf extract was prepared using the ultrasound-assisted extraction method. The leaves are cleaned and washed, then dried in the sun. The leaf powder (50 g) was put in an Erlenmeyer glass, and 96% ethanol solvent (500 ml) was added at a ratio of 1:10, followed by a 2-minute extraction with three repetitions. The filtration products were then evaporated using a rotary evaporator and an oven at 40°C until the extract's texture became concentrated. About 17.7 g of extract was collected. Experimentally, the extract was dissolved in Na-CMC. HistopathologyThe tongue organ was taken by cutting the base of the tongue and fixed in 10% formalin solution, then dehydrated, paraffin-embedded, and hematoxylin-eosin stained. The degree of keratinization was measured by observing 20 fields of view and 400× microscope magnification. The epithelium was observed in each area of view to determine the degree of dysplasia (Takkem et al., 2018). Evaluation OED was classified into mild, moderate, and severe, while OSCC was graded based on moderate or poor differentiation (Mohan et al., 2016). Histological changes of potentially malignant oral lesions (usual leukoplakia) are only about 50% of the lesions that show dysplasia, the rest show non-hyperplasia and hyperkeratosis (Speight, 2007). Mild dysplasia (the changes as mentioned earlier were restricted to the lower third of the epithelium basal and parabasal layers), moderate dysplasia (changes are reaching two-thirds of the epithelium in the middle layer of the squamous layer), and severe dysplasia (tissue and cytologic changes located outside two-thirds of the epithelium) (Ribeiro et al., 2015). Immunohistochemistry for Ki-67The tongue was fixed with 10% formalin buffer, and then paraffin blocks were made. The tissue was immersed in alcohol, xylol, and ethanol. The slides were washed with water and put into a solution of H2O2 in 0.5% methanol (100 ml Methanol + 1.6 ml H2O2) for 20 minutes. The slides were immersed in a diva solution, heated in a decloaking chamber at a temperature of 95°C for 60 minutes, cooled at room temperature for 20–30 minutes, and immersed in phosphate-buffered saline (PBS) for 2–5 minutes. Rat monoclonal primary antibody Ki-67 (6G6) (Bioss, USA) was dripped, incubated for 90 minutes, and washed with PBS for 3–5 minutes. After washing, the slides were leaked with secondary antibody biotinylated goat anti-mouse IgG (no. B0529; Sigma). The slides were dripped with TREKAVIDIN-HRP. The last slide was dripped with 3,3' diaminobenzidine chromogen. Immunohistochemical staining was quantified using ImageJ software (Kačarević et al. 2017). The presence of brownish spots was indicated as a Ki-67 expression. Data analysisThe Ki-67 expression data were analyzed using the one-way analysis of variance parametric statistical test for numerical data. In contrast, the degree of dysplasia was analyzed using the non-parametric chi-square test because the data were ordinal with a 5% confidence level—data analysis using the Statistical Program Service Solution version 26 program. Ethical approvalThis research has been approved by the Health Research Ethics Commission, Faculty of medicine and health sciences, UIN Maulana Malik Ibrahim Malang, with letter number 001/EC/KEPK-FKIK/2021. ResultsThe results showed that DMBA-induced rats in the positive control group and all treatments (T1, T2, and T3) experienced weight loss in the second week after DMBA induction, then increased weight. All treatment groups, T1, T2, and T3, showed an increase in body weight after administration of C. cinerariifolium leaf extract at the end of the seventh week of the study. Mice induced by DMBA developed clinical signs of OSCC, such as leukoplakia (white plaque) and elevation of the rat tongue mucosa. The plaque has a rough texture and a clear border (unpublished data). Histopathological imageHistopathologically described the rat tongue epithelium in the positive control group (C+) showed that the cells had dysplasia. Cells appear irregular stratification and loss of basal cell polarity, and there are variations in cell size and nucleus size. Cells and nuclei in the granulosum layer also undergo variations in shape, and there is a hyperchromatic nucleus. The ratio between the nucleus and the cytoplasm also increases. The epithelial change of the tongue toward dysplasia is about two-thirds the thickness of the epithelium (Fig. 1). In group (C-), the epithelium of the rat’s tongue had a normal histopathological appearance. There appeared to be a regular epithelial stratification among the cells, with healthy cell turnover in each layer. The base layer consists of hyperchromatic nuclei, while the spinosum layer is composed of irregular polygonal cells. Granulosa has oval-shaped cells. Nucleus and cell organelles vanish, followed by a transition to the corneum layer, demonstrating keratinization development (Fig. 2). The histopathology of the tongue epithelium of rats in the T1 group revealed dysplastic cells. Cell form and size variations (anisocytosis and pleomorphism) and irregular epithelial stratification due to nuclear shape and size differences. The ratio of nuclei to cytoplasm rises, and hyperchromatic nuclei are visible. Cell changes affect two-thirds of the thickness of the rat tongue epithelium. Therefore, this is classified as mild dysplasia (Fig. 3). The histopathological appearance of the rat tongue epithelium in the T2 group showed irregular stratified cells. In the basal and parabasal layers, upwards to one-third of the thickness of the epithelium reveals differences in the form and size of cells and nuclei. Hyperchromatic nuclei were found in the epithelium’s basal and parabasal layers, which do not extend beyond the lowest one-third. The layers of spinosum and granulosum had no dysplastic cells (Fig. 4). The histopathological appearance of the rat tongue epithelium in the T3 group was close to that of normal epithelium. It is characterized by regular stratification cells. Variations in the shape and size of the nucleus are slight. Hyperchromatic nuclei are found in the basal and parabasal layers. Irregular polygonal cell shapes in the spinosum layer and oval cell shapes in the granulosum layer were also seen in this group (Fig. 5). The effect of the administration of C. chrysanthemum leaf extract on the grade of dysplasia in DMBA-induced rats based on the Kruskal–Wallis test showed a significant difference between the treatment groups (p < 0.05). The Post Hoc Mann–Whitney analysis results showed significant differences between all treatment groups. Effect of C. cinerariifolium leaves extract on Ki-67
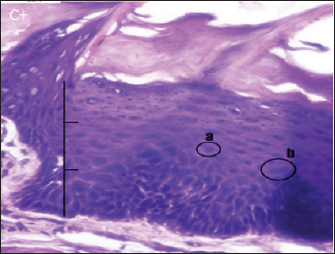
Fig. 1. Histopathological description of the rat tongue epithelium in the positive control group (C+), microscope at ×400. (a) Hyperchromatic nucleus; (b) the size of the nucleus increases so that the ratio between the nucleus and the cytoplasm increases.
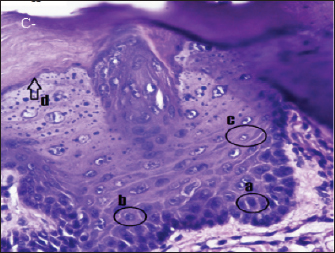
Fig. 2. Histopathologically described the rat tongue epithelium in the negative control group (C-), microscope at ×400. (a) basal layer with a hyperchromatic nucleus and basophilic cytoplasm; (b) spinosum layer with cells of irregular polygonal shape; (c) cells in the granulosum layer with oval cell shape; d. the layer of corneum filled with keratin. Ki-67 is a biological marker for cancer risk (Hutagalung et al., 2014), cancer grading, and prognostic evaluation (Takkem et al., 2018). The results showed that the expression of Ki-67 in the positive control (56.75 ± 1.79) was significantly higher than in the negative control (9.65 ± 0.81) and in all treatment groups (T1, T2, and T3) significantly. The expression of Ki-67 in treatment T1 (45.125 ± 3.66) was significantly higher than in T2 (25.025 ± 3.18) and T3 (16.4 ± 1.92). The higher the extract dose, the lower the Ki-67 expression (Fig. 6). DiscussionDMBA effectThis study used the carcinogenic DMBA to induce the OSCC animal model. Domestic animal OSCC resembles human OSCC in many aspects (Sharma et al., 2021), such as etiopathogenesis, molecular markers, tumor biology, clinical symptoms, treatment, and prognosis so that the inducer or cell culture used may be the same (Rathore et al., 2014). DMBA is a model oral carcinogen because neoplastic alterations are morphologically and histologically similar to those found in human oral carcinoma (Ribeiro et al., 2015). The induction of DMBA causes the body to produce free radicals causing damage to DNA, cell membranes, and proteins and dysregulation of anti-apoptotic mediators (Barroso et al., 2017). DMBA also causes keratinocyte cells to undergo genetic changes, resulting in changes in cell cycle development, DNA repair mechanisms, and apoptosis (Chen et al., 2020). On the basis of clinical examination, DMBA-induced rats in the positive control and treatment groups (T1, T2, and T3) exhibited symptoms of OSCC on the tongue in the form of white plaques or elevations (leukoplakia). The clinical manifestations of OSCC originate from leukoplakia cells (pre-malignant cells). According to Takkem et al. (2018), OSCC symptoms include ulcers with broken or elevated borders, lumps, reddish lesions (erythroplakia), white lesions (leukoplakia), or a combination of both lesions (Rivera and Venegas, 2014). Moreover, lesions may be non-healing extraction sockets or swollen cervical lymph nodes (Markopoulos, 2012).
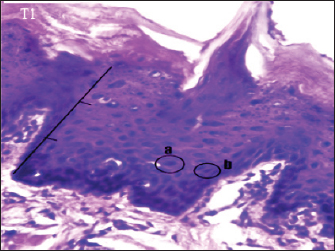
Fig. 3. Histopathological description of the rat tongue epithelium in the T1 group, microscope at ×400. (a) the ratio between the nucleus and the cytoplasm increases, with a hyperchromatic nucleus; (b) the shape of the nucleus showing anisonucleus.
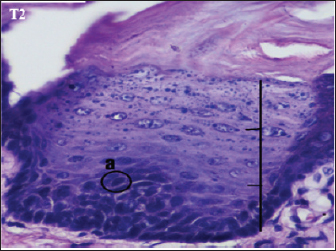
Fig. 4. Histopathological description of the rat tongue epithelium in the T2 group, microscope at ×400. (a) Variations in cell shape with hyperchromatic nuclei. Multiple illnesses and symptoms in OSCC are due to genetic mutations. The early stage is undetectable and painless, then pain with a burning sensation develops, and finally, neoplasia. (Yakob et al., 2014). According to Dost et al. (2014), OSCC is typically preceded by OED, which consists of tissue and cellular alterations consistent with carcinoma but limited to the surface epithelium (OED).
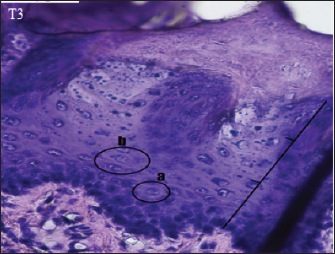
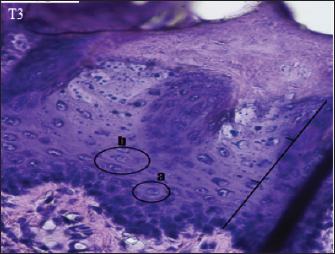
Fig. 5. Histopathological appearance of the rat tongue epithelium in group T3, microscope at ×400. (a) irregular polygonal cell shape in the spinosum layer; (b) oval cell shape in the granulosum layer.
Fig. 6. Effect of the extract of leaves of C. cinerariifolium on Ki-67 expression based on notation. The study results are presented as mean and standard deviation (SD). NS notation (not significant) indicates p > 0.05, notation * indicates p=0.05, notation ** indicates p=0.01, notation *** indicates p=0.001, notation **** indicates p=0.0001. In this study, the injection of DMBA into the rats’ tongues had the painful consequence of lowering their appetite, which could lead to weight loss during the second week. However, the rats’ body weight increased between the third and fifth weeks. DMBA caused weight loss in rats during the second week after induction. DMBA in the body induce pro-inflammatory NF-κβ, resulting in the production of macrophages. Activated macrophages generate pro-inflammatory cytokines, including tumor necrosis factor-alfa (TNF-α) (Wang et al., 2014). In the last week of the research, the administration of C. cinerariifolium leaf extract did not significantly affect weight growth; nevertheless, the T3 groups tended to raise more weight than the other groups (unpublished data). Zhang et al. (2017) stated that the quercetin content, which is also present in C. cinerariifolium extract, has no impact on weight gain despite its potential as an OSCC chemopreventive agent. Histopathological features of OSCCBased histopathologically, OED showed dysplastic oral mucosal tissue (Dost et al., 2014). OSCC is a type of malignant neoplasm that arises in the stratified squamous epithelium of the oral mucosa (Rivera and Venegas, 2014). The formation of OSCC begins with epithelial dysplasia, marked by changes in the proliferation of dysplastic squamous cells on the epithelial surface area and basement membrane degradation. Through the islets and cords of epithelial cells, basement membrane degradation promotes local injury and metastatic invasion of cells into tissues (Fuentes et al., 2012; Rivera and Venegas, 2014). Dysplasia demonstrates a transition from stratified squamous epithelium to malignant epithelium, marked by cellular atypia and loss of cell differentiation and stratification. (Takkem et al., 2018). The long-term accumulation of carcinogens causes dysplasia. Increased dysplasia exacerbates epithelium severity (Mohan et al., 2016). The presence of pleomorphism, or variations in the shape and size of cells, and an increase in the ratio of the nucleus to the cytoplasm, or a disproportional and larger nucleus, are further hallmarks of dysplasia. In normal cells, the ratio between the nucleus and cytoplasm is 1:4 or 1:6; however, in dysplastic cells, the ratio is close to 1: 1. The nucleus develops hyperchromatic due to its high DNA content, resulting in darker staining (Speight, 2007). Histopathological analysis of group C+ oral epithelium revealed dysplasia, characterized by irregular stratification of the oral epithelium due to changes in cell shape and nucleus size. There are also hyperchromatic nuclei in all layers except the basal layer. The rat tongue epithelium had moderate dysplasia in the positive control group. According to Nandini and Subramanyam (2011), in the carcinoma group, most of the nuclei were oval, only a few were indistinguishable, and irregular nuclei. The results of this study follow previous research by Zhang et al. (2017), which proves that DMBA induction can trigger epithelial dysplasia and even cause OSCC with many times and long DMBA exposure, but in this study, only once and short time application. Chemical carcinogens affect the mouth’s epithelial lining and the epithelial lining of the excretory ducts of the salivary glands. Salivary tract epithelial dysplasia can lead to the potential for malignant transformation (Mohan et al., 2016). Histopathological examination of the group (C-) revealed no abnormalities in the stratified or basal epithelium. This group did not observe OED because they were not triggered by DMBA. Normal oral epithelium consists of a basal layer of cuboidal or prismatic epithelial cells with hyperchromatic nuclei and basophilic cytoplasm. There are several cells with irregular polygonal shapes in the spinosum layer. The granulosum layer contains oval-shaped cells. The further above granulosum layer, nucleus is lost, and the layer becomes flatter. The outermost layer is the corneum layer, which contains no nucleus and is composed of keratin. The T1 group had dysplastic alterations in the epithelium of the rat tongue as the (C+) group. This group exhibited mild dysplasia limited to one-third of the epithelium (basal and parabasal layers). According to Speight (2007), moderate dysplasia (grade II) is characterized by abnormal cell proliferation extending to the middle third of the epithelium, more severe cytological alterations than mild dysplasia, and the presence of hyperchromatic cells and prominent nuclei. Loss of basal polarity can be observed near the base of the epithelium, indicating tissue alterations. The administration of C. cinerariifolium leaf extract at a dose of 50 mg/kg bw (T1 groups) was not optimum for reducing the severity of OED. A dose of 100 mg/kg bw (T2 groups) reduces the degree of dysplasia but is not the same as the negative control group. The results of this study contradict those of Ribeiro et al. (2015). They found that administration of hydroalcoholic extract of red propolis (HERP) at doses of 50 and 100 mg/kg bw for 20 weeks inhibited 40% of OSCC growth, promoted a 3-week delay in clinical tumor development, and found no epithelial dysplasia. HERP contains flavonoid formononetin, also found in Chrysanthemum, and functions as an anticancer by inhibiting cancer cell growth and apoptosis via induction of the caspase pathway; decreasing anti-apoptotic proteins bcl-2 and bcl-x; and stimulating cell cycle arrest at the G0/G1 phase. The T2 group showed cell improvement toward mild dysplasia. According to Speight (2007), mild dysplasia (grade I) indicates proliferation or hyperplasia of basal and parabasal layer cells that do not extend beyond the lower third of the epithelium, pleomorphism in cells or nuclei, and minimum tissue changes. In several visual fields, cells in the T3 group had decreased dysplasia, and their condition was similar to that of the negative control group. This suggests that C. cinerariifolium leaf extract at a concentration of 200 mg/kg bw can effectively lower the degree of dysplasia in the oral epithelium. Differences in the terpenoid and flavonoid content of extract and the degree of dysplasia between the treatment groups were reasonably considerable. Other studies have shown that terpineol component in the extract has an anticancer effect by inhibiting the signaling of the transcription factor NF-kB in tumor cells, hence decreasing gene expression in malignancies (Hassan et al., 2010). Terpenoids also function as anticancer agents in human oral cancer cell lines by inducing cell cycle arrest and death in OSCC cells via caspase cascade (Kim et al., 2022). In the extract of C. cinerariifolium leaf, there is also the flavonoid kaempferitin, which can boost the production of the p53 oncogene protein, hence promoting an increase in apoptosis (Siddiqui et al., 2020). The process of inhibiting the production of oncogene proteins and enhancing the expression of tumor suppressor genes can stop aberrant cell proliferation and lower the severity of dysplasia. According to Mutiah et al. (2020), 96% ethanolic extract of C. cinerariifolium (Trev.) leaf based on in silico analysis contains glutamic acid in hydrogen bonds, which inhibits tumor development by blocking angiogenesis. Effect of chrysanthemum leaf extract on Ki-67 expressionIndicators of cell proliferation, such as Ki-67, play a significant part in the biological markers of neoplasms, as they indicate the existence of hyperactive epithelial cells. In addition to OSCC, breast, lung, prostate, cervical, and nerve tissue cancers also utilize KI-67 for prognostic purposes (Takkem et al., 2018). The results demonstrated that the Ki-67 expression in the C+ group (71.6) was substantially greater than in the C- group (70.4). Ki-67 expression was localized in the basal layers of the negative control. In contrast, Ki-67 in both the positive control and dosage treatment (OED) was seen in the basal, suprabasal, and spinous layers (Rivera and Venegas, 2014; Takkem et al., 2018). Based on the non-parametric Kruskal–Wallis test, the expression of ki-67 in T1 (59.15) was greater than in T2 (38.83) and T3 (27.73) groups when C. cinerariifolium leaf extract was administered to the OSCC model. These findings suggest that the extract can inhibit the expression of cell growth as measured by the Ki-67 indicator. Leaf extract of C. cinerariifolium contains terpenoids and flavonoids, which inhibit inflammation and cancer cell proliferation (Zhang et al., 2017). This research was supported by Chodidjah et al. (2013), demonstrating that flavonoids can decrease tyrosine kinase signal pathway levels to suppress cell proliferation. Frequently, these signaling pathways change in cancer cells to confer a selective advantage. Reduced cell proliferation was associated with decreased expression of Ki-67, enhanced cell death, and reduced inflammation (Takkem et al., 2018). Based on the results of previous studies by the authors, the ethanol extract of C. cinerariifolium leaves used in the test UPLC-QToF-MS/MS indicates the active ingredients are genistein and N - [(5-Chloro-1,2,3-thiadiazol-4-yl) methyl] -1- (2-isopropyl-4 -methyl-1,3-thiazol-5-yl) -N-methyl ethanolamine; dan D - (-) - Morphine (Listiyana et al., 2019). The results of the phytochemical screening of C. cinerariifolium leaf extract by thin layer chromatography method, after sprayed under white light with a wavelength of 341–389 nm, showed a yellow color indicating flavonol group compounds and red color exhibiting terpenoid group compounds (Mutiah et al., 2020). Morphine acts directly on the central nervous system to relieve pain and on peripheral tissues to control complications in breast cancer (Gach et al., 2011). Flavonoids act as an anticancer by modulating reactive oxygen species (ROS)-scavenging enzyme, stopping the cell cycle, inducing apoptosis and autophagy, and suppressing the proliferation and invasion of cancer cells to the next stage of malignancy (Kopustinskiene et al., 2020). Genistein acts as a chemotherapeutic drug against various cancer types by modifying (apoptosis, cell cycle, and angiogenesis) and preventing metastasis. Potential synergistic therapy exists between genistein and anticancer medications such as adriamycin, docetaxel, and tamoxifen (Spagnuolo et al., 2015). Inflammatory cells trigger cancer cell proliferation, survival rate, and metastasis. In oral cancer and the saliva of individuals with pre-neoplastic oral lesions, inflammatory mediators such as interleukin (IL)-6, IL-8, and TNF-α are enhanced. Increased TNF-α in the tumor’s microenvironment promotes invasion and shorter survival (Goertzen et al., 2018). TNF-α is cytotoxic to tumor cells, inhibits tumor development, and triggers necrosis, angiogenesis, proliferation, migration, and survival of most cancer cells (Deepthi et al., 2019). Previous research has demonstrated that 50–200 mg/kg of C. cinerariifolium leaf extract can inhibit TNF-α expression (Aishaqeena et al., 2021). Chrysanthemum cinerariifolium suppresses cancer cell growth and invasion, modifies ROS enzymes, and promotes apoptosis and autophagy (Gorlach et al., 2015), improving OSCC conditions. In a recent study, the leaf extract of C. cinerariifolium (Trev.) was discovered to lower the number of macrophages in a mouse model of OSCC (unpublished data). Flavonoids and their derivatives, specifically luteoin, can suppress NF-κβ signals that contribute to inflammation, decreasing TNF-α levels and macrophage cells (Salminen et al., 2008). In rats with OSCC, administration of C. cinerariifolium leaf extract at a concentration of 200 mg/kg bw (T1 groups) reduced the expression of Ki-67, resulting in a decrease in dysplasia similar to normal circumstances. The higher the amount of the extract administered to the OSCC rat model, the more significant the reduction in inflammation and proliferation, which resulted in the regeneration of the oral epithelium. ConclusionChrysanthemum cinerariifolium leaf extract in a dose of 200 mg/kg bw has the potency to be used as a therapeutic for oral squamous cell cancer by lowering the proliferation and degree of dysplasia of the oral epithelium. AcknowledgmentsThe author would like to thank the BOPTN Litabdimas LP2M UIN Maulana Malik Ibrahim Malang grant from the Ministry of Religion of the Republic of Indonesia in 2021. Conflicts of interestNo conflicts of interest among the authors in this research and publication. Author contributionsA.L. and R.A.K.: making research designs; A.L.: analyzing and writing manuscripts; A.L. and R.A.K.: critical revision of the manuscript; L.F.A., A.P.M.A., A.M.F.R., and F.R.I.: technical and material support; F.R.I.: supervision; C.I.: Final approval. ReferencesAishaqeena, A.M., Anik, L. and Kristanti R. 2021. Pengaruh pemberian ekstrak daun krisan (Chrysanthemum cinerariiforium) sebagai antikanker oral squamous cell carcinoma (OSCC) terhadap kadar tumor necrosis factor-alpha (TNF-α) secara in vivo. Malang, Indonesia: Universitas Islam Negeri Maulana Malik Ibrahim. Barroso, P., Verli, F., Rocha, R., Lima, N., Avelar, B.A. and Melo, G.E.B. 2017. Effect of crude latex from Euphorbia tirucalli on DMBA-induced carcinogenesis. J. Histol. Histopathol. 4, 3. Chen, Y., Hsieh, M., Chen, P., Weng, C., Yang, S. and Lin, C. 2020. Erianin induces apoptosis and autophagy in oral squamous cell carcinoma cells. Am. J. Chin. Med. 48, 183–200. Chodidjah, E., Susanto, Hardhono., Sarjadi. and Sarjadi, C.D. 2013. Typhonium flagelliforme decreases tyrosine kinase and Ki67 expression in mice. Univ. Med. 32, 146–154. Deepthi, G., Nandan, S.R.K. and Kulkarni P.G. 2019. Salivary tumour necrosis factor-α as a biomarker in oral leukoplakia and oral squamous cell carcinoma. Asian Pac. J. Cancer Prev. 20, 2087–2093. Dost, F., Lê Cao K., Ford P.J., Ades C. and Farah, C.S. 2014. Malignant transformation of oral epithelial dysplasia: a real-world evaluation of histopathologic grading. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 117, 343–352. Fuentes, B., Duaso J., Droguett D., Castillo C., Donoso W., Rivera C., Venegas B. and Kemmerling U. 2012. Progressive extracellular matrix disorganization in chemically induced murine oral squamous cell carcinoma. ISRN Pathol. 2012, 1–7. Gach, K., Wyrebska, A., Fichna, J. and Janecka, A. 2011. The role of morphine in regulation of cancer cell growth. Naunyn-Schmiedebergs Arch Pharmacol. 384, 221–23. Goertzen, C., Mahdi H., Laliberte C., Meirson T., Eymael D., Gil-Henn H. and Magalhaes M. 2018. Oral inflammation promotes oral squamous cell carcinoma invasion. Oncotarget 9, 29047–29063. Gorlach, S., Fichna, J. and Lewandowska, U. 2015. Polyphenols as mitochondria-targeted anticancer drugs. Cancer Lett. 366(2), 141–149. Gracia, I., Irianiwati, Uroro T., Supriatno, Astuti I., Heriyanto D. and Pramono, D. 2018. Oral squamous cell varcinoma for HPV Genotypes 16 and 18 : a retrospective clinicopathologic study in Yogyakarta, Indonesia. J. Int. Dent. Med. Res. 11, 866–871. Hamdy, R. and Halim A. 2019. Squamous cell carcinoma of the oral tongue: single institution retrospective cohort study from Mansoura University Hospital. Indones. J. Cancer, 12, 102. Hassan, S., Gali-Muhtasib, H., Göransson, H. and Larsson R. 2010. Alpha terpineol: a potential anticancer agent which acts through suppressing NF-κB signalling. Anticancer Res. 30, 1911–1919. Hutagalung, S.B., Mulyadi I.K. and Artha I.G.A. 2014. Ekspresi Ki-67 dan HER-2/neu Berhubungan dengan Derajat Histopatologik Karsinoma Payudara Invasif No Special Type (NST). Maj. Patol. Indones. 23, 45–50. Kačarević, Ž., Grgić A., Šnajder D., Bijelić N., Belovari T., Cvijanović O., Blažičević V. and Radić R. 2017. Different combinations of maternal and postnatal diet are reflected in changes of hepatic parenchyma and hepatic TNF-alpha expression in male rat offspring. Acta Histochem. 119, 719–726. Khan, R., Khurshid Z., Akhbar S. and Moin S. 2016. Advances of salivary proteomics in oral squamous cell carcinoma (OSCC) detection: an update. Proteomes. 4, 1–11. Kim, J., Kim, J., Bandara, B.M., Tilakaratne, W.M. and Kim D. 2022. Leaf extract of osbeckia octandra induces apoptosis in oral squamous cell carcinoma cells. BMC Complement. Med. Ther. 22, 1–10. King, R.J.B. and Robins M.W. 2006. Chemical and radiation carcinogenesis. In: Cancer biology. pp. 96–97. Kopustinskiene, D.M., Jakstas, V., Savickas, A. and Bernatoniene, J. 2020. Flavonoids as anticancer agents. Nutrients. 12, 457. Listiyana, A., Lestari N., Irawati S., Indrawijaya, Y.Y., Annisa, R., Bhagawan, W. and Mutiah, R. 2019. Anticancer activities and metabolite fingerprinting of UPLC-QTOF-MS/MS method from Chrysanthemum cinerariifolium (Trev). J. Islam. Pharm. 4, 19. Lour, G. and Meiyanto E. 2007. Penghambatan karsinogenesis kanker payudara tikus terinduksi DMBA pada fase post inisiasi oleh ekstrak etanolik daun Gynura procumbens ( Lour ), Merr Suppression of DMBA-induced carcinogenesis of breast cancer in post initition stage by ethanolic extract. Carcinogenesis 18, 169–175. Magnano, S., Barroeta, H., Duffy, R., O’Sullivan, J. and Zisterer, D.M. 2021. Cisplatin induces autophagy-associated apoptosis in human oral squamous cell carcinoma (OSCC) mediated in part through reactive oxygen species. Toxicol. Appl. Pharmacol. 427, 115646. Markopoulos, A.K. 2012. Current aspects on oral squamous cell carcinoma. Open Dent. J. 6, 126–130. Mohan, S., Chitturi, R., Ragunathan, Y., Lakshmi, S., Nallusamy, J. and Joseph I. 2016. Minor salivary gland changes in oral epithelial dysplasia and oral squamous cell carcinoma—a histopathological study. J. Clin. Diagn. Res. 10, ZC12–ZC15. Mutiah, R., Indrawijaya, Y.Y. and Puspita D. 2020. Study in silico compounds In 96% ethanol extract of Chrysanthemum cinerariifolium (Trev.) leaves towards alfa estrogen receptors. Indones. J. Cancer Chemoprev. 11, 144. Nandini, D.B. and Subramanyam, R.V. 2011. Nuclear features in oral squamous cell carcinoma: a computer-assisted microscopic study. J. Oral Maxillofac. Pathol. 15, 177–181. Ningsih, I.Y. 2016. Studi Etnofarmasi Penggunaan Tumbuhan Obat Oleh Suku Tengger Di Kabupaten Lumajang Dan Malang, Jawa Timur. Pharmachy 13, 10. Pires, F., Ramos, A., De Oliveira, J.B., Tavares, A., De Luz P.S. and Dos Santos T.C.R. 2013. Oral squamous cell carcinoma: clinicopathological features from 346 cases from a single oral pathology service during an 8-year period. J. Appl. Oral Sci. 21, 460–467. Rathore, K., Alexander, M. and Cekanova, M. 2014. Piroxicam inhibits Masitinib-induced cyclooxygenase 2 expression in oral squamous cell carcinoma cells in vitro. Transl. Res. 164, 158–168. Ribeiro, D.R., Alves, Â.V.F., Dos Santos, E.P., Padilha, F.F., Gomes M.Z., Rabelo, A.S., Cardoso, J.C., Massarioli A., De Alencar, S. and De Albuquerque-Júnior R.L. 2015. Inhibition of DMBA-induced oral squamous cells carcinoma growth by Brazilian Red Propolis in rodent model. Basic Clin. Pharmacol. Toxicol. 117, 85–95. Rivera, C. and Venegas B. 2014. Histological and molecular aspects of oral squamous cell carcinoma. Oncol. Lett. 8, 7–11. Salminen, A., Lehtonen, M., Suuronen, T., Kaarniranta, K. and Huuskonen, J. 2008. Terpenoids: natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell. Mol. Life Sci. 65(19), 2979–2999. Scheff N.N., Ye,Y., Bhattacharya, A., MacRae, J., Hickman, D.N., Sharma, A.K., Dolan J.C. and Schmidt B.L. 2017. Tumor necrosis factor alpha secreted from oral squamous cell carcinoma contributes to cancer pain and associated inflammation. Pain 158(12), 2396–2409. Setiawati, T. 2019. Pengenalan Khasiat Obat Tanaman Krisan Dan Pembuatan Teh Krisan Sebagai Minuman Kesehatan. ETHOS 7, 64–69. Shahrajabian M.H., Sun W., Zandi P. and Cheng Q. 2019. A review of chrysanthemum, the eastern queen in traditional chinese medicine with healing power in modern pharmaceutical sciences. Appl. Ecol. Environ. Res. 17, 13355–13369. Sharma, S., Boston, S.E., Skinner, O., Perry, J.A., Verstraete, F.J.M., Lee, D., Van Stee, L.L.L., Thompson, C., Boylan, M., McKee, T. and Bergman P.J. 2021. Survival time of juvenile dogs with oral squamous cell carcinoma treated with surgery alone: a veterinary society of surgical oncology retrospective study. Vet. Surg. 50, 740–747. Siddiqui, S., Rahman, S., Rupasinghe, H.P. and Vazhappilly C. 2020. Dietary flavonoids in p53-mediated immune dysfunctions linking to cancer prevention. Biomedicines 8, 1–31. Singh, P., Rai, A., Verma, A., Alsahli, M.A., Rahmani, A., Almatroodi, S.A., Alrumaihi, F., Dev, K., Sinha, A., Sankhwar, S. and Dohare R. 2021. Survival-based biomarker module identification associated with oral squamous cell carcinoma (OSCC). Biology (Basel) 10(8), 760. Spagnuolo, C., Russo, G., Orhan, I., Habtemariam, S., Daglia, M., Sureda, A., Nabavi, S., Devi, K N., Loizzo, M R., Tundis R. and Nabavi S. 2015. Genistein and cancer: current status, challenges, and future directions. Adv. Nutr. 6, 408–419. Speight, P.M. 2007. Update on oral epithelial dysplasia and progression to cancer. Head Neck Pathol. 1, 61–66. Suresh, G., Koppad R., Prakash B., Sabitha K. and Dhara P. 2019. Prognostic indicators of oral squamous cell carcinoma. Ann. Maxillofac. Surg. 9, 364–370. Takkem, A., Barakat, C., Zakaraia, S., Zaid, K., Najmeh, J., Ayoub, M. and Seirawan M. 2018. Ki-67 prognostic value in different histological grades of oral epithelial dysplasia and oral squamous cell carcinoma. Asian Pac. J. Cancer Prev. 19, 3279–3286. Tołoczko-Iwaniuk, N., Dziemiańczyk-Pakieła, D., Celińska-Janowicz, K., Zaręba, I., Klupczyńska, A., Kokot, Z.J., Nowaszewska, B., Reszeć J., Borys J. and Miltyk W. 2020. Proline-dependent induction of apoptosis in oral squamous cell carcinoma (OSCC)—the effect of celecoxib. Cancers (Basel) 12(1), 136. Wang, C., Liang, H. and Zen, K. 2014. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front. Immunol. 5, 614. Warshawsky, D. and Killblane C. 2005. Molecular carcinogenesis and the molecular biology of human cancer, molecular carcinogenesis and the molecular biology of human cancer. CRC Press. Yakob, M., Fuentes, L., Wang, M.B., Abemayor, E. and Wong, D.T.W. 2014. Salivary biomarkers for detection of oral squamous cell carcinoma: current state and recent advances. Curr. Oral Heal. Rep. 1, 133–141. Yulianti, H. and Hernowo, B.S. 2015. Hubungan antara Imunoekspresi Ki-67 dan risiko agresivitas tumor pada gastrointestinal stromal tumor. Maj. Kedokt. Bandung 47, 231–236. Zaccone, R., Renzi, A., Chalfon, C., Lenzi, J., Bellei, E., Marconato, L., Ros, E., Rigillo, A., Bettini, G., Faroni, E., Guerra, D. and Sabattini, S. 2022. Environmental risk factors for the development of oral squamous cell carcinoma in cats. J. Vet. Intern. Med. 36, 1398–1408. Zhang, W., Yin, G., Dai, J., Sun, Y., Hoffman, R., Yang, Z. and Fan Y. 2017. Chemoprevention by quercetin of oral squamous cell carcinoma by suppression of the NF-B signaling pathway in DMBA-treated hamsters. Anticancer Res. 37, 4041–4050. | ||
| How to Cite this Article |
| Pubmed Style Listiyana A, Kristanti RA, Rumondang AMF, Ahmad APM, Astari LF, Indradmojo C, Inayatilah FR. Effect of ethanol extract from Chrysanthemum cinerariiforium leaves on Ki-67 proliferation and dysplasia severity in a rat model of oral squamous cell carcinoma. Open Vet J. 2023; 13(1): 99-107. doi:10.5455/OVJ.2023.v13.i1.10 Web Style Listiyana A, Kristanti RA, Rumondang AMF, Ahmad APM, Astari LF, Indradmojo C, Inayatilah FR. Effect of ethanol extract from Chrysanthemum cinerariiforium leaves on Ki-67 proliferation and dysplasia severity in a rat model of oral squamous cell carcinoma. https://www.openveterinaryjournal.com/?mno=128054 [Access: July 03, 2025]. doi:10.5455/OVJ.2023.v13.i1.10 AMA (American Medical Association) Style Listiyana A, Kristanti RA, Rumondang AMF, Ahmad APM, Astari LF, Indradmojo C, Inayatilah FR. Effect of ethanol extract from Chrysanthemum cinerariiforium leaves on Ki-67 proliferation and dysplasia severity in a rat model of oral squamous cell carcinoma. Open Vet J. 2023; 13(1): 99-107. doi:10.5455/OVJ.2023.v13.i1.10 Vancouver/ICMJE Style Listiyana A, Kristanti RA, Rumondang AMF, Ahmad APM, Astari LF, Indradmojo C, Inayatilah FR. Effect of ethanol extract from Chrysanthemum cinerariiforium leaves on Ki-67 proliferation and dysplasia severity in a rat model of oral squamous cell carcinoma. Open Vet J. (2023), [cited July 03, 2025]; 13(1): 99-107. doi:10.5455/OVJ.2023.v13.i1.10 Harvard Style Listiyana, A., Kristanti, . R. A., Rumondang, . A. M. F., Ahmad, . A. P. M., Astari, . L. F., Indradmojo, . C. & Inayatilah, . F. R. (2023) Effect of ethanol extract from Chrysanthemum cinerariiforium leaves on Ki-67 proliferation and dysplasia severity in a rat model of oral squamous cell carcinoma. Open Vet J, 13 (1), 99-107. doi:10.5455/OVJ.2023.v13.i1.10 Turabian Style Listiyana, Anik, Risma Aprinda Kristanti, Al Mazida Fauzil Rumondang, Anggun Putri Maulana Ahmad, Lina Fitria Astari, Christyaji Indradmojo, and Fidia Rizkiah Inayatilah. 2023. Effect of ethanol extract from Chrysanthemum cinerariiforium leaves on Ki-67 proliferation and dysplasia severity in a rat model of oral squamous cell carcinoma. Open Veterinary Journal, 13 (1), 99-107. doi:10.5455/OVJ.2023.v13.i1.10 Chicago Style Listiyana, Anik, Risma Aprinda Kristanti, Al Mazida Fauzil Rumondang, Anggun Putri Maulana Ahmad, Lina Fitria Astari, Christyaji Indradmojo, and Fidia Rizkiah Inayatilah. "Effect of ethanol extract from Chrysanthemum cinerariiforium leaves on Ki-67 proliferation and dysplasia severity in a rat model of oral squamous cell carcinoma." Open Veterinary Journal 13 (2023), 99-107. doi:10.5455/OVJ.2023.v13.i1.10 MLA (The Modern Language Association) Style Listiyana, Anik, Risma Aprinda Kristanti, Al Mazida Fauzil Rumondang, Anggun Putri Maulana Ahmad, Lina Fitria Astari, Christyaji Indradmojo, and Fidia Rizkiah Inayatilah. "Effect of ethanol extract from Chrysanthemum cinerariiforium leaves on Ki-67 proliferation and dysplasia severity in a rat model of oral squamous cell carcinoma." Open Veterinary Journal 13.1 (2023), 99-107. Print. doi:10.5455/OVJ.2023.v13.i1.10 APA (American Psychological Association) Style Listiyana, A., Kristanti, . R. A., Rumondang, . A. M. F., Ahmad, . A. P. M., Astari, . L. F., Indradmojo, . C. & Inayatilah, . F. R. (2023) Effect of ethanol extract from Chrysanthemum cinerariiforium leaves on Ki-67 proliferation and dysplasia severity in a rat model of oral squamous cell carcinoma. Open Veterinary Journal, 13 (1), 99-107. doi:10.5455/OVJ.2023.v13.i1.10 |