| Case Report | ||
Open Vet J. 2024; 14(2): 743-749 Open Veterinary Journal, (2024), Vol. 14(2): 743-749 Case Report Simultaneous surgical repair of a cardiac myxoma causing left ventricular outflow tract obstruction and a ventricular septal defect in a small dogKippei Mihara1, Isamu Kanemoto1*, Takanori Ando1,2, Koudai Kawase1,3, Kazuhito Iguchi1,4, Satoko Yokoyama1,5, Atsushi Asai6 and Katsuichiro Hoshi71Cardiac Surgery Team, Chayagasaka Animal Hospital, Nagoya, Aichi, Japan 2Heart-Will Animal Hospital, Kita-Kyushu, Hukuoka, Japan 3Sapporo Night Animal Hospital Emergency and Critical Care, Sapporo, Hokkaido, Japan 4Momijiyama Douri Hospital Pet Clinic, Tokyo, Japan 5Miyashita Animal Hospital, Kure, Hiroshima, Japan 6Asai Animal Hospital, Niigata, Japan 7Mituke Animal Hospital, Mituke, Niigata, Japan *Corresponding Author: Isamu Kanemoto. Chayagasaka Animal Hospital, Nagoya, Aichi, Japan. Email: kanemoto [at] ta2.so-net.ne.jp Submitted: 10/10/2023 Accepted: 21/01/2024 Published: 29/02/2024 © 2024 Open Veterinary Journal
AbstractBackground: Cardiac myxomas are benign tumours that can occur in any heart chamber or valve. They are extremely rare in dogs. We present a novel case involving a cardiac myxoma in the left ventricular outflow tract (LVOT) and a ventricular septal defect (VSD) in a small dog. Case Description: A female miniature dachshund (age, 7 months; weight, 2.88 kg) presented with growth insufficiency, lethargy, and a cardiac murmur. Echocardiography revealed a small polypoid mass in the LVOT and a membranous VSD. Simultaneous surgeries were performed to resect the mass (aortotomy) and close the VSD (right atriotomy) using low-flow cardiopulmonary bypass with surface-cooling hypothermia and retrograde cardioplegia. The tumour was histopathologically identified as a myxoma. The dog survived with no cardiac complications for 11 years after surgery. Conclusion: To our knowledge, this is the first report of ante-mortem diagnosis and simultaneous surgical repair of a cardiac myxoma obstructing the LVOT and a VSD in a small-breed dog. In addition to describing this complicated case, this report presents what we believe is the first reported use of retrograde cardioplegia during open-heart surgery in a small-breed dog. Keywords: Cardiac myxoma, Left ventricular outflow tract obstruction, Retrograde cardioplegia, Small-breed dog, Ventricular septal defect. IntroductionCardiac myxomas are benign tumours that can occur in any heart chamber or valve. According to Machida et al. (2023), the tricuspid valve in the right heart chamber is the most common site of occurrence of cardiac myxoma in dogs. They are extremely rare in dogs. According to Ware and Hopper (1999), a veterinary medical database search from 1982 to 1995 identified 1,383 dogs with tumours of the heart out of 729,265 dogs (0.19% incidence). However, there are no reports on cardiac myxoma incidence. As noted by Mellish et al. (2022), none of the cardiac tumours in three retrospective case series involving 1,775 dogs were myxomas (Walter and Rudolph, 1996; Ware and Hopper, 1999; Aupperle et al., 2007). In fact, only 12 cases of cardiac myxoma in dogs have been published, nine involving the right side of the heart (Roberts, 1959; Darke and Gordon, 1974; Bright et al., 1990; Ori et al., 1994; Machida et al., 2003; Akkoc et al., 2007; Šimundić et al., 2019; Alfaro et al., 2020; Mellish et al., 2022) and three involving the left side (Fernandez-del Palacio et al., 2011; de Nijs et al., 2016; Stack et al., 2021). In two reports, left-sided cardiac myxomas obstructed the left ventricular outflow tract (LVOT), which can result in sudden death (de Nijs et al., 2016; Stack et al., 2021). To date, there are no reports of LVOT myxomas in dogs with a ventricular septal defect (VSD). Successful surgical repair of a myxoma in the right ventricular outflow tract has been reported for a large dog (Bright et al., 1990), whereas surgical repair of an LVOT myxoma has not been reported for any dog. The present report describes the diagnosis of an LVOT myxoma and VSD in a small dog and their simultaneous surgical repair using low-flow cardiopulmonary bypass (CPB) with surface-cooling hypothermia (sHT). Case DetailsA seven-month-old miniature dachshund weighing 2.88 kg was presented to our hospital for a heart murmur, lethargy, and poor weight gain. Examination findingsAuscultation revealed a heart rate of 150 beats/minutes and a grade 4/6 systolic murmur in the precordial region near the sternum. Electrocardiography revealed a mean electrical axis of +82º and a mitral P wave in the II, III, and aVF leads. Thoracic radiography showed moderate heart enlargement (vertebral heart score, 10 vertebrae; cardio-thoracic ratio [CTR]: 70%) and increased opacity of the pulmonic field. B-mode echocardiography revealed a small polypoid mass at the dorsal part of the interventricular septum below the aortic valve (Fig. 1a). Two areas of flow acceleration were evident, leading to the mosaic pattern on colour flow Doppler echocardiography: one due to a membranous VSD of 3.17 mm in diameter and the other due to LVOT obstruction caused by the mass (Fig. 1b). In the continuous-wave Doppler (CWD) mode, the LVOT blood flow velocity was 4.55 m/seconds, with a calculated systolic pressure gradient (ΔP) of 83 mmHg between the left ventricle (LV) and aorta (Fig. 2a), and the VSD blood flow velocity was 4.55 m/seconds, with a calculated systolic ΔP of 83 mmHg between the LV and right ventricle (RV) (Fig. 2b). Aortic regurgitation (AR) (Fig. 2a) was also observed. In the CWD mode, the AR blood flow velocity was 3.11 m/seconds, with a calculated diastolic ΔP of 39 of mmHg between the aorta and LV (Fig. 2a). Based on the results of these examinations, the dog was diagnosed with a small membranous VSD, moderate LVOT obstruction due to a small polypoid mass, mild AR, and slight PR. Due to the predicted risk of worsening obstruction or embolism of the mass and arrhythmias leading to sudden death, resection of the tumour in the LVOT and closure of the VSD were proposed and agreed upon by the dog’s owner. Surgical methodsWe performed low-flow CPB with sHT as previously described by Kanemoto et al. (2010) and (2021). The dog was anesthetized and placed in the left recumbent position. A venous cannula (12 Fr) and arterial cannula (8 Fr) were inserted into the right jugular vein and right carotid artery, respectively, and both were connected to the CPB circuit. After right 4th intercostal space thoracotomy, the azygos vein was ligated, followed by wrapping a tourniquet around the cranial and caudal vena cava and inserting a one-stage drainage cannula (12 Fr) from the right atrial appendage to the caudal vena cava via the right atrium (Fig. 3). After fixing an aortic root cannula for cardioplegia, the cranial and caudal vena cava were snared, and the pump was started. An aortic cross clamp (ACC) was applied, and a cardioplegic solution (St. Thomas No. 2 solution, 10 mL/kg, 4°C) was manually infused antegrade into the coronary artery via the aortic root cannula using a syringe. The right atrium was opened transversely, and a balloon-tipped urethral catheter (6F) was inserted into the coronary sinus ostium (CSO) (Fig. 3a). Using this catheter, the cardioplegic solution was infused retrograde into the coronary vein (first infusion, 10 ml/kg; additional infusions every 20 minutes, 5 ml/kg). We found a membranous VSD underneath the cranial part of the septal leaflet of the tricuspid valve (Fig. 3a), communicating on the LV side between the aortic valve and the mass. We then performed the oblique aortotomy and found a pinkish fibrous tumour (10 × 8 × 4 mm) immediately below the right coronary cusp (RCC) (Fig. 3b). Firstly, a polypropylene suture (Prolene; Ethicon) was placed into the fibrous tumour, and gentle traction on the tumour allowed it to be dissected free from the underlying septal myocardium using ophthalmic scissors. To directly close the VSD foramen, two mattress sutures of 5–0 Prolene with expanded polytetrafluoroethylene plegets were placed between the right side of the lower edge of the VSD foramen and the annulus of the tricuspid valve and tied (Fig. 3a). Finally, the aorta was closed using double continuous Prolene 5–0 sutures. After removing the air from the LV and releasing the ACC, self-beating was immediately restored by applying two DC countershocks (initially 5 joules, secondarily continued with 10 joules). The right atrium and chest were closed in a routine manner. The ACC time was 65 minutes, the lowest oesophageal temperature was 21.1°C, and the pump time was 2 hours and 30 minutes. Tracheal extubation was performed 3 hours after surgery. For surgical prophylaxis, cefazolin sodium was administered 20 mg/kg intravenously just before and after surgery. Postoperatively, it was administered subcutaneously 10 mg/kg twice/day for 3 days and orally for 4 days.
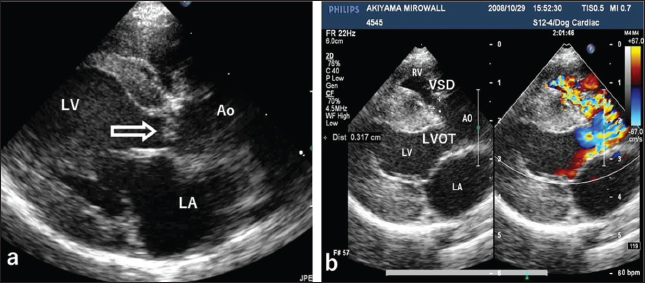
Fig. 1. Echocardiography of the right parasternal long-axis view. (a) B-mode echocardiography shows a small polypoid mass (arrow) at the top of the interventricular septum below the aortic valve. (b) Color Doppler echocardiography of the same view shows two mosaic flows during systole due to a membranous ventricular septal defect (VSD, 3.17 mm in diameter) at the top of the ventricular septum and obstruction of the left ventricular outflow tract (LVOT), respectively. LV: left ventricle, AO: aorta, LA: left atrium, RV: right ventricle.
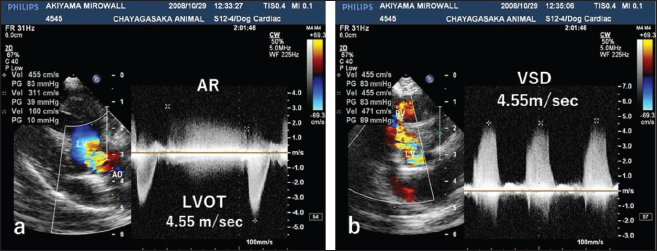
Fig. 2. CWD echocardiography of the right parasternal long-axis view. (a) The blood flow velocity in the left ventricular outflow tract (LVOT) was 4.55 m/seconds, with a calculated systolic pressure gradient (ΔP) of 83 mmHg between the LV and aorta (AO). The AR blood velocity was 3.11 m/seconds, with a calculated diastolic ΔP of 39 mmHg between the LV and AO. (b) The blood flow velocity in the ventricular septal defect (VSD) was 4.55 m/seconds, with a calculated systolic ΔP of 83 mmHg between the LV and RV. The peak velocity measurement of LVOT, AR, and VSD is not accurate, because of measurements taken using the “beard” instead of the “chin” (see “Study Limitations”).
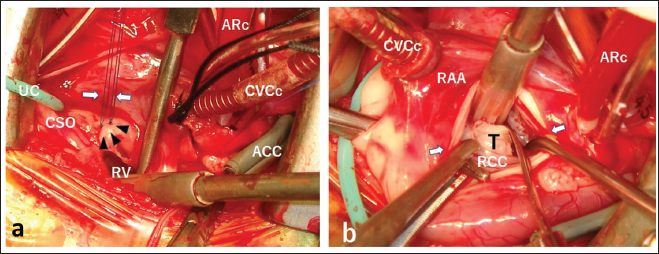
Fig. 3. Surgical procedures and views. (a) After right atriotomy, a balloon-tipped urethral catheter (UC) was inserted into the CSO for retrograde cardioplegia. A membranous VSD foramen (three black-headed arrows) was found under the cranial part of the septal leaflet of the tricuspid valve. Two white arrows show two ligated 5–0 Prolene sutures using the mattress suture technique for direct closure of VSD. (b) After oblique aortotomy (arrows), a pinkish fibrous tumour (T) (10 × 8 × 4 mm) was observed immediately below the RCC. RV: right ventricle, ACC: aortic cross clamp, CVCc: caudal vena cava cannula, ARc: aortic root cannula, RAA: right atrial appendage.
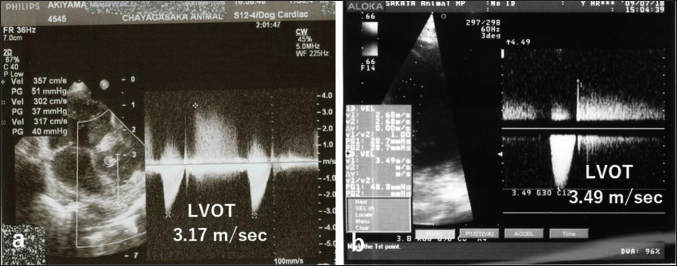
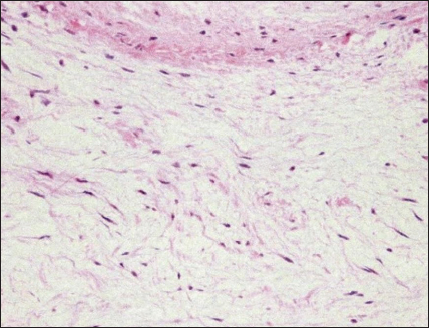
Fig. 4. CWD echocardiography after surgery. (a) Five days after surgery, the systolic pressure gradient (ΔP) between the LV and aorta (AO) had decreased from 83 mmHg (the preoperative value) to 40 mm Hg. The corresponding values for left ventricular outflow tract (LVOT) velocity were 4.55 and 3.17 m/seconds, respectively. (b) Thereafter, the values were essentially unchanged and were 48.7 mmHg (ΔP) and 3.49 m/seconds (LVOT) at 8.5 months. Postoperative courseThe dog was monitored daily with physical examinations, including echocardiography, and the postoperative course was good. However, the dog remained in our hospital for 10 days until discharge to accommodate the owner, who lived remotely. Postoperative auscultation revealed a persistent grade 2/6 systolic murmur, and colour Doppler echocardiography revealed slight residual shunt blood flow through the VSD. As shown via thoracic radiography, the CTR was lower after surgery (66% at 10 days and 65.9% at 8.5 months) than before surgery (70%). As shown via CWD echocardiography, the systolic ΔP between the LV and aorta was also lower after surgery (40 mmHg at 5 days) than before surgery (83 mmHg) (Fig. 4a); after 5 days, it remained essentially unchanged and was 48.7 mmHg at 8.5 months (Fig. 4b). At 8.5 months, the dog had no clinical signs of heart disease and her body weight had increased to 3.9 kg. According to the referral hospital, the dog had no cardiac complications during the next 11 years. Histopathological examinationHaematoxylin and eosin staining showed spindle-shaped fibroblast-like cells sparsely distributed throughout a faintly eosinophilic myxoid matrix (Fig. 5). The tumour was diagnosed as a myxoma (mesenchymal benign tumour), and histopathology confirmed tumour-free margins. DiscussionDiagnosis and evaluationEchocardiography is the best tool for the detection of intracardiac tumour, especially obstructive myxomas (Bright et al., 1990; Ori et al., 1994; Fernandez-del Palacio et al., 2011; Šimundić et al., 2019; Stack et al., 2021; Mellish et al., 2022). In the present case, auscultation, electrocardiography, and thoracic radiography were also useful. B-mode, colour (Fig. 1a, b), and CWD (Fig. 2a, b) echocardiography clearly revealed a small polyploid mass that moderately obstructed the LVOT, as well as AR and a membranous VSD. The VSD likely caused the AR. In our study, CWD echocardiography was especially useful for monitoring blood flow velocity by calculating ΔP before and after surgery. As determined using this modality, the systolic ΔP between the LV and aorta was 83 mmHg immediately before surgery. According to the referral hospital, it was 50.7 mmHg 2 months before surgery. This slow increase in the ΔP presumably reflected the slow growth of the tumour, which was likely benign (Han et al., 2012). Five days after surgery, the ΔP had decreased to 40 mmHg (Fig. 4a), with little change over the next 8.5 months (Fig. 4b). Although echocardiography may indicate whether a tumour is benign or malignant, biopsy is required for definitive diagnosis of a cardiac myxoma. Open heart surgeryOpen heart surgery is more difficult in small dogs than in big dogs (Kanemoto et al., 2021). The preoperative body weight of the dog in our case was 2.88 kg, and two anomalies, namely a tumour in the LVOT and a membranous VSD, were present. The treatment method used in our case was low-flow CPB combined with sHT (Kanemoto et al., 2010). Difficulties are expected when two surgical procedures are performed simultaneously; however, both operations were safely and successfully completed. Therefore, low-flow CPB with sHT appears to be a viable perfusion technique during open-heart surgery for small-breed dogs.
Fig. 5. Histopathology of the cardiac myxoma. Spindle-shaped fibroblast-like cells are sparsely distributed throughout a faintly eosinophilic myxoid matrix. Haematoxylin and eosin, 200× magnification. In the present case, cardioplegia was essential to minimize the risk of air embolization through the VSD or aortotomy. To perform cardioplegia, a cardioplegic solution was first perfused antegrade into the coronary artery and then continued retrograde into the coronary sinus; this procedure was effective. In this case, direct coronary ostia perfusion was difficult owing to the narrow surgical view in the aorta. For retrograde cardioplegia, the infusion pressure must not exceed 40 mmHg; otherwise, damage to the coronary vein, myocardial bleeding, and oedema may occur (Lolley and Hewitt, 1980). In our case, two direct pledgeted mattress sutures were used to close the small membranous VSD; this method was chosen because it is easy to perform and causes minimal damage to the cardiac conduction system in small-breed dogs with narrow surgical views (Ohara et al., 2001). Although the patch method has been used to close large VSDs in humans, a complete right bundle branch block may occur postoperatively (Fukuda et al., 2002). In the study by Shimizu et al. (2007), three mattress sutures were used to close a large subpulmonary valve-type VSD in a small dog undergoing CPB. In our case, slight residual shunt blood flow through the VSD was detected via colour Doppler echocardiography after surgery but was not considered clinically problematic. No damage to the cardiac conduction system was observed. There are two reports of surgically treated cardiac myxomas in dogs. In one, the myxoma originated in the tricuspid valve and was resected using CPB; the dog died 36 hours after surgery (Machida et al., 2003). The other report included two cases of myxoma, both in the right ventricular outflow tract (Bright et al., 1990). In one case, the dog was euthanized at the owner’s request. On the other hand, the myxoma was surgically removed via pulmonary arteriotomy using venous inflow occlusion; the dog had no cardiac signs for 2 years postoperatively. The dog in our case survived without cardiac complications for 11 years after surgery, at which time she died of a non-cardiac disease. Early diagnosis and removal of benign cardiac tumours may permit recovery in selected cases (Bright et al.,1990; de Nijs et al., 2016). To our knowledge, this is the first report of ante-mortem diagnosis and simultaneous surgical repair of a cardiac myxoma causing LVOT obstruction and a membranous VSD in a small dog (weight, <3 kg). In addition to describing this complicated case, this report presents what we believe is the first documentation of retrograde cardioplegia during open-heart surgery in a small-breed dog. Study LimitationsFor CWD echocardiography, Kyranis et al. (2018) identified the measurement site for peak velocities on Doppler wave to ensure the exact value is taken at the “chin” and not the “beard”. In this case (Fig. 2a and b), we measured the peak velocities at the beard, not the chin. Therefore, the exact volume appears to be slightly lower. However, this cannot be corrected because of a lack of original image data. On echocardiographic assessment of the LVOT, pulsed-wave Doppler (PWD) is used for LVOT flow evaluation when no flow acceleration is noted to accurately measure the specific velocity at the region of interest. When there is a fixed obstruction or dynamic obstruction (as is possible in this case owing to the mobile mass) leading to flow acceleration, then aliasing of PWD can occur, which forces us to use CWD for evaluation of the peak velocity and peak gradient (Koplitz et al., 2006). However, in our case, we did not obtain PWD data. AcknowledgmentsThis study was presented in part at the 2010 American College of Veterinary Surgery Symposium in Seattle, WA, USA. We thank Dr. Hideaki Takahashi at Amanecer (www.amanecer.co.jp) for the histopathological diagnosis and Editage (www.editage.jp) for English language editing. Authors contributionsKM and IK performed the examinations and surgical operations. IK wrote the manuscript. TA, KK, KI, and SY assisted with the open-heart surgery. AA and KH were a primary care veterinarian and a board-certified veterinary cardiologist, respectively, who referred the dog to our hospital. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research received no specific grant. Data availabilityThe data that support the findings of this study are available from the authors upon request. ReferencesAkkoc, A., Ozyigit, M.O. and Cangul, I.T. 2007. Valvular cardiac myxoma in a dog. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 54, 356–358. Alfaro, L., Enciso, A. and Enciso, J. 2020. Cardiac myxoma in the pulmonary trunk of a canine: presence of mesenchymal stem-like cells. Thai J. Vet. Med. 50, 431–434. Aupperle, H., März, I., Ellenberger, C., Buschatz, S., Reischauer, A. and Schoon, H.A. 2007. Primary and secondary heart tumours in dogs and cats. J. Comp. Pathol. 136, 18–26. Bright, J.M., Toal, R.L. and Blackford, L.A.M. 1990. Right ventricular outflow obstruction caused by primary cardiac neoplasia. Clinical features in two dogs. J. Vet. Intern. Med. 4, 12–16. Darke, P.G. and Gordon, L.R. 1974. Cardiac myxoma in a dog. Vet. Rec. 95, 565–567. de Nijs, M.I., Vink, A., Bergmann, W. and Szatmári, V. 2016. Left ventricular cardiac myxoma and sudden death in a dog. Acta Vet. Scand. 58, 41–44. Fernandez-del Palacio, M.J., Sanchez, J., Talavera, J. and Martínez, C. 2011. Left ventricular inflow tract obstruction secondary to a myxoma in a dog. J. Am. Anim. Hosp. Assoc. 47, 217–223. Fukuda, T., Suzuki, T., Kashima, I., Sato, M. and Morikawa, Y. 2002. Shallow stitching close to the rim of the ventricular septal defect eliminates injury to the right bundle branch. Ann. Thorac. Surg. 74, 550–555. Han, D., Kim, H. and Hyun, C. 2012. Echocardiographic diagnosis of intracardiac masses in Yorkshire Terrier dogs: 2 cases. J. Vet. Clin. 29, 483–487. Kanemoto, I., Mihara, K. and Sato, K. 2021. Open-heart techniques and mitral valve plasty for mitral regurgitation in toy- and small-breed dogs: a review. Open Vet. J. 11, 14–26. Kanemoto, I., Taguchi, D., Yokoyama, S., Mizuno, M., Suzuki, H. and Kanamoto, T. 2010. Openheart surgery with deep hypothermia and cardiopulmonary bypass in small and toy dogs. Vet. Surg. 39, 674–679. Koplitz, S.L., Meurs, K.M. and Bonagura, J.D. 2006. Echocardiographic assessment of the left ventricular outflow tract in the Boxer. J. Vet. Intern. Med. 20, 904–911. Kyranis, S.J., Latona, J., Platts, D., Kelly, N., Savage, M., Brown, M., Hamilton-Craig, C., Scalia, G.M. and Burstow, D. 2018. Improving the echocardiographic assessment of pulmonary pressure using the tricuspid regurgitant signal—the “chin” vs. the “beard”. Echocardiography 35, 1085–1096. Lolley, D.M. and Hewitt, R.L. 1980. Myocardial distribution of asanguineous solutions retroperfused under low pressure through the coronary sinus. J. Cardiovasc. Surg. (Torino). 21, 287–294. Machida, N., Hoshi, K., Kobayashi, M., Katsuda, S. and Yamane, Y. 2003. Cardiac myxoma of the tricuspid valve in a dog. J. Comp. Pathol. 129, 320–324. Machida, N., Sasaki, T. and Kimura, Y. 2023. Pathological features of primary cardiac myxoid tumour in dogs: a review of 11 cases (2002–2020). J. Comp. Pathol. 207, 50–58. Mellish, C., Côtĕ, É., Aburto, E. and Lichtenberger, J. 2022. Mineralized, obstructive cardiac myxoma with chondroid differentiation in a cocker spaniel. Can. Vet. J. 63, 411–415. Ohara, K., Kanemoto, I., Masumoto, T., Suzuki, H., Itoh, T. and Nakanishi, A. 2001. A case of ventricular septal defect in a dog surgically repaired under surface-induced hypothermia. J. Jpn. Vet. Med. Assoc. 54, 380–382. Ori, J., Yamaguchi, T., Sasa, Y., Komiya, M. and Narama, I. 1994. A case of canine cardiac myxoma. J. Jpn. Vet. Med. Assoc. 47, 499–501. Roberts, S.R. 1959. Myxoma of the heart in a dog. J. Am. Vet. Med. Assoc. 134, 185–188. Shimizu, M., Tanaka, R., Hoshi, K., Hirao, H., Kobayashi, M., Shimamura, S. and Yamane, Y. 2006. Surgical correction of ventricular septal defect with aortic regurgitation in a dog. Aust. Vet. J. 84, 117–121. Šimundić, M., Domanjko Petrič, A., Pavlin, D., Zemljič, T., Firm, I., Gombač, M., Srečnik, Š., Stojov, M., Šimenc, L. and Švara, T. 2019. Cardiac myxoma in a dog. Slov. Vet. Res. 56, 133–138. Stack, J.P., Fries, R.C. and Samuelson, J.P. 2021. Subaortic cavitated myxoma causing severe left ventricular outflow tract obstruction in a young dog. Case Rep. 5, 340–345. Walter, J.H. and Rudolph, R. 1996. Systemic, metastatic, eu- and heterotope tumours of the heart in necropsied dogs. Zentralbl. Veterinarmed. A. 43, 31–45. Ware, W.A. and Hopper, D.L. 1999. Cardiac tumours in dogs: 1982–1995. J. Vet. Intern. Med. 13, 95–103. | ||
| How to Cite this Article |
| Pubmed Style Mihara K, Kanemoto I, Ando T, Kawase K, Iguchi K, Yokoyama S, Asai A, Hoshi K. Simultaneous surgical repair of a cardiac myxoma causing left ventricular outflow tract obstruction and a ventricular septal defect in a small dog. Open Vet J. 2024; 14(2): 743-749. doi:10.5455/OVJ.2024.v14.i2.15 Web Style Mihara K, Kanemoto I, Ando T, Kawase K, Iguchi K, Yokoyama S, Asai A, Hoshi K. Simultaneous surgical repair of a cardiac myxoma causing left ventricular outflow tract obstruction and a ventricular septal defect in a small dog. https://www.openveterinaryjournal.com/?mno=171570 [Access: June 30, 2025]. doi:10.5455/OVJ.2024.v14.i2.15 AMA (American Medical Association) Style Mihara K, Kanemoto I, Ando T, Kawase K, Iguchi K, Yokoyama S, Asai A, Hoshi K. Simultaneous surgical repair of a cardiac myxoma causing left ventricular outflow tract obstruction and a ventricular septal defect in a small dog. Open Vet J. 2024; 14(2): 743-749. doi:10.5455/OVJ.2024.v14.i2.15 Vancouver/ICMJE Style Mihara K, Kanemoto I, Ando T, Kawase K, Iguchi K, Yokoyama S, Asai A, Hoshi K. Simultaneous surgical repair of a cardiac myxoma causing left ventricular outflow tract obstruction and a ventricular septal defect in a small dog. Open Vet J. (2024), [cited June 30, 2025]; 14(2): 743-749. doi:10.5455/OVJ.2024.v14.i2.15 Harvard Style Mihara, K., Kanemoto, . I., Ando, . T., Kawase, . K., Iguchi, . K., Yokoyama, . S., Asai, . A. & Hoshi, . K. (2024) Simultaneous surgical repair of a cardiac myxoma causing left ventricular outflow tract obstruction and a ventricular septal defect in a small dog. Open Vet J, 14 (2), 743-749. doi:10.5455/OVJ.2024.v14.i2.15 Turabian Style Mihara, Kippei, Isamu Kanemoto, Takanori Ando, Koudai Kawase, Kazuhito Iguchi, Satoko Yokoyama, Atsushi Asai, and Katsuichiro Hoshi. 2024. Simultaneous surgical repair of a cardiac myxoma causing left ventricular outflow tract obstruction and a ventricular septal defect in a small dog. Open Veterinary Journal, 14 (2), 743-749. doi:10.5455/OVJ.2024.v14.i2.15 Chicago Style Mihara, Kippei, Isamu Kanemoto, Takanori Ando, Koudai Kawase, Kazuhito Iguchi, Satoko Yokoyama, Atsushi Asai, and Katsuichiro Hoshi. "Simultaneous surgical repair of a cardiac myxoma causing left ventricular outflow tract obstruction and a ventricular septal defect in a small dog." Open Veterinary Journal 14 (2024), 743-749. doi:10.5455/OVJ.2024.v14.i2.15 MLA (The Modern Language Association) Style Mihara, Kippei, Isamu Kanemoto, Takanori Ando, Koudai Kawase, Kazuhito Iguchi, Satoko Yokoyama, Atsushi Asai, and Katsuichiro Hoshi. "Simultaneous surgical repair of a cardiac myxoma causing left ventricular outflow tract obstruction and a ventricular septal defect in a small dog." Open Veterinary Journal 14.2 (2024), 743-749. Print. doi:10.5455/OVJ.2024.v14.i2.15 APA (American Psychological Association) Style Mihara, K., Kanemoto, . I., Ando, . T., Kawase, . K., Iguchi, . K., Yokoyama, . S., Asai, . A. & Hoshi, . K. (2024) Simultaneous surgical repair of a cardiac myxoma causing left ventricular outflow tract obstruction and a ventricular septal defect in a small dog. Open Veterinary Journal, 14 (2), 743-749. doi:10.5455/OVJ.2024.v14.i2.15 |