| Research Article | ||
Open Vet J. 2024; 14(2): 707-715 Open Veterinary Journal, (2024), Vol. 14(2): 707–715 Original Research Fertility testing of preserved epididymal sperm by microinjection: A model for the rescue and utilization of genetically superior animalsSigit Prastowo1*, Rini Widyastuti2, Jaswandi Jaswandi3 and Arief Boediono41Department of Animal Science, Faculty of Animal Science, Universitas Sebelas Maret, Surakarta, Indonesia 2Department of Animal Production, Faculty of Animal Husbandry, Universitas Padjajaran, Bandung, Indonesia 3Department of Reproduction Biotechnology, Faculty of Animal Science, Universitas Andalas, Padang, Indonesia 4Department of Anatomy, Physiology and Pharmacology, School of Veterinary and Biomedical, IPB University, Bogor, Indonesia *Corresponding Author: Sigit Prastowo. Department of Animal Science, Faculty of Animal Science, Universitas Sebelas Maret, Surakarta, Indonesia. Email: prastowo [at] staff.uns.ac.id Submitted: 07/12/2023 Accepted: 21/01/2024 Published: 29/02/2024 © 2024 Open Veterinary Journal
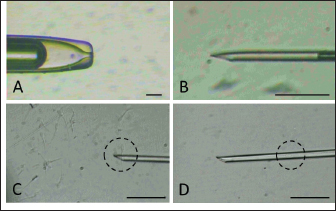
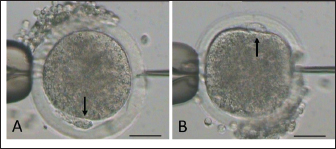
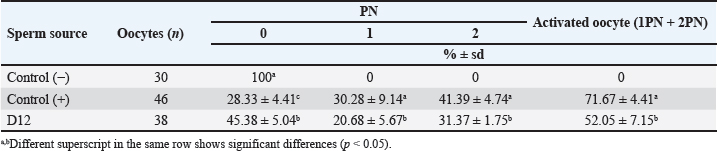
AbstractBackground: Epididymal sperm preservation is a simple conservation approach that can help prevent the loss of high genetic quality of farm animals. The chance of loss increases, especially during disease outbreaks or other interruptions to normal reproduction function. Aim: This study looked into the ability of preserved ram epididymal sperm to fertilize oocytes. Due to motility becoming an issue following sperm storage for fertilization, the sperm microinjection known as intracytoplasmic sperm injection approach was employed. Methods: The study was divided into two parts. First, involved the preservation of epididymal sperm at 5°C for 12 days. During preservation, sperm quality parameters namely motility, viability, intact membrane, acrosome, and Deoxyribonucleic acid (DNA) are evaluated every three days. For the fertility test in the second experiment, matured oocytes were injected with immotile sperm discovered in the last days of preservation. The presence of pronucleus development following in vitro culture is used as an indicator of sperm’s ability to activate and fertilize oocytes. Results: All sperm quality parameters significantly (p < 0.05) declined during preservation time. On day 12, motility was discovered to be 0%, but viable sperm, sperm with intact membrane, acrosome, and DNA remained at 41.86% ± 9.30%, 31.18% ± 5.15%, 21.88% ± 1.93%, and 33.35% ± 8.74%, respectively. On the fertility test, we inject immotile sperm from day 12 of preservation, which has the lowest motility found, into matured oocytes. Those sperms are able to activate (52.05% ± 7.15%) and fertilize (31.37% ± 1.75%) the injected oocytes, but their fertilizing ability is significantly lower (p < 0.05) when compared to the sperm derived from the ejaculate. Conclusion: In this study, simple preservation of epididymal sperm reduces all sperm quality criteria, particularly motility. Using the microinjection approach preserved sperm which had no motility, still demonstrated its ability to activate and fertilize the oocytes. According to that, this study provides potential approaches and tools for using genetically superior animals that have lost their ability to execute regular fertilization, and also prolong reproduction function. Keywords: Epididymal sperm, Genetic rescue, Sperm microinjection, Sperm preservation. IntroductionIn animal reproduction, the fragility of male genetic material can be jeopardized by many factors, ranging from diseases to accidents. The consequence of the loss of such genetic material extends beyond the individual, posing potential economic implications when the male has a high genetic quality (elite sire) or is an endangered species. Diseases and accidents, both unexpected and unavoidable, can cause damage to the male reproductive system, culminating in the inability to achieve successful fertilization. Addressing these critical issues, the preservation of epididymal sperm emerges as a promising technique to safeguard male genetic material. Simple sperm preservation methods have the potential to prolong sperm viability and mitigate the risk of reproductive failure. Nevertheless, the journey toward prolonged reproduction is not without its intricacies. As sperm quality, especially motility experiences a reduction over time, the assistance of advanced reproductive technologies becomes an essential tool. Intracytoplasmic sperm injection (ICSI) is a microinjection technique that involves mechanically injecting a single sperm with the help of a micromanipulator into a mature oocyte (Unnikrishnan et al., 2021). This is an assisted reproduction technology offering a viable solution to overcome the challenges posed by low sperm quality. One of the advantages of ICSI is using immotile sperm because the zona pellucida is mechanically penetrated by an injection needle. In domestic animals, ICSI has been used to study ways to overcome problems that are faced during in vitro fertilization (IVF), such as the inability of spermatozoa to penetrate oocytes, capacitation, and so on. This technique is more efficient than IVF and artificial insemination (AI) because ICSI only uses one intact spermatozoon to fertilize an oocyte. At the same time, IVF and AI require millions of spermatozoa. Successful ICSI fertilization to generate offspring has been well documented in rabbits (Iritani and Hosoi, 1989), cows (Goto et al., 1990), and ultimately in humans (Palermo et al., 1996). Based on these findings, ICSI has been studied and used in cattle (Horiuchi et al., 2002), horses (Lazzaria et al., 2002), sheep (Gómez et al., 1998; Jiménez-Macedo et al., 2006), cats (Comizzoli et al., 2006), pigs (García-Roselló et al., 2006), and quail (Mizushima et al., 2007). These studies have resulted in different embryonic development stages and the birth of live offspring. Rescue and preservation of epididymal spermatozoa can be performed by preservation or cryopreservation (Boediono et al., 2004). Preserving spermatozoa at 5°C is considered a simple and low-cost procedure in the field. However, the consideration of testing the fertilization ability of spermatozoa from preservation is the decline of sperm quality, mainly motility. ICSI could overcome this issue as it does not require motile spermatozoa but is quite viable. Looking at the possibilities for rescue, preservation, and usage of epididymal spermatozoa, this study sought to observe the viability of sheep epidydimal sperm during preservation for several days. Furthermore, the ICSI procedure was used to examine the fertilization ability of matured oocytes. Hence, this study is expected to yield information regarding the storage and fertilization abilities of sheep epididymis spermatozoa, which can then be used for animal embryo production or as a model for rescue, preservation, and use of epididymal spermatozoa in other animals. Materials and MethodsTwo experiments were carried out in this study. The quality of preserved epididymal sperm was assessed, followed by a fertility test using the ICSI method in the second experiment. Experiment 1Epididymal preservation and sperm retrieval During travel to the laboratory, ram epididymis was stored in a 0.9% NaCl solution with the addition of 100 IU/ml Penicillin and 100 g/ml Streptomycin. Once it arrives in the laboratory, epididymis is transferred to the same solution and stored at 5°C for up to 12 days. The preservation solution was changed every three days, and sperm quality parameters were evaluated. There are four time points for observing sperm quality: day 0 (D0), day 3 (D3), day 9 (D9), and day 12 (D12). Epididymal sperms were retrieved by dissecting the cauda and firmly pressing the distal region. A total of 2 μl of sperms was collected and then diluted by 998 μl of pre-warmed (37°C) phosphate buffer saline (PBS) solution. Sperm were carefully mixed using gentle pipetting and left in the heating block for 10 minutes before being examined for quality (Prastowo et al., 2021). Sperm quality evaluationSperm motility The motility of spermatozoa was subjectively observed using a 400× magnification microscope in at least five fields of view. A drop of diluted epididymal sperm was placed on a warmed slide glass (37°C) covered with glass slip. The percentage of motile sperm was scored on a scale of 0% (immotil) to 100% (motil). Sperm viability Sperm viability, which means the percentage of live spermatozoa, was determined by the staining method. In total 2 μl of diluted epididymal sperm were stained with 8 μl of 2% Eosin-Nigrosin staining solution, smeared on slide glass, and air dried. A total of 200 spermatozoa were examined under a 400× magnification microscope. The white or light pink sperm head indicated live sperm, while the red or dark head indicated dead sperm (Prastowo et al., 2021). The percentage of live sperms is then calculated as a percentage of the total number of observed sperms. Sperm membrane integrity The Hypoosmotic Swelling Test (HOST) was used to assess the parameter of sperm membrane integrity (Pérez-Llano et al., 2001). In total 2 μl of diluted epididymal sperm were mixed with 18 μl of hypoosmotic solution (0.90 g D-fructose and 0.490 g Trisodium citrate in 100 ml aquadest with osmolarity 150 m osmoles), which was then incubated at 37°C for 30 minutes. After incubation, 5 μl of sperm stained with 2% Eosin-Nigrosin was smeared on the slide glass, air dried, and examined under a 400× magnification microscope. A minimum of 200 sperms were evaluated. Sperm with intact membranes will have a swollen or coiling tail, whereas damaged sperm will have an unswollen tail (Tartaglione and Ritta, 2004). Sperms with intact membranes were calculated as a percentage of total sperm count. Sperm acrosome intact The acrosome hood’s integrity is determined by mixing epididymal sperm with a formol saline solution. Five micro litter of spermatozoa were added to 10 μl of formolsaline and incubated at 38°C for 10 minutes. The acrosome was then examined using a phase contrast microscope. Intanct acrosomes have a black line color at the head. A minimum of 200 sperms were examined, and the percentage of intact acrosome heads was calculated. Sperm Deoxyribonucleic acid (DNA) integrity Sperm DNA integrity is assessed using a combination staining of Hoechst 33258 and Propidium Iodine (PI) at 1 g/ml concentrations. Based on the satue of the nucleus and membrane, this staining attempts to determine whether sperm cells are alive or dead (Cai et al., 2005). A total of 18 μl of sperm samples plus 1 μl of Hoechst 33258 and 1 μl of PI, were gently mixed using a vortex and incubated in the dark for 10 minutes at 38°C. After incubation, 10 μl of sperm samples were placed on a glass slide and covered before being examined under a fluorescence microscope. Sperm with red heads is considered dead (Martínez-Pastor et al., 2010), whereas sperm with blue to violet heads is considered alive with viable nuclei or Hoechst positive. A total of 200 sperm were examined, and the percentage of sperm with intact DNA was calculated. Experiment 2Oocyte collection and in vitro maturation Ovaries from sheep were collected from a nearby slaughterhouse and transported to the laboratory in a warm (37°C) 0.9% NaCl solution with 100 IU/ml Penicillin and 100 g/ml Streptomicyn. Oocytes were collected from the ovaries by slicing method in a PBS medium supplemented with 5% fetal calf serum (FCS). The oocytes were then selected based on their uniform cytoplasmic states. Compact of cumulus cells (more than two layers), matured in vitro in 50 μl drop of maturation medium containing TCM-199 (Gibco—USA) 10% FCS and 0.01 AU/ml follicle stimulating hormone, coated with mineral oil. Each maturation medium drop contains 10–15 oocytes and is incubated at 38°C 5% CO2 for 20–24 hours. The following day, oocytes were evaluated by observing the polar body I (PB I) extrusion. Only oocytes with PB I were used in ICSI. Before ICSI, expanded cumulus cells are removed by exposing them to 100–300 IU/ml Hyaluronidase (Sigma) for 20–30 seconds with careful pipetting. Handling of sperm and oocytes during ICSI and in vitro culture In the second experiment, we use ICSI to test the fertilizing abilities of immotile sperm extracted from preserved epididymis. Because ICSI requires immotile sperm with an intact plasma membrane, a HOST was performed before ICSI. During the second experiment, three groups were established: control (−), control (+), and sperm from the last day of experiment 1. Oocytes in the control (−) group are mechanically injected with a microinjection needle, while oocytes in the control (+) group are injected with sperm from the ejaculate. Sperm injection was carried out using an inverted microscope equipped with a heating stage and a set of micromanipulators (Narishige-Japan). The manipulation media (PBS plus 5% FCS) was prepared in a three-drop format on the manipulation dish. On the left, a drop of manipulation media containing oocytes is formed and filled with 2−3 oocytes each. On the right, a drop manipulation media containing sperm. Three drops of sperm manipulation (10 μl each) were made elongated, two drops contained manipulation medium, and the third contained 5% PVP, which was used to clean the holding (Fig. 1A) at the left side and injection needles (Fig. 1B) at the right side. The procedure of ICSI begins by immobilizing motile sperm and disrupting the membrane by pressing the tail of the sperm with an injection needle in the midpiece area, followed by sperm aspiration (Fig. 1C and D). The oocyte is held in place and directed so that PB I is at the 6 (Fig. 2A) or 12 (Fig. 2B) o’clock position. Sperm is slowly injected at 3 o’clock (Fig. 2) and withdrawn after the zona pellucida and olema are pierced. Following microinjection, oocytes were washed 2–3 times in culture media (TCM-199 + 10% FCS + 10 μl/ml Gentamycin sulfate), transferred to culture media, and incubated for 18 at 38°C, 5% CO2. Assessment of oocyte development following ICSI The pronucleus (PN) formation of oocytes was evaluated 18 hours after ICSI. To access the PN formation, oocytes were fixed in a solution containing 1 part of ethanol and 3 parts of acetic acid solutions for 48 hours. Oocytes were then stained with acetoorcein for 10–15 minutes, they were fixed on the slide and covered with cover glass. Observation of PN formation was done under a phase contrast microscope. In this study, activated oocytes are identified by the presence of 1 and 2 PN, whereas fertilized oocytes are identified by the presence of only 2 PN. Furthermore, the fertility test results were presented as a ration of developed to the number of injected oocytes per PN development observed.
Fig. 1. Holding pipette (A) and injection needle (B) setting. Sperm (in dashed circle) are immobilized by touching the tail (C) followed by aspiration (D).
Fig. 2. Mature oocyte handled during sperm injection according to its polar body (PB) position, as shown by arrow (A: PB at 6 and B: PB at at 12 o’clock). (bar=50 μm). Table 1. Epididymal sperm quality parameter during preservation.
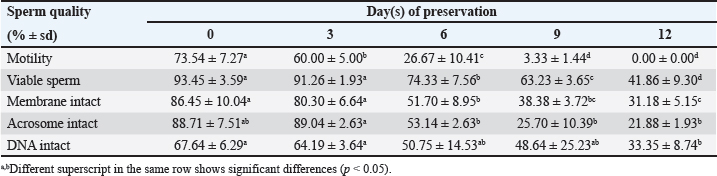
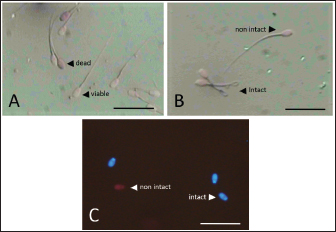
Data Analysis The preservation and microinjection of ram epididymal sperm were repeated three times as a replicate. In this study, statistical analysis was done by analysis of variance, followed by the Duncan test as post hoc analysis. The threshold for significant differences was set at p < 0.05, and all data were presented as mean ± sd. Ethical approval There were no treatments given to the animals in this study. We collected sheep epididymis and ovaries from a local abattoir that follows Indonesian regulations to avoid animal discomfort and pain, as outlined in government law number 18 on animal husbandry and health, which was issued in 2009. A veterinarian also supervises sample collection to ensure that it is suitable for use in the study. ResultsExperiment 1Table 1 shows the quality of epididymal sperm during preservation. It demonstrates that all quality parameters decreased significantly (p < 0.05) from D0 to D12 of preservation. The motility parameter showed a significant decrease (p < 0.05) from D0 to D9, with no motility (0%) observed at D12. Despite the lack of motility at D12, we still see viable (life) sperm, sperm with intact membrane, acrosome, and DNA at 41.86% ± 9.30%, 31.18% ± 5.15%, 21.88% ± 1.93%, and 33.35% ± 8.74%, respectively. Figure 3 shows that viable sperms have a pale and clear head (Fig. 3A). In the HOST result, sperm with intact membranes were characterized by a curved and/or swallow tail (Fig. 3B), whereas sperm with intact DNA emitted blue fluorescence under a microscope (Fig. 3C). We decided to use D12 as the source of preserved sperm for the fertility test in experiment 2 based on the results of experiment 1. The reason was that we discovered the lowest sperm motility in D12 when compared to another day of preservation.
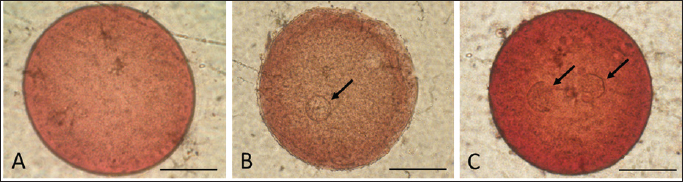
Fig. 3. Photograph of sperm quality parameter. Sperm viability (A), sperm membrane integrity (B), and sperm DNA integrity (C) (Bar=50 μm). Experiment 2Table 2 shows the results of the fertility test using preserved epididymal sperm. There are three treatment groups based on sperm source: no sperm (control −), ejaculate sperm (control +), and immotile epididymal sperm preserved from D12. We found that epididymal sperm is preserved until D12 can still able to activate and fertilize the matured oocyte. We notice that D12 sperm can fertilize matured oocytes by observing 2 PN formation at 31.37% ± 1.75%. On the other hand, D12 sperm can activate oocytes at 52.05% ± 7.15% as shown (accumulation number) by the presence of 1 and 2 PN formation. Moreover, we also found 45.38% ± 5.04% of oocytes which cannot be activated by D12 sperm as 0 PN observed. Furthermore, D12 sperm is comparatively lower when compared to the control (+), as we found more activated (52.05% ± 7.15% vs. 71.67% ± 4.41%; p < 0.05) and fertilized oocyte (31.37% ± 1.75% vs. 41.39% ± 4.74%: p < 0.05). Figure 4 depicts the formation of PN in oocytes following ICSI. Oocytes that did not develop PN (Fig. 4A) were classified as not activated due to activation failure. The oocytes that developed 1PN (Fig. 4B) and 2PN (Fig. 4C) were classified as activated. Only oocytes with 2PN, on the other hand, were considered fertilized. The finding could explain why preserved epididymal sperm has the same ability as ejaculate sperm in activated and fertilized oocytes. It should be noted that immotile sperm used is sperm that shows positive results on the HOST. Table 2. Oocyte development after ICSI.
Fig. 4. Oocyte with no (A), one (B), and two (C) PN (shown by arrows) after sperm injection. (bar=50 μm). We used control (−) to test the effect of mechanical injection on oocyte activation in this study, and we found no formation of PN (Table 2). This suggests that in this study, sperm was the only factor that activated and fertilized matured oocytes. As a result, this demonstrates that ICSI without sperm cannot activate oocytes, as evidenced by the lack of PN development. DiscussionThe simple preservation of epididymal sperm using 0.9% NaCl at 5°C aims to maintain sperm viability by slowing down cell metabolism. Preservation, on the other hand, resulted in a decrease in sperm motility and viability as we discovered in the current study (Table 1). It can be seen that during the preservation process, the motility of epididymal sperm decreased markedly from the first day (D0) to the twelfth day (D12). Earlier studies also reported the same finding of sperm quality decline during preservation time in various animals and different methods in ram (Lone et al., 2011; Paul et al., 2018), in bull (Bertol et al., 2013; Rahimizadeh et al., 2021), in equine (Monteiro et al., 2013), in cat (Mardatillah et al., 2020), and in bucks (Abu et al., 2016) for example. The most plausible explanation for sperm quality decline during preservation is oxidative stress caused by reactive oxygen species (ROS) accumulation. During the preservation process, epididymal sperm continues to carry out metabolism processes to stay alive by carrying out oxidation processes resulting in ROS. As a result, the accumulated ROS causes lipid peroxidation (LPO), which damages the plasma membrane and, as a result, reduces sperm motility, sperm viability, and related sperm membrane function (Baumber et al., 2000; Garrido et al., 2004; Pujianto et al., 2021; Sedaghatizadeh et al., 2023). It is also stated that ROS is produced by the natural mechanism of cells. ROS is an oxidative agent categorized as oxygen-derived free radicals produced by living cells from oxygen metabolism (Sikka, 1996). A number of studies (Sikka, 1996; Ford, 2004; Agarwal et al., 2006; Maneesh and Jayalekshmi, 2006; Aitken et al., 2022) found that free radicals such as superoxide anion (O2-), hydrogen peroxide (H2O2), peroxyl radicals and hydroxyl radicals (OH-) are produced during ROS production and highly reactive. In addition, free radicals are also produced in the form of nitrid oxyd (NO-) and peroxynitrite anion (ONOO-), which appear to be important in reproduction and fertilization. The ROS product, hydrogen peroxide plays an essential role in reducing sperm quality (Pujianto et al., 2021; Sedaghatizadeh et al., 2023). Furthermore, hydrogen peroxide damages the sperm membranes. Damage to the sperm membrane can be explained by the fact that the sperm plasma membrane is composed of many chains of unsaturated fatty acids, making it susceptible to LPO influence by hydrogen peroxide. This phenomenon was confirmed in our result (Table 1), which shows the decrease in motility, percentage of sperm viability, sperm with intact membrane, acrosome, and DNA as the days of preservation passed-presumably, more hydrogen peroxide which leads to LPO which disrupt cell membrane function. The continuous production of ROS resulted in a significant (p < 0.05) decrease in sperm qualities. According to previous research, adding or supplementing antioxidants during sperm preservation may be beneficial in protecting sperm from ROS, as reported in earlier studies (Agarwal et al., 2006; Aitken and Drevet, 2020; ChaithraShree et al., 2020). In addition, it is stated that high concentrations of hydrogen peroxide cause significant DNA damage to sperm (Kumar and Muralidhara, 2007). It is explained that peroxidation damages the DNA base in the guanine base. Our results (Table 1) show a significant (p < 0.05) decrease in sperm with intact DNA during preservation, as confirmed by Hoescht and PI dye observations. The number of sperms with DNA damage increased with the number of days of preservation, indicating that more free radicals were produced. If the DNA-damaged sperm is used for fertilization, it will result in failure of fertilization of the oocytes. Aside from the negative impact, ROS in controlled amounts will affect sperm hypermotility, capacitation, and acrosomal reactions in sperm (De Lamirande et al., 1997; Henkel et al., 2018; Mannucci et al., 2022). On the other hand, ROS under controlled conditions plays a role in ovulation and fertilization in female animals, implying that ROS is also important in the reproductive process (Fujii et al., 2005). Cells naturally counteract the harmful effects of ROS production by secreting antioxidants. Antioxidants, such as superoxide dismutase and glutathione peroxidase (GPx) are secreted on a regular basis in the epididymis epithelium in living animals (Potts et al., 1999; Luberda, 2005). These antioxidants would mitigate the negative effects of ROS on the cell. The goal of simple preservation of epididymal sperm is to keep it viable for a set period of time so that it can be used for biological processes, specifically fertilization. Fertilization occurs naturally when mature oocytes are by sperms, followed by a cascade process such as PN formation and later embryo development. As shown in Table 2, preserved epididymal sperms from D12 that are immotile still able to activate and fertilize mature oocytes after ICSI by the presence and development of PN. However, when compared to control (+), ICSI with ejaculated sperm produces significantly lower results (p < 0.05). It has been reported that failed fertilization after ICSI is mainly attributed to the sperm’s inability to induce oocyte activation, and Phospholipase C zeta (PLCζ) is one of the factors for the induction of oocyte activation (Aghajanpour et al., 2011), during fertilization process (Nomikos, 2015; Machaty, 2016). The oocyte activation is driven by intracellular calcium (Ca2+) oscillations induced by PLCζ (Jones et al., 2022). Significant correlation between sperm concentration, motility, and abnormal morphology with the percentage of PLCζ positive spermatozoa (Tavalaee et al., 2017). This demonstrated that PLCζ is considered a biomarker for fertilization capacity (Kashir et al., 2020). PLCζ is distributed in the sperm head of mammalian sperm in areas near the equatorial region, which is where the fusion between gametes is proposed (Escoffier et al., 2014). The presence of PLCζ on spermatozoa is reduced during or after preservation (Moreau et al., 2019), with membrane alterations being the most likely cause. As a consequence, sperm with low sperm quality can be assumed to have low PLCζ. This report is in line with our findings in Table 1. In this study, simple preservation reduced sperm quality parameters, particularly motility and related membrane sperm function which decreased significantly (p < 0.05) during preservation time (D0 to D12). It can be suggested that, due to membrane disruption, the presence of PLCζ in preserved sperm heads has deteriorated, resulting in less amount of that protein leading to its incapabilities to initiate Ca2+ oscillation in the oocytes. Second, this explains why control (+) gives a better activation and fertilization rate compared to sperm from D12. Ejaculate sperm, which has more PLCζ on its head, is more likely to activate mature oocytes better than preserved sperm which has low sperm quality. The calcium oscillation promotes oocyte activation, as evidenced by the resumption of meiosis and the formation of male and female pronuclei of the mature oocyte. In most mammalian species, an ovulated oocyte is arrested at Metaphase-II. Oocyte activation typically occurs immediately following sperm penetration of the oocyte (Anifandis et al., 2016), and is triggered by a series of calcium waves (Nomikos et al., 2012). It has been established that a sperm-specific PLCζ (located in the sperm head) is the sperm-derived oocyte activating factor. In our study, sperm from D12, though not motile, still has an intact membrane particularly related to PLCζ, which is intact of acrosome in the sperm head. We propose that an intact acrosome can be used as an indicator of the presence of PLCζ, which is later able to initiate oocyte activation and later PN formation. Since microinjection of sperm heads lacking PLCζ failed to activate oocytes, a recent study revealed that additional artificial activation of oocytes is required (Hirose et al., 2023). According to this viewpoint, artificial oocyte activation methods can be used to improve the outcome of ICSI. To summarize, epididymis sperm can be preserved for up to 12 days, but its motility and viability will be reduced; however, preserved sperm can be used to activate and fertilize oocytes using ICSI. In this regard, ICSI is a promising tool for the genetic rescue of high-quality animals, as well as for the conservation of endangered and wild species. This study’s findings are expected to serve as the foundation for developing rescue procedures and using epididymis sperm in a variety of animals, with some modifications. AcknowledgmentsThe corresponding author received an Indonesian Research Collaboration grant for the publication of this manuscript in fiscal year 2023. Conflict of interestThe authors declare that there is no conflict of interest in the current study. FundingIndonesian Research Collaboration grant. Authors contributionsSP and RW were responsible for the study’s design, data curation, statistical analysis, and original manuscript drafting. J is the data tabulator and critic. AB assisted with technical lab guidance and manuscript critique. The final manuscript was read and approved by all the authors. Data availabilityThe data supporting the finding in this study, are available upon request to the corresponding author. ReferencesAbu, A.H., Kisani, A.I. and Ahemen, T. 2016. Evaluation of sperm recovered after slaughter from cauda epididymides of red Sokoto bucks. Vet. World. 9, 1440–1444. Agarwal, A., Gupta, S. and Sikka, S. 2006. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol. 18(3), 325–332. Aghajanpour, S., Ghaedi, K., Salamian, A. Deemeh, M.R., Tavalaee, M., Moshtaghian, J., Parrington, J. and Nasr-Esfahani, M.H. 2011. Quantitative expression of phospholipase C zeta, as an index to assess fertilization potential of a semen sample. Hum Reprod. 26(11), 2950–2956. Aitken, R.J., Bromfield, E.G. and Gibb, Z. 2022. Oxidative stress and reproductive function: the impact of oxidative stress on reproduction: a focus on gametogenesis and fertilization. Reproduction. 164(6), F79–F94. Aitken, R.J. and Drevet, J.R. 2020. The importance of oxidative stress in determining the functionality of mammalian spermatozoa: a two-edged sword. Antioxidants. 9(2), 111. Anifandis, G., Messini, C.I., Dafopoulos, K., Daponte, A. and Messinis, I.E. 2016. Sperm contributions to oocyte activation: more that meets the eye. J Assist Reprod Genet. 33(3), 313–316. Baumber, J., Ball, B.A., Gravance, C.G., Medina, V. and Davies-Morel, M.C.G. 2000. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 21(6), 895–902. Bertol, M.A.F., Weiss, R.R., Thomaz-Soccol, V., Kozicki, L.E., Fujita, A.S., de Abreu, R.A. and Green, K.T. 2013. Viability of bull spermatozoa collected from the epididymis stored at 18°C–20°C. Braz Arch Biol Technol. 56, 777–783. Boediono, A., Herdis, H. and Rizal, M. 2004. Preservation of garut rams spermatozoon as a source of male germ plasm. Biotropia. 23, 40–46. Cai, K., Yang, J., Guan, M., Ji, W., Li, Y. and Rens, W. 2005. Single UV excitation of Hoechst 33342 and propidium iodide for viability assessment of rhesus monkey spermatozoa using flow cytometry. Arch Androl. 51(5), 371–383. ChaithraShree, A.R., Ingole, S.D., Dighe, V.D., Nagvekar, A.S., Bharucha, S.V., Dagli, N.R., Kekan P.M. and Kharde, S.D. 2020. Effect of melatonin on bovine sperm characteristics and ultrastructure changes following cryopreservation. Vet. Med. Sci. 6(2), 177–186. Comizzoli, P., Wildt, D.E. and Pukazhenthi, B.S. 2006. In vitro development of domestic cat embryos following intra-cytoplasmic sperm injection with testicular spermatozoa. Theriogenology 66(6–7), 1659–1663. De Lamirande, E., Jiang, H., Zini, A., Kodama, H. and Gagnon, C. 1997. Reactive oxygen species and sperm physiology. Rev. Reprod. 2(1), 48–54. Escoffier, J., Yassine, S., Lee, H.C., Martinez, G., Delaroche, J., Coutton, C., Karaouzéne, T., Zouari, R., Metzler-Guillemain, C., Pernet-Gallay, K., Hennebicq, S., Ray, P.F., Fissore, R. and Arnoult, C. 2014. Subcellular localization of phospholipase Cz in human sperm and its absence in DPY19L2-deficient sperm are consistent with its role in oocyte activation. Mol. Hum. Reprod. 21(2), 157–168. Ford, W.C.L. 2004. Regulation of sperm function by reactive oxygen species. Hum. Reprod. Update. 10(5), 387–399. Fujii, J., Iuchi, Y. and Okada, F. 2005. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrinol. 3, 28. García-Roselló, E., Coy, P., García Vázquez, F.A., Ruiz, S. and Matás, C. 2006. Analysis of different factors influencing the intracytoplasmic sperm injection (ICSI) yield in pigs. Theriogenology 66(8), 1857–1865. Garrido, N., Meseguer, M., Alvarez, J., Simón, C., Pellicer, A. and Remohí, J. 2004. Relationship among standard semen parameters, glutathione peroxidase/glutathione reductase activity, and mRNA expression and reduced glutathione content in ejaculated spermatozoa from fertile and infertile men. Fertil. Steril. 82, 1059–1066. Gómez, M.C., Catt, J.W., Evans, G. and Maxwell, W.M.C. 1998. Cleavage, development and competence of sheep embryos fertilized by intracytoplasmic sperm injection and in vitro fertilization. Theriogenology 49(6), 1143–1154. Goto, K., Kinoshita, A., Takuma, Y. and Ogawa, K. 1990. Fertilisation of bovine oocytes by the injection of immobilised, killed spermatozoa. Vet. Rec. 127(21), 517–520. Henkel, R., Samanta, L. and Agarwal, A. 2018. Oxidants, antioxidants and impact of the oxidative status in male reproduction. Amsterdam, The Netherlands: Elsevier. Hirose, N., Kikuchi, Y., Kageyama, A., Sugita, H., Sakurai, M., Kawata, Y., Terakawa, J., Wakayama, T., Ito, J. and Kashiwazaki, N. 2023. Successful production of offspring derived from phospholipase C zeta-deficient sperm by additional artificial activation. Life. 13(4), 980. Horiuchi, T., Emuta, C., Yamauchi, Y., Oikawa, T., Numabe, T. and Yanagimachi, R. 2002. Birth of normal calves after intracytoplasmic sperm injection of bovine oocytes: a methodological approach. Theriogenology 57(3), 1013–1024. Iritani, A. and Hosoi, Y. 1989. Microfertilization by various methods in mammalian species. Prog. Clin. Biol. Res. 294, 145–149. Jiménez-Macedo, A.R., Anguita, B., Izquierdo, D., Mogas, T. and Paramio, M.T. 2006. Embryo development of prepubertal goat oocytes fertilised by intracytoplasmic sperm injection (ICSI) according to oocyte diameter. Theriogenology 66(5), 1065–1072. Jones, C., Meng, X. and Coward, K. 2022. Sperm facrors and egg activation. Phospholipase C zeta (PLCZ1) and the clinical diagnosis of oocyte activation deficiency. Reproduction 164(1), F53–F66. Kashir, J., Mistry, B.V., BuSaleh, L., Abu-Dawas, R., Nomikos, M., Ajlan, A., Abu-Dawud, R., AlYacoub, N., AlHassan, S., Lai, F.A., Assiri, A.M. and Coskun, S. 2020. Phospholipase C zeta profiles are indicative of optimal sperm parameters and fertilisation success in patients undergoing fertility treatment. Andrology 8(5), 1143–1159. Kumar, T.R. and Muralidhara. 2007. Induction of oxidative stress by organic hydroperoxides in testis and epididymal sperm of rats in vivo. J. Androl. 28(1), 77–85. Lazzaria, G., Crotti, G., Turini, P., Duchi, R., Mari, G., Zavaglia, G., Barbacini, S. and Galli, C. 2002. Equine embryos at the compacted morula and blastocyst stage can be obtained by intracytoplasmic sperm injection (ICSI) of in vitro matured oocytes with frozen-thawed spermatozoa from semen of different fertilities. Theriogenology 58(2), 709–712. Lone, F.A., Islam, R., Khan, M.Z. and Sofi, K.A. 2011. Effect of transportation temperature on the quality of cauda epididymal spermatozoa of ram. Anim. Reprod. Sci. 123(1–2), 54–59. Luberda, Z. 2005. The role of glutathione in mammalian gametes. Reprod Biol. 5(1), 5–17. Machaty, Z. 2016. Signal transduction in mammalian oocytes during fertilization. Cell Tissue Res. 363, 169–183. Maneesh, M. and Jayalekshmi, H. 2006. Role of reactive oxygen species and antioxidants on pathophysiology of male reproduction. Indian J. Clin. Biochem. 21(2), 80–89. Mannucci, A., Argento, F.R., Fini, E., Coccia, M.E., Taddei, N., Becatti, M. and Fiorillo, C. 2022. The impact of oxidative stress in male infertility. Front Mol Biosci. 8, 799294. Mardatillah, K., Widyastuti, R., Pristihadi, D., Gunawan, A., Prastowo, S., Wahyudin and Boediono, A. 2020. The potential of gamete collected from cat (Felis catus) testes as model for feline reproductive technology. Vet. Prac. 21, 281–283. Martínez-Pastor, F., Mata-Campuzano, M., Álvarez-Rodríguez, M., Álvarez, M., Anel, L. and de Paz, P. 2010. Probes and techniques for sperm evaluation by flow cytometry. Reprod. Domest. Anim. 45, 67–78. Mizushima, S., Takagi, S., Ono, T., Atsumi, Y., Tsukada, A., Saito, N. and Shimada, K. 2007. Possible role of calcium on oocyte development after intracytoplasmic sperm injection in quail (Coturnix japonica). J. Exp. Zool. A Ecol. Genet. Physiol. 307(11), 647–653. Monteiro, G.A., Guasti, P.N., Rocha, A.S., Martin, I., Sancler-Silva, Y.F.R., Freitas Dell’Aqua, C.P., Dell’Aqua, J.A. and Papa, F.O. 2013. Effect of storage time and temperature of equine epididymis on the viability, motion parameters, and freezability of epididymal sperm. J. Equine Vet. 33(3), 169–173. Moreau, J., Fargeon, S., Gatimel, N., Parinaud, J. and Léandri. R.D., 2019. Expression of phospholipase PLC Zeta in human spermatozoa: impact of cryopreservation. Andrology 7(3), 315–318. Nomikos, M. 2015. Novel signalling mechanism and clinical applications of sperm-specific PLCζ. Biochem. Soc. Trans. 43(3), 371–376. Nomikos, M., Swann, K. and Lai, F.A. 2012. Starting a new life: Sperm PLC-zeta mobilizes the Ca 2+ signal that induces egg activation and embryo development: an essential phospholipase C with implications for male infertility. Bioessays 34, 126–134. Palermo, G.D., Schlegel, P.N., Colombero, L.T., Zaninovic, N., Moy, F. and Rosenwaks, Z. 1996. Aggressive sperm immobilization prior to intracytoplasmic sperm injection with immature spermatozoa improves fertilization and pregnancy rates. Hum. Reprod. 11(5), 1023–1029. Paul, R.K., Balaganur, K., Bahire, S.V., Kumar, D. and Singh, R. 2018. Supplementation of cauda epididymal plasma improves sperm characteristics following liquid preservation of ram semen at 3°C–5°C. Reprod. Fertil. Dev. 30(11), 1389–1401. Pérez-Llano, B., Lorenzo, J.L., Yenes, P., Trejo, A. and García-Casado, P. 2001. A short hypoosmotic swelling test for the prediction of boar sperm fertility. Theriogenology 56(3), 387–398. Potts, R.J., Mjefferies, T. and Notarianni, L.J. 1999. Antioxidant capacity of the epididymis. Hum. Reprod. 14(10), 2513–2516. Prastowo, S., Nugroho, A.F. and Widyastuti, R. 2021. Epididymal sperm quality of Kacang goat preserved in low temperature for genetic material utilization in assisted reproductive technologies. IOP Conf. Ser.: Earth Environ. Sci. 902, 12004. Pujianto, D., Oktarina, M., Sharma Sharaswati, I. and Yulhasri, Y. 2021. Hydrogen peroxide has adverse effects on human sperm quality parameters, induces apoptosis, and reduces survival. J. Hum. Reprod. Sci. 14(2), 121–128. Rahimizadeh, P., Rezaei Topraggaleh, T., Bucak, M.N., Ziarati, N., Hasirbaf, A., Taher-Mofrad, S.M.J., Maroufizadeh, S. and Shahverdi, A. 2021. Effect of bovine serum albumin supplementation in tris-soybean lecithin-based extender on quality of chilled ram epididymal spermatozoa. Biopreserv. Biobank. 19(1), 33–40. Sedaghatizadeh, K., Tabari, M.G., Bishehkolaei, R., Ghadikolaii, F.P. and Nemati, F. 2023. Protective effect of spirulina and phycocyanin on testicular tissue and sperm parameters in adult rats treated with hydrogen peroxide. J. Mazandaran. Univ. Med. Sci. 32(216), 49–60. Sikka, S.C. 1996. Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front. Biosci. 1, 78–86. Tartaglione, C.M. and Ritta, M.N. 2004. Prognostic value of spermatological parameters as predictors of in vitro fertility of frozen-thawed bull semen. Theriogenology 62(7), 1245–1252. Tavalaee, M., Kiani-Esfahani, A. and Nasr-Esfahani, M.H. 2017. Relationship between phospholipase C-zeta, semen parameters, and chromatin status. Syst. Biol. Reprod. Med. 63(4), 259–268. Unnikrishnan, V., Kastelic, J. and Thundathil, J. 2021. Intracytoplasmic sperm injection in cattle. Genes 12(2), 1–18. | ||
| How to Cite this Article |
| Pubmed Style Prastowo S, Widyastuti R, Jaswandi J, Boediono A. Fertility testing of preserved epididymal sperm by microinjection: A model for the rescue and utilization of genetically superior animals. Open Vet J. 2024; 14(2): 707-715. doi:10.5455/OVJ.2024.v14.i2.11 Web Style Prastowo S, Widyastuti R, Jaswandi J, Boediono A. Fertility testing of preserved epididymal sperm by microinjection: A model for the rescue and utilization of genetically superior animals. https://www.openveterinaryjournal.com/?mno=180246 [Access: July 06, 2025]. doi:10.5455/OVJ.2024.v14.i2.11 AMA (American Medical Association) Style Prastowo S, Widyastuti R, Jaswandi J, Boediono A. Fertility testing of preserved epididymal sperm by microinjection: A model for the rescue and utilization of genetically superior animals. Open Vet J. 2024; 14(2): 707-715. doi:10.5455/OVJ.2024.v14.i2.11 Vancouver/ICMJE Style Prastowo S, Widyastuti R, Jaswandi J, Boediono A. Fertility testing of preserved epididymal sperm by microinjection: A model for the rescue and utilization of genetically superior animals. Open Vet J. (2024), [cited July 06, 2025]; 14(2): 707-715. doi:10.5455/OVJ.2024.v14.i2.11 Harvard Style Prastowo, S., Widyastuti, . R., Jaswandi, . J. & Boediono, . A. (2024) Fertility testing of preserved epididymal sperm by microinjection: A model for the rescue and utilization of genetically superior animals. Open Vet J, 14 (2), 707-715. doi:10.5455/OVJ.2024.v14.i2.11 Turabian Style Prastowo, Sigit, Rini Widyastuti, Jaswandi Jaswandi, and Arief Boediono. 2024. Fertility testing of preserved epididymal sperm by microinjection: A model for the rescue and utilization of genetically superior animals. Open Veterinary Journal, 14 (2), 707-715. doi:10.5455/OVJ.2024.v14.i2.11 Chicago Style Prastowo, Sigit, Rini Widyastuti, Jaswandi Jaswandi, and Arief Boediono. "Fertility testing of preserved epididymal sperm by microinjection: A model for the rescue and utilization of genetically superior animals." Open Veterinary Journal 14 (2024), 707-715. doi:10.5455/OVJ.2024.v14.i2.11 MLA (The Modern Language Association) Style Prastowo, Sigit, Rini Widyastuti, Jaswandi Jaswandi, and Arief Boediono. "Fertility testing of preserved epididymal sperm by microinjection: A model for the rescue and utilization of genetically superior animals." Open Veterinary Journal 14.2 (2024), 707-715. Print. doi:10.5455/OVJ.2024.v14.i2.11 APA (American Psychological Association) Style Prastowo, S., Widyastuti, . R., Jaswandi, . J. & Boediono, . A. (2024) Fertility testing of preserved epididymal sperm by microinjection: A model for the rescue and utilization of genetically superior animals. Open Veterinary Journal, 14 (2), 707-715. doi:10.5455/OVJ.2024.v14.i2.11 |