| Case Report | ||
Open Vet J. 2019; 9(4): 294-300 doi: 10.4314/ovj.v9i4.3 Open Veterinary Journal, (2019), Vol. 9(4): 294–300 Case Report DOI: http://dx.doi.org/10.4314/ovj.v9i4.3 Unilateral phacoemulsification in a captive African elephant (Loxodonta africana)Katherine E. L. Manchip1*, Ghislaine Sayers2, John C. M. Lewis3 and James W. Carter11South Devon Veterinary Hospital, The Old Cider Works, Abbotskerswell TQ12 5GH, UK 2Paignton Zoo Environmental Park, Paignton TQ4 7EU, UK 3International Zoo Veterinary Group, Station House, Keighley BD21 4NQ, UK *Corresponding Author: Katherine E. L. Manchip. South Devon Veterinary Hospital, The Old Cider Works, Abbotskerswell TQ12 5GH, UK. Email: kat.manchip [at] southdevonvets.co.uk Submitted: 07/05/2019 Accepted: 18/09/2019 Published: 26/10/2019 © 2019 Open Veterinary Journal AbstractBackground: The following case reports describe the clinical presentation, surgical protocol, post-operative care, and long-term follow-up of an African elephant (Loxodonta Africana) presenting with a unilateral cataract. Case description: A 42-year-old female African elephant presented for the assessment of ocular discomfort and visual deterioration in the left eye. Pre-surgical treatment included topical anti-inflammatory medication for 20 days prior to surgery. On the day of surgery, following anesthetic induction, a two-handed phacoemulsification technique was performed in the left eye. She was left aphakic post-operatively. Nine days post-operatively, the patient had an intact menace response, dazzle reflex, and direct pupillary light reflex. Fundoscopy at that stage was unremarkable. Follow-up information was available for 5 years, from the time of surgery to the present day. Conclusion: Despite remaining aphakic, this case presents a successful visual outcome. To the best of the authors’ knowledge, there is no other published report of phacoemulsification in a captive elephant. Keywords: African elephant, Cataract, Loxodonta Africana, Phacoemulsification. IntroductionElephants are a flagship species among the Animal Kingdom. These highly mobile mammals, with their unusual anatomy, strong family bonds, and longevity have been a source of fascination for many years. Habitat fragmentation, obstruction of migratory corridors, and poaching to supply the international ivory trade are key threats to the survival of the species, which is currently listed as “Vulnerable” by the International Union for the Conservation of Nature Redlist (Lindsay et al., 2017). Despite the long and complex relationship between humans and captive elephants, much information about their husbandry and veterinary care remains anecdotal. Within the literature, there is some information about ophthalmic anatomy and physiology, but relatively little is published about ocular disease and treatment. The elephant eye is small in relation to its body size, with a mean axial length of 35 mm in African elephants and 38.75 mm in Asian elephants (Murphy et al., 1992; Suedmeyer, 2006). The ventral eyelid is more developed than the dorsal, with a greater distance of excursion. Similar to cats, elephants do not possess true cilia but have large accessory cilia up to 110 mm, which extend over the eye (Samuelson, 2013; Super, 1975). A unique feature is the absence of a lacrimal apparatus. Instead, the tear film is directed to the medial canthus where it flows onto the face and along a groove, known as the medial canthal groove (Duke-Elder, 1958; Murphy et al., 1992; Suedmeyer, 2006). Also, unlike many other terrestrial animals, elephants have a distinct Bowman’s layer (Hayashi et al., 2002). They possess a tapetum lucidum fibrosum similar in structure and function to other ungulates and are paurangiotic (Stone and Halasz, 1989; Shoshani, 2006). Topographic mapping of the retina of African elephants identified a horizontal streak of increased retinal ganglion cell density inferior to the optic disk, comparable to other mammalian species, and an additional superior temporal region of increased ganglion cell density, which has been postulated to monitor movements of the trunk (Stone and Halasz, 1989). Cataract formation has been documented in both African and Asian elephants, within both wild and captive populations (McCullagh and Gresham, 1969; Schmidt, 1986; Godagama and Ratnasooriya, 1999; Kraiwong et al., 2016). This case report describes cataract surgery by phacoemulsification in a female African elephant Loxodonta africana. Case DetailsClinical historyA 42-year-old, 4,100 kg, female African elephant L. africana presented for the assessment of ocular discomfort and visual deterioration in the left eye. The eye had been monitored over the previous 3 years for the progression of cataract formation, and the patient was already receiving topical 0.5% ketorolac trometamol (Acular; Allergan, Marlow, UK) twice daily. Her keeper had noticed a deterioration in the vision for approximately 2 months prior to the reassessment by a veterinary ophthalmologist and had noted an increased blink rate, redness, epiphora, and sand around the eye (Fig. 1). It was suspected that she was throwing sand at the eye in an attempt to relieve ocular discomfort. Topical medication was increased to three times daily, with the subsequent addition of oral flunixin meglumine (Equinixin; Norbrook Laboratories Ltd. Newry, UK) 1.02 mg/kg once daily. The right eye was enucleated 3 years previously owing to uncontrolled glaucoma secondary to lens-induced uveitis. The exhibit at that time opted for enucleation rather than cataract surgery. The elephant had resided at the same park for 35 years and was, otherwise, systemically well, with no other pertinent medical history. Ophthalmic examinationOphthalmic examination was performed in the conscious patient by an RCVS boarded Specialist in Veterinary Ophthalmology (JC). The patient was already trained to walk forward to the perimeter wire of the enclosure and lower her head so that her eye was at standing eye height for the examiner. From here, personnel were able to approach her from outside the wire and perform the examination (Fig. 2). She was also trained to tilt her head to make the application of topical medication easier. The eye was examined using a slit-lamp biomicroscope (Keeler PSL Classic; Keeler Ltd, Windsor, UK) and direct ophthalmoscope (Keeler Practitioner direct ophthalmoscope; Keeler Ltd, Windsor, UK). Neuro-ophthalmic assessment revealed an absent menace response, with an intact dazzle reflex (DR) and direct pupillary light reflex (PLR). Ocular examination revealed a serious ocular discharge and conjunctival hyperemia. The cornea was clear and healthy, with no opacities or defects, and there was no fluorescein uptake. There was no aqueous flare present or indication of anterior segment inflammation. The pupil was round and mid-size, and there was anterior and posterior subcapsular cataract formation with cortical extensions (Fig. 3). Posterior segment examination was not possible owing to lens pathology. Despite training, attempts to measure intraocular pressure with rebound tonometry (ICare® Tonovet tonometer, Tiolat, Helsinki, Finland) were unsuccessful owing to repetitive, voluntary, movement of the nictitans membrane when the Tonovet was moved toward the eye. Similarly, an attempt to measure a Schirmer tear test value was not tolerated by the patient.
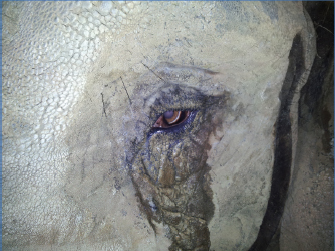
Fig. 1. Indications of ocular discomfort included epiphora and increased blink rate. Ocular B-mode ultrasonographyOcular ultrasound was performed following the application of topical Proxymetacaine (Minims Proxymetacaine Hydrochloride 0.5%, Bausch & Lomb UK Limited). B-mode scan ultrasonography was carried out using an 8 MHz probe, to assess potential posterior segment abnormalities. The anteroposterior dimension of the lens was 12.5 mm, and its equatorial distance was 19.5 mm. The distance from the anterior lens to retina was measured at 34.5 mm. Pretreatment and pre-surgical trainingTreatment with topical neomycin-polymyxin B-dexamethasone 0.1% suspension (Maxitrol®; Alcon laboratories) was commenced four times daily, and topical ketorolac trometamol (Acular; Allergan, Marlow, UK) was increased to four times daily for 20 days prior to surgery. Training in preparation for intramuscular injection of etorphine involved getting the patient used to presenting the gluteal region and having the area cleaned. Owing to the significant health and safety implications, of both the patient size and administration of etorphine, training had to be completely satisfactory prior to the day of surgery, with extensive logistical planning also necessary. On the morning of surgery, topical atropine (Minims Atropine sulfate 1%; Bausch & Lomb UK Limited) was applied twice at 30 minute intervals prior to induction. A small bolus of food was given to administer oral flunixin meglumine (Equinixin; Norbrook Laboratories Ltd. Newry, UK). Anesthesia for surgeryThe patient was immobilized using a total dose of 10.045 mg etorphine hydrochloride and 41 mg acepromazine maleate (4.1 ml Immobilon®; Novartis Animal Health UK Limited) given intramuscularly in the gluteal region. The injection site was immediately washed with a water spray to reduce the handling risk to staff afterward. The patient settled into right lateral recumbency, so further repositioning was not necessary (Fig. 4A). The patient was nasally intubated with 18 and 22 mm cuffed endotracheal (ET) tubes, and the pharynx was packed with towels. Anesthesia was maintained with isoflurane and oxygen delivered from two large animal circle circuits, each fitted with two 30 liter rebreathing bags (Fig. 4B). Retrobulbar anesthesia was achieved using a four-point nerve block, with a total of 10 ml bupivacaine 0.5% injectable solution (MarcainÒ; AstraZeneca, UK). Intravenous access was obtained with a 12-gauge catheter in both ears, and the patient received Hartmann’s solution throughout the procedure. Anesthetic monitoring included pulse oximetry, electrocardiography, and capnography. Intravenous marbofloxacin 2 mg/kg (Forcyl; Vetoquinol, UK) was administered perioperatively. At the end of surgery, all monitoring equipment was removed and the patient extubated. To antagonize the etorphine 1 mg diprenorphine (Revivon; C-Veterinary Products, Leyland, UK) was administered subcutaneously and a further 12.3 mg intravenously, after which the remaining intravenous catheter was removed. Between extubation and standing, oxygen was administered via an equine demand valve on inspiration to maintain SPO2 values at greater than 95%.
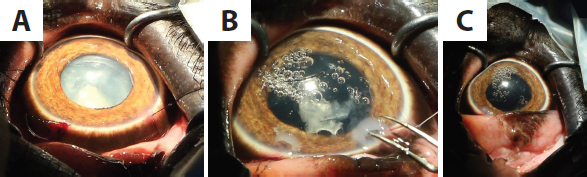
Fig. 2. The patient was managed using “protected contact” and was examined from the other side of a barrier fence. Recovery was relatively uneventful except for mild dysphoria, with the patient regaining tonicity of the trunk a few hours after surgery, and eating and responding to vocal commands normally that evening. PhacoemulsificationAn equine Castroviejo eyelid speculum was used to improve the globe exposure for the procedure (Fig. 5A). With the aid of a portable microscope (Leica Wild M-690 Microscope) (Seen in Fig. 4A), three stay sutures were placed using 7–0 braided silk (Mersilk®; Ethicon Inc., Somerville, NJ). A side-port incision was made using a 69 Beaver scalpel blade and 3.2 mm keratome, and trypan blue was injected into the anterior segment to stain the anterior lens capsule. The anterior segment was then irrigated with Balanced Salt Solution containing adrenaline (1:10,000) and heparin (2 IU/ml), increasing the pupil diameter. The anterior segment was reformed using viscoelastic agents (2% sodium hyaluronate; Acrivet Syn 2.0%; S&V Technologies, Acrivet Veterinary Division, Hennigsdorf, and 2% hydroxypropyl methylcellulose; Acrivet Viscose; S&V Technologies, Acrivet Veterinary Division, Hennigsdorf), which further increased the pupil diameter. A clear corneal stepped incision was then made approximately 90° to the right of the side-port incision. The same keratome used to enter the anterior segment was used to make the initial anterior capsular incision. The incision was extended using Vannas scissors, followed by the completion of a continuous curvilinear capsulorrhexis using Utrata capsulorrhexis forceps. The target diameter was approximately 10 mm, which was achieved. A two-handed technique was performed using an Acrivet Alexos portable phacoemulsification unit (Acrivet Alexos, Salt Lake City, UT) with a 23 mm long, 0.9 mm diameter phacoemulsification needle, and residual cortical material was removed with automated irrigation/aspiration. The total ultrasound time was 416 seconds. Viscodissection with 2% sodium hyaluronate (Acrivet Syn 2.0%; S&V Technologies, Acrivet Veterinary Division, Hennigsdorf) was utilized to move lens fragments closer to the phacoemulsification needle so that they could be emulsified and aspirated. Some small lens fragments that did not easily engage with the phacoemulsification needle were removed using Utrata forceps (Fig. 5B). A posterior capsular rupture occurred during the surgery, and an intraocular lens was not implanted. The primary corneal incision was closed using a continuous reverse saw-tooth pattern with 8-0 polyglactin 910 (Vicryl®; Ethicon Inc., Somerville, NJ, USA), and the side-port incision was closed with a simple interrupted suture using the same material (Fig. 5C).
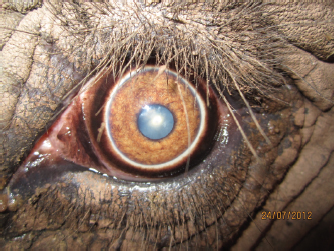
Fig. 3. Photograph of the eye taken prior to surgery. Note the conjunctival hyperaemia and late-immature cataract formation. Cornea appears to be healthy.
Fig. 4. (A): Patient in right lateral recumbency, with portable microscope in position. (B): Nasal intubation with two cuffed ET tubes. Each ET tube was then connected to a large animal circle circuit. Post-operativelyPost-operatively, the patient received topical neomycin-polymyxin B-dexamethasone 0.1% suspension (Maxitrol®; Alcon laboratories, UK), and ketorolac-trometamol (Acular; Allergan, Marlow, UK) four times daily for 9 d. Oral marbofloxacin 1 mg/kg (Marbocyl®; Vetoquinol, UK) and flunixin-meglumine 1 mg/kg (EquinixinÒ; Norbrook Laboratories Ltd. Newry, UK) were continued for 9 days post-operatively. Medications were tapered over 3 months and then discontinued.
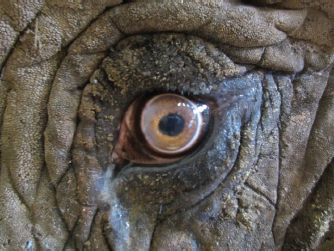
Fig. 5. (A): An equine Castroviejo eyelid speculum provided good exposure of the globe. Stay sutures facilitated manipulation of the globe. (B): A few of the smaller lens fragments were removed manually using Utrata forceps. (C): At the conclusion of surgery. Shortly after recovery, she was seen to throw sand at the eye and so access to the sandpit was restricted for the first 24 hours. Subsequent to this, there was no evidence of the patient interfering with the surgical site at any point. At 24-hours following the surgery, there was mild conjunctival hyperemia and subtle aqueous flare. A DR was intact with a poor menace response and absent direct PLR. After 72 hours, post-operatively, a menace response was intact, and the patient was capable of tracking the movement of a target stick but was unable to touch the target with her trunk. There was a positive DR, but the pupil was miotic. There was some mild corneal oedema associated with the corneal sutures (Fig. 6). Nine days post-operatively, the patient had an intact menace response, DR, and PLR. Fundoscopy at that stage was unremarkable. Vision continued to improve over the following 3 to 4 weeks, and the patient was able to target small objects and accurately pick them up with her trunk. Mild ocular discomfort for the first 2 weeks post-operatively was assumed owing to a slightly increased blink rate and intermittent foamy ocular discharge. Follow-up information was available for 5 years, from the time of surgery to the present day. At the time of writing, the patient was visual and comfortable without the requirement of any long-term topical medication. DiscussionPhacoemulsification has been performed in a variety of wild and exotic animals (Cooley, 2001; Colitz et al., 2002; Kelly et al., 2005; Montiani-Ferreira et al., 2010). There are a few reports of ocular surgery on elephants (Schmidt, 1986; Kraiwong et al., 2016), but these are limited to news and online reports with limited detail provided. To the best of the authors’ knowledge, this is the first published case report describing ocular ultrasound and phacoemulsification in this species. The causes of cataract formation include congenital defects, nutritional imbalances, oxidative damage, hereditary defects of the lens, systemic disease, age-related change, trauma and environmental effects (Kern, 2013). Although more limited than the information available about domestic species, there are various reports of cataract formation in wild and exotic species, with a range of different etiologies proposed (Vainisi et al., 1981; de Villiers et al., 2001; Bakal et al., 2005; Beltran et al., 2007). Cataract formation has been documented on occasion in African and Asian species of elephant, in both wild and captive animals. Cataracts were identified in 16 of 300 elephants culled from a population in Kenya; concurrent ocular abnormalities included uveitis and lens luxation (McCullagh and Gresham, 1969). The authors speculated that excess infrared and visible-radiation due to lack of shade and heat reflection from the ground may be a contributing factor. The temperate and largely indoor environment of the patient in this report does not support this theory, and considering the latter population of wild elephants in Kenya potentially precludes husbandry related causes or nutritional deficiencies, which may spring to mind in a captive animal. Owing to the lack of historical information about the patient in this case report, it was not possible to determine a definitive cause, although the history of bilateral ocular disease also makes a traumatic etiology less likely. In this case, the patients’ age and appearance of the cataract formation were consistent with senile cataract formation.
Fig. 6. After 72 hours post-operatively; a DR was intact. There was some mild perilimbal edema associated with the corneal sutures. A survey of anterior ocular abnormalities in captive Asian elephants identified various stages of cataractogenesis, uveitis, and both anterior and posterior lens luxation. One-third of the elephants with anterior uveitis had cataracts, in which the authors suggested demonstrated that lens-induced uveitis was possible during various stages of cataract development (Kraiwong et al., 2016); however, there was no further investigation into other possible causes of anterior uveitis. As the patient in this report had no other signs of systemic disease, unfortunately, no further diagnostic testing was performed at the time, although it was strongly suspected that the uveitis was lens-induced. As with any large migratory species in captivity, there is an ethical discussion. In light of the potential improvement to the patient’s welfare in terms of vision and comfort, and the safety implications of a blind elephant, it was decided to perform phacoemulsification. The decision was made to enucleate the right eye previously, rather than perform cataract surgery, as the prognosis for phacoemulsification was deemed too poor given the level of uveitis, corneal edema, and suspected damage to the retina by the time of diagnosis. At the time, the left eye was still visual and comfortable, and it was considered that the other elephant in the enclosure provided enough support to cope with unilateral vision. To produce globe akinesia and analgesia and maintain a central eye position for the surgery, a four-point retrobulbar block was performed, as described in the horse (Stoppini and Gilger, 2017) with a successful outcome. Subsequently, neuromuscular blockade was not required. The limitation of retrobulbar anesthesia in this case was that the patient was blind on recovery and initially stressed, although calmed in response to vocal cues. The choice of topical and systemic drugs was based on the experience of both the surgeon and zoo veterinary staff. The patient had undergone a successful enucleation 3 years previously, and so a similar anesthetic/analgesic protocol was selected. The lack of interference with the surgical sight post-operatively suggests that the patient experienced only mild discomfort. The biggest challenge encountered during the surgery was the size of the globe. Even with a long phacoemulsification needle, it was not possible to reach the posterior aspect of the lens. Using a divide and conquer technique, following removal of the first segment of the lens, the remainder dropped to the bottom of the capsule and was then difficult to engage. This was overcome by floating the lens fragments to the top of the capsule using 2% sodium hyaluronate, as has been described during equine phacoemulsification (Brooks et al., 2014). It was probably during this stage that the posterior capsular rupture occurred. Following this, the surgeon converted to a bimanual technique to facilitate directing irrigation fluids away from the tear, and viscoelastic material was utilized to tamponade the vitreous, bringing phacoemulsification closer to the surgeon (Wilkie and Willis, 1999). Intraocular lenses have been developed for various domestic species (Cooley, 2001). However, owing to the size limitations of available lenses and the cost of a custom-made one, the decision was made pre-operatively to leave the patient aphakic. Despite this, the improvement in vision post-surgery was remarkable, with the patient able to navigate her surroundings more effectively and accurately pick up objects. She became less vocal, and a marked improvement in her welfare was appreciated by her keepers. Case reports such as this contribute to the limited literature available on ocular disease and treatment in captive elephants. It highlights the need for regular ophthalmic examinations of captive elephants to ensure the early detection and successful treatment of ocular diseases. AcknowledgmentThe authors wish to thank Optivet Referrals for the use of their portable microscope and phacoemulsification machine. Author contributionsJames W. Carter performed the surgery and post-operative rechecks; Ghislaine Sayers was the head vet at the zoological park; John C. M. Lewis conducted the anesthesia; Katherine E. L. Manchip was the primary author of the case report; all authors contributed to the writing and the revision of this manuscript. ReferencesBakal, R.S., Hickson, B.H., Gilger, B.C., Levy, M.G., Flowers, J.R. and Khoo, L. 2005. Surgical removal of cataracts due to Diplostomum species in Gulf sturgeon (Acipenser oxyrinchus desotoi). J. Zoo Wildl. Med. 36, 504–508. Beltran, W.A., Vanore, M., Ollivet, F., Nemoz-Bertholet, F., Clerc, B. and Chahory, S. 2007. Ocular findings in two colonies of gray mouse lemurs (Microcebus murinus). Vet. Ophthalmol. 10, 43–49. Brooks, D.E., Plummer, C.E., Carastro, S.M. and Utter, M.E. 2014. Visual outcomes of phacoemulsification cataract surgery in horses: 1990–2013. Vet. Ophthalmol. 17, 117–128. Colitz, C.M.H., Lewbart, G. and Davidson, M.G. 2002. Phacoemulsification in an adult Savannah monitor lizard. Vet. Ophthalmol. 5, 207–209. Cooley, P.L. 2001. Phacoemulsification in a clouded leopard (Neofelis nebulosa). Vet. Ophthalmol. 4, 113–117. de Villiers, C., Seier, J.V. and Dhansay, M.A. 2001. Probable genetic origin for a large number of cataracts among captive-bred vervet monkeys (Chlorocebus aethiops). Am. J. Primatol. 55, 43–48. Duke-Elder, S. 1958. The eye in evolution. MO: Mosby. pp: 429–501. Godagama, W.K. and Ratnasooriya, W.D. 1999. Prevalence of eye defects in domesticated Sri Lanken elephants (Elephas maximus maximus). Ceylon J. Sci. 27, 41–5. Hayashi, S., Osawa, T. and Tohyama, K. 2002. Comparative observations on corneas, with special reference to Bowman’s layer and Descemet’s membrane in mammals and amphibians. J. Morphol. 254, 247–258. Kelly, T.R., Walton, W., Nadelstein, B. and Lewbart, G.A. 2005. Phacoemulsification of bilateral cataracts in a loggerhead sea turtle (Caretta caretta). Vet. Rec. 156, 774–777. Kern, T.C.C. 2013. Exotic animal ophthalmology. In Veterinary ophthalmology, 5th ed. Eds., Gelatt, K.N., Brian, G.C. and Kern, T.J. Ames, IA: Blackwell Pub, pp: 1750–1819. Kraiwong, N., Sanyathitiseree, P., Boonprasert, K., Charoenphan, P., Pintawong, W. and Thayananuphat, A. 2016. Anterior ocular abnormalities of captive Asian elephants (Elephas maximus indicus) in Thailand. Vet. Ophthalmol. 19, 269–74. Lindsay, K., Chase, M., Landen, K. and Nowak, K. 2017. The shared nature of Africa’s elephants. Biol. Conserv. 215, 260–267. McCullagh, K.G. and Gresham, G.A. 1969. Eye lesions in the African elephant (Loxodonta africana). Res. Vet. Sci. 10, 587–589. Montiani-Ferreira, F., Lima, L., Bacellar, M., D’Otaviano Vilani, R.G., Fedullo, J.D. and Lange, R.R. 2010. Bilateral phacoemulsification in an orangutan (Pongo pygmaeus). Vet. Ophthalmol. 13, 91–99. Murphy, C.J., Kern, T.J. and Howland, H.C. 1992. Refractive state, corneal curvature, accommodative range and ocular anatomy of the Asian elephant (Elephas maximus). Vis. Res. 32, 2013–2021. Samuelson, D. 2013. Ophthalmic Anatomy. In Veterinary ophthalmology, 5th edn. Eds., Gelatt, K.N., Brian, G.C. and Kern, T.J. Ames, IA: Blackwell Pub, pp: 39–170. Schmidt, M. 1986. Elephants (Proboscidea): disease description. In Zoo and wild animal medicine. Ed., Fowler, M.E. Philadephia: W. B Saunders, pp: 915–927. Shoshani, J. 2006. Taxonomy, classification, history and evolution of elephants. In Biology, medicine and surgery of elephants. Eds., Fowler, M.E. and Mikota, S.K. Blackwell Pub, pp: 3–14. Stone, J. and Halasz, P. 1989. Topography of the Retina in the Elephant Loxodonta africana. Brain Behav. Evol. 34, 84–95. Stoppini, R. and Gilger, B.C. 2017. Equine ocular examination basic techniques. In Equine ophthalmology. Ed., Gilger, B.C. Ames, IA: Wiley Blackwell, pp: 1–39. Suedmeyer, W. 2006. Special senses. In Biology, medicine and surgery of elephants. Eds., Fowler, M.E. and Mikota, S.K. Ames, IA: Blackwell Pub, pp: 399–407. Super, S. 1975. Optometric examination of the African elephant Loxodonta africana africana in Southwest Africa. Madoqua. 9, 45–51. Vainisi, S.J., Edelhauser, H.F., Wolf, E.D., Cotlier, E. and Reeser, F. 1981. Nutritional cataracts in timber wolves. J. Am. Vet. Med. Assoc. 179, 1175–1180. Wilkie, D.A. and Willis, A.M. 1999. Viscoelastic materials in veterinary ophthalmology. Vet. Ophthalmol. 2, 147–153. | ||
| How to Cite this Article |
| Pubmed Style Manchip KEL, Sayers G, Lewis JCM, Carter JW. Unitlateral phacoemulsification in a captive African elephant (Loxodonta africana) . Open Vet J. 2019; 9(4): 294-300. doi:10.4314/ovj.v9i4.3 Web Style Manchip KEL, Sayers G, Lewis JCM, Carter JW. Unitlateral phacoemulsification in a captive African elephant (Loxodonta africana) . https://www.openveterinaryjournal.com/?mno=47519 [Access: July 01, 2025]. doi:10.4314/ovj.v9i4.3 AMA (American Medical Association) Style Manchip KEL, Sayers G, Lewis JCM, Carter JW. Unitlateral phacoemulsification in a captive African elephant (Loxodonta africana) . Open Vet J. 2019; 9(4): 294-300. doi:10.4314/ovj.v9i4.3 Vancouver/ICMJE Style Manchip KEL, Sayers G, Lewis JCM, Carter JW. Unitlateral phacoemulsification in a captive African elephant (Loxodonta africana) . Open Vet J. (2019), [cited July 01, 2025]; 9(4): 294-300. doi:10.4314/ovj.v9i4.3 Harvard Style Manchip, K. E. L., Sayers, . G., Lewis, . J. C. M. & Carter, . J. W. (2019) Unitlateral phacoemulsification in a captive African elephant (Loxodonta africana) . Open Vet J, 9 (4), 294-300. doi:10.4314/ovj.v9i4.3 Turabian Style Manchip, Katherine Edith Leonora, Ghislaine Sayers, John C M Lewis, and James W Carter. 2019. Unitlateral phacoemulsification in a captive African elephant (Loxodonta africana) . Open Veterinary Journal, 9 (4), 294-300. doi:10.4314/ovj.v9i4.3 Chicago Style Manchip, Katherine Edith Leonora, Ghislaine Sayers, John C M Lewis, and James W Carter. "Unitlateral phacoemulsification in a captive African elephant (Loxodonta africana) ." Open Veterinary Journal 9 (2019), 294-300. doi:10.4314/ovj.v9i4.3 MLA (The Modern Language Association) Style Manchip, Katherine Edith Leonora, Ghislaine Sayers, John C M Lewis, and James W Carter. "Unitlateral phacoemulsification in a captive African elephant (Loxodonta africana) ." Open Veterinary Journal 9.4 (2019), 294-300. Print. doi:10.4314/ovj.v9i4.3 APA (American Psychological Association) Style Manchip, K. E. L., Sayers, . G., Lewis, . J. C. M. & Carter, . J. W. (2019) Unitlateral phacoemulsification in a captive African elephant (Loxodonta africana) . Open Veterinary Journal, 9 (4), 294-300. doi:10.4314/ovj.v9i4.3 |