| Original Article | ||
Open Vet J. 2022; 12(6): 965-974 Open Veterinary Journal, (2022), Vol. 12(6): 965–974 Original Research Impact of vitamin AD3E treatment before application of controlled intra-vaginal drug releaser on clinical and hematologic findings, ovarian hormones, and calving rates in she-camels (Camelus dromedarius)Ragab H. Mohamed1, Arafat Khalphallah2, Fatma Ali3, Ebtsam S. Abdel-lah4, Abdulrahman Abdulkarim5* and Enas Elmeligy6 1Theriogenology Department, Faculty of Veterinary Medicine, Aswan University, Aswan, Egypt 2Division of Internal Medicine, Department of Animal Medicine, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt 3Physiology Department, Faculty of Veterinary Medicine, Aswan University, Aswan, Egypt 4Department of Pharmacology, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt 5Faculty of Veterinary Medicine, Omar Almukhtar University, Bayda, Libya 6Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt Submitted: 29/05/2022 Accepted: 11/11/2022 Published: 12/12/2022 *Corresponding Author: Abdulrahman Abdulkarim. Faculty of Veterinary Medicine, Omar Almukhtar University, Bayda, Libya. Email: abdulrahman.tahir [at] omu.edu.ly © 2022 Open Veterinary Journal
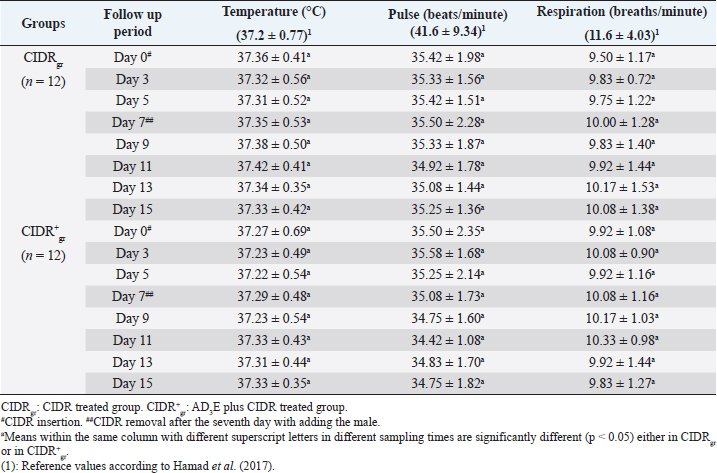
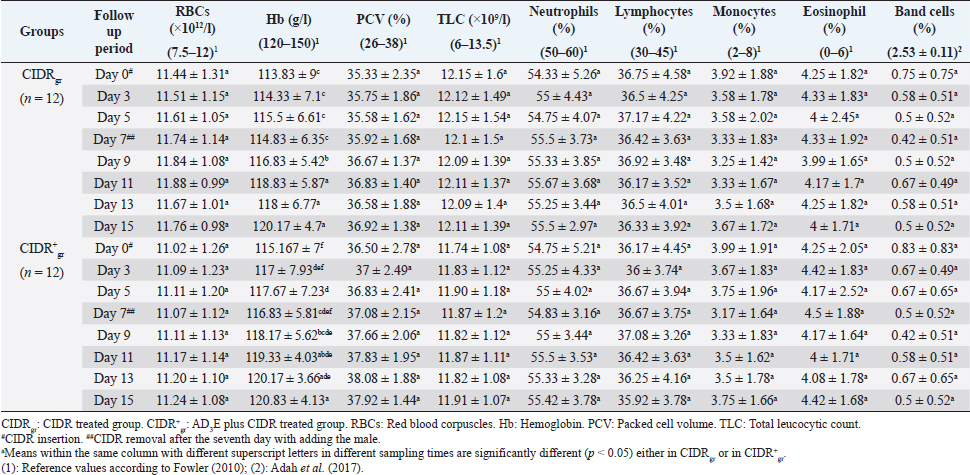
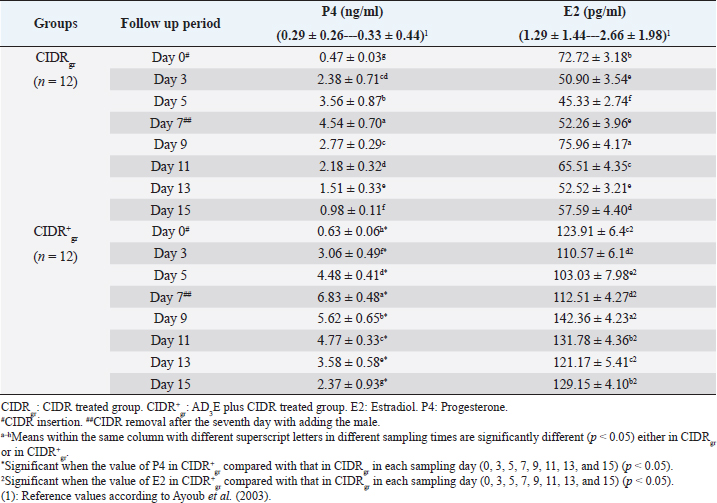
AbstractBackground: The scope of using controlled intra-vaginal drug releasers (CIDR) in the camel industry was small relative to that of cow, sheep, and goat. Aim: The purpose of this study was to determine the effects of intramuscular injection of AD3E (25 ml/400 kg) as a premedication protocol before CIDR treatment on ovarian hormones profile and calving rate in dromedary she-camels (Camelus dromedarius) through measuring concentrations of progesterone (P4) and estradiol (E2) before, during, and after CIDR use. Methods: The study was conducted on she-camels (C. dromedarius) (n=24) aged between 6 and 14 years. The animals were assigned into two equal groups: CIDR treated group (CIDRgr) and AD3E plus CIDR treated group (CIDR+gr). Results: Except for hemoglobin, the clinical and hematological findings showed no remarkable change either within each independent group or between CIDRgr and CIDR+gr. Serum P4 and E2 concentrations differed significantly between the first 7 days (during CIDR use) and days from 9 to 15 (after CIDR use) in dromedary camels. Serum concentrations of P4 and E2 showed significant elevation at CIDR+gr at day 0 compared with CIDRgr. This significant increase in serum of P4 and E2 at CIDR+gr compared to those of CIDRgr was also observed either during CIDR use (days 3, 5, and 7) or after CIDR use (days 9, 11, 13, and 15). During CIDR use, serum concentrations of P4 were negatively correlated with those of E2 either in CIDRgr or CIDR+gr, however, they were positively correlated after CIDR removal either in CIDRgror CIDR+gr. Regarding the calving rate, it was highest in CIDR+gr whereas CIDRgr showed the lowest one. Conclusion: It was concluded that the AD3E pretreatment protocol of she-camels before insertion of CIDR could be used successfully during the breeding season to improve fertility in she-camels whereas the serum ovarian hormones profile and calving rates were clearly changed due to pretreatment of she-camels with AD3E before CIDR insertion. Keywords: Camel estrus synchronization, AD3E, CIDR, Calving rates, Ovarian hormones. IntroductionRegarding reproduction, camels show low reproductive efficacy compared to farm animals mainly due to periods of reproductive inactivity (Kaufmann, 2005). In camels, ovulation could be induced by natural mating or hormonal treatments (Al-Sobayil, 2003). Hormones play a major role where they synchronize she-camel estrous, hence various exogenous elements were reported to improve camel reproductive fertility such as pregnant mare serum gonadotropin (Anouassi and Ali, 1990; Al-Fatlawi and Al-Hamedawi, 2017), equine chorionic gonadotropin (Al-Sobayil, 2003; Ararooti et al., 2018), and gonadotropin-releasing hormone (Bono et al., 1990; Manjunatha et al., 2018; Skidmore, 2018). During the pre-estrus period, the levels of reactive oxygen species (ROS) increase due to the increase in steroidogenic activity and metabolic rate (Agarwal et al., 2005). Moreover, estrus synchronization through the application of a controlled intra-vaginal drug releaser (CIDR) increases oxidative stress (Kuru et al., 2016; Martinez-Ros et al., 2018). Increased rates of ROS can negatively affect ovulation, the quality of oocytes, and progesterone (P4) production (Al-Gubory et al., 2010). It is well known that vitamins A, D3, and E which are fat-soluble vitamins that the animal body cannot produce make the external supply essential for covering the animal’s requirements (Hafez, 2012). Reproductive disorders associated with vitamin A deficiency in animals involve low conception rate, high embryo mortality, and low ovarian activities in females (Smith et al., 1984; Hurley and Doane, 1989; Corah and Ives, 1991; Clagett-Dame and DeLuca, 2002) as well as negatively affect male reproduction (Smith et al., 1984). Vitamin D plays an important role in the enhancement of animal immunity (Holick, 2006). The precise role of vitamin D is the regulation of calcium and phosphorus metabolism. Recently it is been reported that vitamin D plays a role in reproduction as there are vitamin D receptors in female reproductive organs such as ovaries, uterus, and endometrium (Muscogiuri et al., 2017; Hornstein, 2019). Also, phosphorus deficiency causes irregular estrus and anestrus and decreases the production of ovarian follicles which in turn decreases fertility and conception rate (Ahuja and Parmar, 2017, Muscogiuri et al., 2017). Vitamin E helps in maintaining the animal’s reproductive status (Maranesi et al., 2018; Mutalip et al., 2018) such as ovulation rate (Harrison et al., 1984), conception rate (Arechiga et al., 1994), and body resistance (Lee and Han, 2018; Mutalip et al., 2018). Lack of vitamin E causes reproductive system failure as the accumulation of free radical damages cell membranes as well as disrupts the synthesis of steroids and prostaglandins (Hemler and Lands, 1980; Staats et al., 1988; Lee and Han, 2018; Mutalip et al., 2018). It was observed that vitamin E supplementation increases the pregnancy rate (Laflamme and Hidiroglou, 1991; Awawdeh et al., 2019) and improves animal immunity (Lee and Han, 2018). The scope of using CIDR in the camel industry is small relative to that of cows, sheep, and goats. Results of trials conducted in camels demonstrate that long-term CIDR can be utilized in dromedary camels to synchronize follicular waves during the breeding season which enables the scheduling of calving periods and breeding (Swelum and Alowaimer, 2015). However, CIDR failed to synchronize follicular waves during the nonbreeding season in dromedary camels and couldn’t stimulate estrus out of season (Monaco et al., 2013). Considering this information, it was hypothesized that the combination of AD3E and short-term CIDR could have a positive effect on the induction of cyclicity followed by calving than that obtained after the use of short-term CIDR alone. Therefore, this study aimed to determine the effects of intramuscular injection of AD3E as a premedication protocol before CIDR treatment on ovarian hormones profile and calving rate in dromedary she-camels (Camelus dromedarius) through measuring concentrations of progesterone (P4) and estradiol (E2) before, during and after CIDR use concerning changes in clinical findings and hematological indices. Materials and MethodsAnimals and therapeutic strategyThe experiment was conducted on 24 clinically healthy adult she-camels (C. dromedarius) belonging to private farms in Aswan governorate, Egypt during their breeding season in December 2018. Healthy multiparous dromedary she-camels (n=24) weighing 410–460 kg and with an age range between 6 and 14 years old were randomly and equally assigned to two experimental groups (n=12 for each). One group was treated with CIDR alone as CIDR treated group (CIDRgr) while the other was treated with AD3E plus CIDR during the breeding season at a dose of AD3E as 25 ml/400 kg/every other day through intramuscular route (IM), for hence called AD3E plus CIDR treated group (CIDR+gr). In CIDR+gr and before CIDR treatment, she-camels were also previously treated with AD3E as IM vitamin preparation at a dose of 25 ml/400 kg/every other day for 3 weeks. AD3E was an intramuscularly injected vitamin preparation (QA11CB, Merial, France). CIDR was removed after the seventh day in the two groups and hence allowing the male camels with she-camels for breeding. After conducting the study, the investigated she-camels were released. During the study, camels had free access to food and water. Before starting the experiment, all camels were examined to exclude the ones with genital organ disease or pathology. All camels were examined clinically, and their blood samples were tested in the laboratory to remove the diseased camels and confirm their health status. Clinical examinationThe clinical examinations included measurements of heart and respiratory rates and recording of rectal temperatures was done as described by Fowler (2010) as followings; before CIDR insertion i.e., at day 0, during CIDR use i.e., at days 3, 5, and 7, and after CIDR removal i.e., at days 9, 11, 13 and 15. CIDR insertion and removalFor the intra-vaginal insertion of CIDR, after fixation of she-camels, the perineum of each she-camel was cleaned then disinfected then CIDR (1.38 g P4, Pfizer®, Italy) was gently inserted in the camel in a standing position and left for 7 days. The observation of the animals showed that none of them has lost the CIDR. For natural mating, an experienced male was introduced to she-camels after the removal of CIDR on day 7. All she-camels accepted the male showing signs of estrus that included increased amounts of vaginal mucous secretions, hyperemia, and congestion of vaginal epithelium and endometrium, the cervix was relaxed. During the experiment either before, during, or even after CIDR use, the females were undergoing a thorough gynecological examination to clarify the absence of abnormal secretions and reproductive disorders. Samples and laboratory analysisBlood samples (whole blood and serum) were collected every other day as follows; before CIDR insertion i.e., at day 0, during CIDR use (at days 3, 5, and 7), and after CIDR removal (at days 9, 11, 13). For laboratory evaluations, two blood samples were collected from the jugular veins of each camel; the first sample was placed in a vacutainer tube containing ethylene diamine tetra-acetic acid as an anticoagulant for hematological analysis, and the second sample was collected in a plain tube for biochemical analysis (Coles, 1986). Complete blood picture including red blood corpuscles (RBCs), total leucocytic count (TLC), differential leukocytic count (DLC), hemoglobin (Hb), packed cell volume (PCV), mean corpuscular values were manually estimated according to Harvey (2001) and Latimer et al. (2011). After centrifugation of the second blood sample, serum samples were collected and then frozen at −20°C until hormone levels measurements. P4 and E2 concentrations were determined using commercial radioimmunoassay kits E2 (ParameterTM, Cod; KGE014, USA) and P4 (Oxford Biomedical Research, Cod; EA 74, USA). Statistical analysisStatistics were conducted by Computer Software (SPSS version 16.0, Chicago). Three types of statistical analyses were used for analyzing the data obtained from the clinical findings and biochemical analyses throughout this study including general linear model repeated measures analysis of variances (ANOVA), independent-sample t-test, and correlation coefficient (Pearson Correlation). Data were presented as mean ± standard error values. These statistical analyses were carried out during three periods as followings: before CIDR insertion, during CIDR insertion, and after CIDR removal. Firstly, the obtained data were analyzed by general linear model repeated measures ANOVA and the significance level of results was set at p < 0.05. The significance of differences was evaluated between the means at selected sampling days (days 0, 3, 5, 7, 9, 11, 13, and 15) in each independent group (CIDRgr or CIDR+gr). Secondly, the obtained data were also analyzed by independent-sample t-test. The significance of differences between the means at CIDRgr and CIDR+gr on days 0, 3, 5, 7, 9, 11, 13, and 15, was evaluated by Dunnett’s test at p < 0.05. The correlation coefficient was calculated using Pearson Correlation at p < 0.05 or p < 0.01 between P4 and E2 on days 0, 3, 5, 7, 9, 11, 13, and 15. Ethical approvalAll animal procedures performed in this study were done according to Institutional Animal Care and Use Committee guidelines the animals during the experiment were handled according to the Use and Animal Care Committee of Aswan University and Assiut University which agree with the Guide for the use and care of Laboratory Animals of the National Institutes of Health-USA (NIH publication No. 86-23, revised 1996). The authors obtained written informed consent to use the animals in your study from the owners of the examined animals. ResultsClinical findingsThe clinical findings including temperature, pulse, and respiration either in CIDRgr or CIDR+gr were not significantly changed between days 0, 3, 5, 7, 9, 11, 13, and 15. No significant changes in clinical parameters were reported between CIDRgr and CIDR+gr either before, during, or after CIDR use. All clinical findings values were within the physiological reference range. The normal clinical findings indicated that all she-camels had good health and were free from any clinical abnormalities throughout this study (Table 1). Whole blood pictureExcept for Hb, the present results referred that the whole blood pictures including RBCs, PCV, TLC, and DLC either in CIDRgr or CIDR+gr were not significantly changed between days 0 (before CIDR insertion), 3, 5, 7, 9 (After CIDR removal), 11, 13 and 15. Hb concentrations were remarkably (p < 0.05) improved in both of CIDRgr and CIDR+gr between days 0, 3, 5, 7, 9, 11, 13, and 15. Hb levels were gradually increased after CIDR insertion at day 0 in the two groups until reaching day 7 in which Hb levels were relatively decreased and thus CIDR removal and allowing the male camels with she-camels for breeding occurred. Afterward, Hb concentrations were significantly (p < 0.05) elevated at days 9, 11, 13, and 15 when Hb values were compared with their values at day 0 either in CIDRgr or in CIDR+gr. No remarkable changes in the whole blood picture indices were demonstrated between CIDRgr and CIDR+gr either at day 0, 3, 5, 7, 9, 11, 13, or 15 (Table 2). Serum biochemical assaysThere were significant (p < 0.05) changes either increase or decrease in serum concentration of both P4 and E2 between days 0, 3, 5, 7, 9, 11, 13, and 15 either at CIDRgr or in CIDR+gr (Table 3). Regarding the impact of CIDR treatment on serum P4 concentration in dromedary camels, the overall mean P4 concentrations differed significantly during CIDR use (0–7 days) and after CIDR removal (9–15 days). Serum P4 concentrations were significantly (p < 0.05) elevated by CIDR insertion in both groups (CIDRgr and CIDR+gr) at D5 till reaching their highest values at D7. After CIDR removal in both groups and the introduction of a male camel for breeding, levels of P4 remarkably (p < 0.05) declined at D9 compared to their levels at D0, D3, D5, and D7. This significant reduction (p < 0.05) was observable in the following days until reached the lowest concentration at D15 (Table 3). Regarding AD3E treatments effects on serum P4 concentration, the findings revealed that At D0 the serum P4 concentration showed significant (p < 0.05) elevations in CIDR+gr (AD3E treated group) compared with CIDRgr (AD3E non-treated group). Also this significant (p < 0.05) increase in serum P4 concentrations in CIDR+gr was observed during CIDR use (at days 3, 5, and 7) and after CIDR removal (at days 9, 11, 13, and 15). Thus, treatment with AD3E produced a significantly greater P4 peak in CIDR+gr compared with CIDRgr through the experiment (Table 3). Table 1. Mean values (M ± SD) of temperature, pulse, and respiration in CIDRgr and CIDR+gr.
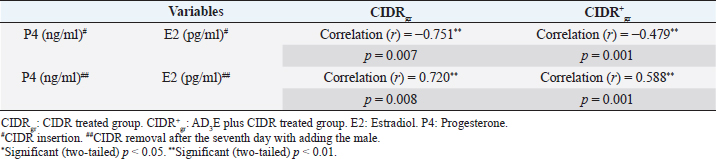
Regarding the impact of CIDR treatment on serum E2 concentration in dromedary camels, The overall means of E2 concentrations differed significantly between the first 7 days (During CIDR use) and days from 9 to 15 (After CIDR use) in both groups (Table 3). The results showed that serum E2 levels were significantly (p < 0.05) reduced after CIDR removal in CIDRgr and CIDR+gr at D3, D5, and D7 compared with levels at D0 (before CIDR insertion). At D9, in both groups, after CIDR removal the serum E2 concentrations were highly (p < 0.05) increased compared with concentrations at D0, D3, D5, and D7. This significant elevation in serum E2 was not observed after the ninth day in both examined groups. As well as serum E2 concentrations were significantly decreased (p < 0.05) either in CIDRgr or in CIDR+gr at D11, D13, and D15 compared with serum E2 values at D9. Moreover, only in CIDR+gr, the serum E2 levels at D15 (after CIDR removal) were still significantly (p < 0.05) higher than those at D0 (during CIDR use). Regarding AD3E treatments effects on serum E2 concentration, at D0 the findings revealed a significant (p < 0.05) elevation of serum E2 in CIDR+gr (AD3E treated group) values compared with CIDRgr (AD3E non-treated group). This significant (p < 0.05) elevation in serum concentrations of E2 at CIDR+gr compared to their values at CIDRgr was also observed at D3, D5, D7, D9, D11, D13, and D15 (Table 3). Serum concentrations of P4 were negatively correlated with serum concentrations of E2 in each of CIDRgr (r=−0.751, p=0.007) and CIDR+gr (r=−0.479, p=0.001) during CIDR use where the elevation in serum P4 during CIDR use was associated with a reduction in serum levels of E2 until the seventh day. On the other hand, serum concentrations of P4 were positively correlated with those of E2 in CIDRgr (r=0.720, p=0.008) and in CIDR+gr (r=−0.588, p=0.001) after CIDR removal and adding male animal for natural mating after the seventh day, and thus a reduction in serum P4 after CIDR removal was associated with a drop in serum level of E2 (Table 4). Calving ratesThe calving rates were improved in she-camels that received AD3E before CIDR compared with those that were not received AD3E. The calving rates obtained at CIDR+gr were higher compared to those of CIDRgr (Table 5). DiscussionThe physiological conditions had more effect biochemical and hormonal rather than hematological indices in camels raised under traditional conditions (Muhammad et al., 2011). The clinical parameters whether in CIDRgr or CIDR+gr were not significantly changed throughout the experiment. No remarkable changes in these clinical findings were observed between CIDRgr and CIDR+gr on any of these sampling days. All clinical findings values were within the physiological reference ranges reported by Bhatt et al. (1960), Schmit-Nielson (1964), Fowler (2010), and Hamad et al. (2017). Table 2. Mean values (M ± SD) of whole blood picture indices in CIDRgr and CIDR+gr.
Table 3. Mean values (M ± SD) of serum P4 and E2 concentrations in CIDRgr and CIDR+gr.
Table 4. Pearson correlation coefficient between P4 and E2 either at CIDRgr or at CIDR+gr during CIDR insertion.
Blood was an important index for several metabolic processes in camels which might in one animal species be variable due to age, sex, physiological status, and environmental factors (Ayoub et al., 2003). It was noticed through this work that no significant changes were described in whole blood indices either within the same investigated group (CIDRgr or CIDR+gr) or between the two groups except for Hb. Hb concentrations were remarkably improved in each of CIDRgr and CIDR+gr due to CIDR insertion and AD3E treatment. Furthermore, all blood picture indices were within the physiological reference ranges reported by Fowler (2010), Poonia et al. (2016), and Adah et al. (2017). The pattern of progesterone and estradiol-17β secretion was well-documented in cattle, buffalo, sheep, goats, mares, and pigs but was still needing more clarification in camels (Ayoub et al., 2003). Camels were different as a result of their induced ovulation rather than the spontaneous type in most species (Sumar, 2000). Rhythmic secretion of these sex steroids had a definite correlation with sexual behavior and receptivity of the male by females in other livestock species. In camelids, the periods of estrous and non-receptivity did not necessarily concur with ovarian status and levels of estradiol-17β and progesterone (Quzy et al., 2013). Throughout this study, profiles of P4 and E2 varied significantly during and after CIDR treatments in both groups. Also, CIDR+gr showed a maintained higher circulating P4 and E2 when compared to CIDRgr. The calving rate obtained after the combination of AD3E and CIDR was significantly increased (above 60%). The tendency for increased calving results in CIDR+gr might be related to high concentrations of P4 during the program of synchronization. Serum P4 concentrations in the present work in both groups were increased rapidly after CIDR insertion, reached the highest concentrations on day 7 then significantly decreased after CIDR removal. This finding may be attributed to the effect of the vaginal device (CIDR) in releasing P4 in a controlled manner after that was absorbed by vaginal epithelium, therefore, circulating P4 decreases by vaginal devices removal (Homeida and Mubarak, 2009; Lima et al., 2009; Swelum and Alowaimer, 2015). Serum concentrations of P4 were higher than their physiological reference values mentioned by Ayoub et al. (2003) throughout this study. It was important to mention that E2 concentrations in both she-camels treated groups either CIDRgr or CIDR+gr, were decreased after CIDR insertion, reached the lowest concentrations at day 7 then significantly increased after CIDR removal. This finding may be attributed to decreased P4 concentrations by device removal, which was associated with increased LH pulse frequency that initiates follicle growth after corpus luteum (CL) regression. This result was consistent with the findings of Bergfeld et al. (1996) and Hussein et al. (2015) who stated that the lowest P4 concentrations are correlated with the highest E2 concentrations and act as an indicator for CL regression. This study stated that serum concentrations of E2 were higher than their physiological reference values reported by Ayoub et al. (2003). Previous reports showed that in camels stimulated with short-term CIDR, the estrous response was less affected additionally the observed E2 concentrations after CIDR treatment for 10 days were higher than that after CIDR treatment for 7 days during the breeding season (Hussein et al., 2015). Therefore, it should be clarified how to manage and improve short-term CIDR to affect fertility. In favor of the role of AD3E, the findings in this study showed that P4 and E2 concentrations in she-camel were remarkably higher in CIDR+gr than in those in CIDRgr, injection of AD3E increased CIDR performance and maintaining high P4 and E2 concentrations during their use. The blood levels of P4 and E2 were significantly higher at CIDR+gr at day 0 (before CIDR use) or on days 9, 11, 13, and 15 (after CIDR removal) compared with those of CIDRgr. Awawdeh et al. (2019) referred that injection of vitamin E injection can improve reproductive performance, antioxidant status, and P4 concentration in the estrus-synchronized animal. These results are in contrast to the findings by Farahavar et al. (2020) who found that injection of vitamin E and selenium injection can’t improve reproductive performance in the estrus-synchronized ewe. Here, the obtained results indicate that the use of AD3E does significantly affect the efficacy of short-term CIDRs in camels. The exact mechanism by which AD3E improves CIDR performance is not completely known but it may be related to improving animal immunity and synthesis of the gonadotropin. Interestingly, it is reported that for most camels intra-vaginally insertion of CIDR or progesterone-releasing intravaginal device (PRID) may be associated with vaginitis and vaginal discharge (Al-Sobayil, 2008; Swelum and Alowaimer, 2015). Moreover, estrus synchronization through the application of CIDR increases oxidative stress (Kuru et al., 2016; Martinez-Ros et al., 2018). Increased rates of ROSs around mating can negatively affect ovulation and the quality of oocytes and P4 production (Al-Gubory et al., 2010). The physiological role of vitamins A, D, and E in the regulation of immunity has been puzzling as several reports pointed to a critical impact of A, D, e in maintain and enhancement animal immunity (Holick, 2006; Lee and Han, 2018; Mutalip et al., 2018). Treatment with CIDR+gr produced a significantly greater P4 peak compared to CIDRgr starting on the 7th, 9th, 11th, 13th, and 15th days through the experiment. This obtained findings about the greater P4 concentrations in CIDR+gr than in CIDRgr could be explained that vitamin D plays a role in reproduction due to vitamin D receptors were found in the ovary, uterus, and endometrium (Muscogiuri et al., 2017; Hornstein, 2019) and it increases the secretion of ovarian P4 secretion through the stimulation of 3β-HSD expression in ovary granulosa cells (Muscogiuri et al., 2017; Farahavar et al., 2020). This study reported that serum concentrations of P4 were negatively correlated with serum concentrations of E2 in each of CIDRgr and CIDR+gr during CIDR use. In contrast, the two hormones were positively correlated in the two examined groups of she-camels after CIDR removal (after day 7) and adding the male for natural. Hussein et al. (2015) added that the lowest P4 concentrations are correlated with the highest E2 concentrations and act as an indicator for CL regression. She camels in this study that had received AD3E before CIDR insertion had a higher calving rate compared with those that had not received AD3E. Therefore, the calving rates obtained at CIDR+gr were higher compared to those of CIDRgr. These animals had higher levels of P4 and E2 either before, during, or after CIDR use. This may be attributable to the findings reported by Lima et al. (2009), Bisinotto et al. (2010), Wiltbank et al. (2011a,b), and Denicol et al. (2012) who mentioned that cows with high circulating P4 during the growth of follicles have been linked to high conception results. Furthermore, the mechanisms of P4 in improving fertility are a result of the positive influence of the uterine endometrium condition, oocyte quality, and hypothalamus–hypophysis–gonad axis sensitivity (Bisinotto et al., 2010; Cerri et al., 2011a,b; Wiltbank et al., 2011a,b). Muscogiuri et al. (2017) added that vitamin D plays a role in successful implantation as it regulates HOXA10 expression in the endometrial stroma cells as well as helps in the maintenance of the fetoplacental unit through stimulation of the placental lactogen expression. Thus, calving results in this study seemed very acceptable for both treatments used. Moreover, the previous reports mentioned that female reproductive disorders associated with vitamin A deficiency are low conception rate, high embryo mortality, and low ovarian activities (Smith et al., 1984; Hurley and Doane, 1989; Corah and Ives, 1991; Clagett-Dame and DeLuca, 2002). For instance, different studies have described a positive relationship between vitamin E and animal reproductive statuses such as ovulation rate and conception rate (Harrison et al., 1984; Arechiga et al., 1994). The clearest effect of vitamin E supplementation is increasing P4 concentration (Farahavar et al., 2020). Lack of vitamin E causes reproductive system failure and disrupts the synthesis of steroids and prostaglandins (Hemler and Lands, 1980; Staats et al., 1988). ConclusionThe study did not report any effects of AD3E pretreatment protocol in examined she-camels on clinical findings and hematological indices with the exception of Hb. The AD3E pretreatment protocol of she-camels before insertion of CIDR could be used successfully during the breeding season to improve fertility in she-camels whereas the serum ovarian hormones and calving rates were greatly changed by pretreatment of she-camels with AD3E before CIDR use. AD3E treatment before CIDR insertion in she-camels enhanced its performance to produce an increase in P4 concentrations and a decrease in E2 concentrations (P4 and E2 were negatively correlated). After CIDR withdrawal, the levels of P4 and E2 decreased inducing the emergence of a new follicular wave (P4 and E2 were positively correlated). Therefore, AD3E plus CIDR protocol can be used to synchronize the follicular waves in dromedary camels. The findings generate information on a possible effective reproductive strategy to manage and improve CIDR performance in camel management. AcknowledgmentNone. Conflict of interestThe authors declare that they have no conflict of interest. FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Authors’ contributionRHM, EE, and AK prepared the conception and design of the study; AK, AA, EE, RHM, and ESA conducted the field study and camel examination; RHM, FA, and EE collected laboratory samples and conducted biochemical analyses; AA, EE, and ESA manipulate and statistically analyzed the data. AK, ESA, and FA conducted data curation and interpretation of data; RHM, FA, and AK drafted the manuscript; AK, AA, EE, and RHM carried out final writing, critical review, and revision. All authors have read and approved the final manuscript. Availability of data and materialsThe datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request. ReferencesAdah, A.S., Ayo, J.O., Rekwot, P.I., Aluwong, T. and Arimie, D.I. 2017. Haematological profile of the one-humped camel subjected to packing (load-carrying) in the harmattan season in the semi-arid region of Nigeria. Bangladesh J. Vet. Med. 15(1), 39–44. Agarwal, A., Gupta, S. and Sharma, R.K. 2005. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 3(1), 28. Ahuja, A. and Parmar, D., 2017. Role of minerals in reproductive health of dairy cattle: a review. Int. J. Livest. Res. 7(10), 16–26. Al-Fatlawi, A. and Al-Hamedawi, T. 2017. Induction of fertile estrus in Iraqi camel (Camelus dromedarius) during seasonal anoestrus. Kufa j. vet. Sci. 8(1), 58–63. Al-Gubory, K.H., Fowler, P.A. and Garrel, C., 2010. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 42(10), 1634–1650. Al-Sobayil, K. 2003. Hormonal treatments for inducing fertile oestrus in young dromedary females at the end of their seasonal anoestrus in Quseem region, Saudi Arabia. J. Camel Pract. Res. 10(2), 179–181. Al-Sobayil, K.A. 2008. The use of estrus synchronization and timed artificial insemination in dromedary she-camels in Saudi Arabia. J. Agri. Vet. Sci. Qassim Univ. 1(1), 3–9. Anouassi, A. and Ali, A. 1990. Embryo transfer in camel (Camelus dromedarius). Proc. Unite de Coordination pour LElevage Camelin. In: Is it possible to improve the reproductive performance of the camel. Paris, France, pp: 327–331. Ararooti, T., Niasari-Naslaji, A., Asadi-Moghaddam, B., Razavi, K. and Panahi, F. 2018. Superovulatory response following FSH, eCG-FSH and hMG and pregnancy rates following transfer of hatched blastocyst embryos with different diameter and shape in dromedary camel. Theriogenology 106, 149–156. Arechiga, C., Ortiz, O. and Hansen, P. 1994. Effect of prepartum injection of vitamin E and selenium on postpartum reproductive function of dairy cattle. Theriogenology 41(6), 1251–1258. Awawdeh, M., Eljarah, A. and Ababneh, M. 2019. Multiple injections of vitamin E and selenium improved the reproductive performance of estrus-synchronized Awassi ewes. Trop. Anim. Health Prod. 51(6), 1421–1426. Ayoub, M. A., El-Khouly, A.A. and Mohamed, T.M. 2003. Some hematological and biochemical parameters and steroid hormone levels in the one-humped camel during different physiological conditions. Emir. J. Food Agric. 15, 44–55. Bhatt, F.L., Kholi, R.N. and Rathore, U.S. 1960. The normal body temperature, respiratory frequency and heart rate of the camel. Indian Vet. J. 37, 456–462. Bergfeld, E., Kojima, F., Cupp, A.S., Wehrman, M., Peters, K., Mariscal, V., Sanchez, T. and Kinder, J. 1996. Changing dose of progesterone results in sudden changes in frequency of luteinizing hormone pulses and secretion of 17β-estradiol in bovine females. Biol. Reprod. 54(3), 546–553. Bisinotto, R., Chebel, R. and Santos, J. 2010. Follicular wave of the ovulatory follicle and not cyclic status influences fertility of dairy cows. J. Dairy Sci. 93(8), 3578–3587. Bono, G.D., Moallim, A. and Comin, A. 1990. Seasonal effects on the endocrine reproductive activity of the dromedary camels. In: Proceedings of UCDEC Workshop, is it possible to improve the reproductive performance in the camel. Paris, France, pp: 107–115. Cerri, R., Chebel, R., Rivera, F., Narciso, C., Oliveira, R., Amstalden, M., Baez-Sandoval, G., Oliveira, L., Thatcher, W. and Santos, J. 2011a. Concentration of progesterone during the development of the ovulatory follicle: II. Ovarian and uterine responses. J. Dairy Sci. 94(7), 3352–3365. Cerri, R., Chebel, R., Rivera, F., Narciso, C., Oliveira, R., Thatcher, W. and Santos, J. 2011b. “Concentration of progesterone during the development of the ovulatory follicle: I. Ovarian and embryonic responses. J. Dairy Sci. 94(7), 3342–3351. Clagett-Dame, M. and DeLuca, H.F. 2002. The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 22(1), 347–381. Coles, EH. 1986. Veterinary Clinical Pathology. 4th ed. Philadelphia, PI: W.B. Saunders, pp: 132–139. Corah, L.R. and Ives, S. 1991. The effects of essential trace minerals on reproduction in beef cattle. Vet. Clin. North Am. Food Anim. Pract. 7(1), 41–57. Denicol, A., Lopes Jr, G., Mendonça, L., Rivera, F., Guagnini, F., Perez, R., Lima, J., Bruno, R., Santos, J. and Chebel, R. 2012. Low progesterone concentration during the development of the first follicular wave reduces pregnancy per insemination of lactating dairy cows. J. Dairy Sci. 95(4), 1794–1806. Farahavar, A., Rostami, Z., Alipour, D. and Ahmadi, A. 2020. The effect of pre-breeding vitamin E and selenium injection on reproductive performance, antioxidant status, and progesterone concentration in estrus-synchronized Mehraban ewes. Trop. Anim. Health Prod. 52(4), 1797–1786. Fowler, M.E. 2010. Medicine and surgery of Camelids. 3rd ed. Ames, IA: Blackwell Publishing, Ltd; A John Wiley & Sons, pp: 89–109, vol. 408. Hafez, Y. 2012. Enhancing milk production in periparturient buffalo fed protected fat with and without vitamin ad3e treatment. E.J.A.P. 49(3), 249–256. Hamad, B., Aggad, H., Hadef, L. and Adaika, A. 2017. Effect of cold and hot seasons on thermoregulation and hemogram blood parameters of dromedary camel (Camelus dromedarius) in Algeria. Livestock Res. Rural Dev. 29(7), 1–8. Harrison, J.H., Hancock, D.D. and Conrad, H. 1984. Vitamin E and selenium for reproduction of the dairy cow 1, 2. J. Dairy Sci. 67(1), 123–132. Harvey, J.H. 2001. Atlas of veterinary hematology. Philadelphia, PI: Elsevier, WB Saunders Company, pp: 3–74. Hemler, M. and Lands, W. 1980. Evidence for a peroxide-initiated free radical mechanism of prostaglandin biosynthesis. J. Biol. Chem. 255(13), 6253–6261. Holick, M.F. 2006. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin. Proc. 81(3), 353–373. Homeida, A. and Mubarak, A. 2009. Effect of administration of different progesterone formulations on the plasma progesterone concentrations in she-camel. Res. Pharmacol. (1), 19–21. Hornstein, M.D. 2019. Vitamin D and infertility: the evidence. Fertil. Reprod. 1(1), 31–33. Hurley, W. and Doane, R. 1989. Recent developments in the roles of vitamins and minerals in reproduction. J. Dairy Sci. 72(3), 784–804. Hussein, F.M., Metwelly, K.K., Mona, A.M. and Ragab, M.H. 2015. Effect of CIDR application duration (7-10-14 days) on circulating estrogen and progesterone during breeding and non-breeding season in she-camels. Alex. J. Vet. Sci. 44(1), 125–129. Kaufmann, B.A. 2005. Reproductive performance of camels (Camelus dromedarius) under pastoral management and its influence on herd development. Livest. Prod. Sci. 92(1), 17–29. Kuru, M., Ögün, M., Oral, H., Kükürt, A., Makav, M. and Kulaksız, R. 2016. The use of controlled internal drug release for synchronization augmented oxidative and nitrosative stress and leptin levels in Georgian goats. J. Cell Neurosci. Oxid. Stress 8(1), 541–542. Laflamme, L. and Hidiroglou, M. 1991. Effects of selenium and vitamin E administration on breeding of replacement beef heifers. Annal. Vet. Res. 22(1), 65–69. Latimer, K.S., Mahaffey, E.A. and Prasse, K.W. 2011. Duncan and prasse’s veterinary laboratory medicine: clinical pathology. 5th ed. Ames, IA: Blackwell Publishing Ltd; Wiley-Blackwell, pp: 3–82. Lee, G.Y. and Han, S.N. 2018. The role of vitamin E in immunity. Nutrients 10(11), 1614. Lima, J., Rivera, F., Narciso, C., Oliveira, R., Chebel, R. and Santos, J. 2009. Effect of increasing amounts of supplemental progesterone in a timed artificial insemination protocol on fertility of lactating dairy cows. J. Dairy Sci. 92(11), 5436–5446. Manjunatha, B., Al-Hosni, A. and Al-Bulushi, S. 2018. Resynchronization of synchronized follicular wave in dromedary camels of unknown pregnancy status (Camelus dromedarius). Theriogenology 119, 208–213. Maranesi, M., Castellini, C., Dall’Aglio, C., Petrucci, L., Mattioli, S., Boiti, C. and Zerani, M. 2018. Effects of PUFAs on animal reproduction: male and female performances and endocrine mechanisms. Phytochem. Rev. 17(4), 801–814. Martinez-Ros, P., Lozano, M., Hernandez, F., Tirado, A., Rios-Abellan, A., López-Mendoza, M.C. and Gonzalez-Bulnes, A. 2018. Intravaginal device-type and treatment-length for ovine estrus synchronization modify vaginal mucus and microbiota and affect fertility. Animals 8(12), 226. Monaco, D., Lacalandra, G.M. and El-Bahrawy, K.A. 2013. Ovarian monitoring and effects of controlled intravaginal drug releaser (CIDR) on vaginal environment and follicular activity in dromedary camels, during non-breeding season in Egypt. Emir. J. Food Agric. 25(4), 296–300. Muhammad, B.F., Aliyu, D., Njidda, A.A. and Madigawa, I.L. 2011. Some haematological, biochemical and hormonal profile of pregnant and non-pregnant she-camels (Camelus dromedarius) raised in a Sudan savanna zone of Nigeria. J. Camel Prac. Res. 18, 73–77. Muscogiuri, G., Altieri, B., de Angelis, C., Palomba, S., Pivonello, R., Colao, A. and Orio, F. 2017. Shedding new light on female fertility: the role of vitamin D. Rev. Endocr. Metab. Disord. 18(3), 273–283. Mutalip, M., Syairah, S., Ab-Rahim, S. and Rajikin, M.H. 2018. Vitamin E as an antioxidant in female reproductive health. Antioxidants 7(2), 22. Poonia, R., Srivastava, A., Sena, S., Srivastava, M., 2016. Study on certain blood and serum parameters of camel Camelus dromedarius maintained on different diets. UK. J. Pharm. Biosci. 4(6), 13. Quzy, I., Anwar, S. and Purohit, G.N. 2013. Hormonal management of ovarian activity in breeding camels 2 months ahead of the natural breeding season. Camel Int. J. Vet. Sci. 1, 37–49. Schmit-Nielson, K. 1964. Desert animals. Physiological problems of heat and water. London, UK: Oxford Clarendon Press, 277 p. Skidmore, J. 2018. Reproduction in dromedary camels: an update. Anim. Reprod. 2(3), 161–171. Smith, K.L., Harrison, J., Hancock, D., Todhunter, D. and Conrad, H. 1984. Effect of vitamin E and selenium supplementation on incidence of clinical mastitis and duration of clinical symptoms. J. Dairy Sci. 67(6), 1293–1300. Staats, D., Lohr, D. and Colby, H. 1988. Effects of tocopherol depletion on the regional differences in adrenal microsomal lipid peroxidation and steroid metabolism. Endocrinology 123(2), 975–980. Sumar, J.B. 2000. Liama and alpacas: In: Reproduction in Farm Animals. Eds., Hafez, B. and Hafez, E.S.E. 7th ed. Philadelphia, PI: Lippincott Williams and Wilkins; Wolter Kluwer Co., pp: 218–236. Swelum, A.A. and Alowaimer, A.N. 2015. The efficacy of controlled internal drug release (CIDR) in synchronizing the follicular wave in dromedary camels (Camelus dromedarius) during the breeding season. Theriogenology 84(9), 1542–1548. Wiltbank, M.C., Carvalho, P.D., Kaskin, A., Hackbart, K.S., Meschiatti, M.A., Bastos, M.R., Guenther, J.N., Nascimento, A.B., Herlihy, M.M. and Amundson, M.C. 2011a. Effect of progesterone concentration during follicle development on subsequent ovulation, fertilization, and early embryo development in lactating dairy cows. In: Proc. 44th Annu. Mtg. Soc. Study Reprod., Portland, Oregon, Madison, WI: Society for the Study of Reproduction. Wiltbank, M.C., Souza, A.H., Carvalho, P.D., Bender, R.W. and Nascimento, A.B. 2011b. Improving fertility to timed artificial insemination by manipulation of circulating progesterone concentrations in lactating dairy cattle. Reprod. Fertil. Dev. 24(1), 238–243. | ||
| How to Cite this Article |
| Pubmed Style Mohamed RH, Khalphallah A, Ali F, Abdel-lah ES, Abdulkarim A, Elmeligy E. Impact of vitamin AD3E treatment before application of controlled intra-vaginal drug releaser (CIDR) on clinical and hematologic findings, ovarian hormones and calving rates in she camels (Camelus dromedarius). Open Vet J. 2022; 12(6): 965-974. doi:10.5455/OVJ.2022.v12.i6.24 Web Style Mohamed RH, Khalphallah A, Ali F, Abdel-lah ES, Abdulkarim A, Elmeligy E. Impact of vitamin AD3E treatment before application of controlled intra-vaginal drug releaser (CIDR) on clinical and hematologic findings, ovarian hormones and calving rates in she camels (Camelus dromedarius). https://www.openveterinaryjournal.com/?mno=50352 [Access: April 04, 2025]. doi:10.5455/OVJ.2022.v12.i6.24 AMA (American Medical Association) Style Mohamed RH, Khalphallah A, Ali F, Abdel-lah ES, Abdulkarim A, Elmeligy E. Impact of vitamin AD3E treatment before application of controlled intra-vaginal drug releaser (CIDR) on clinical and hematologic findings, ovarian hormones and calving rates in she camels (Camelus dromedarius). Open Vet J. 2022; 12(6): 965-974. doi:10.5455/OVJ.2022.v12.i6.24 Vancouver/ICMJE Style Mohamed RH, Khalphallah A, Ali F, Abdel-lah ES, Abdulkarim A, Elmeligy E. Impact of vitamin AD3E treatment before application of controlled intra-vaginal drug releaser (CIDR) on clinical and hematologic findings, ovarian hormones and calving rates in she camels (Camelus dromedarius). Open Vet J. (2022), [cited April 04, 2025]; 12(6): 965-974. doi:10.5455/OVJ.2022.v12.i6.24 Harvard Style Mohamed, R. H., Khalphallah, . A., Ali, . F., Abdel-lah, . E. S., Abdulkarim, . A. & Elmeligy, . E. (2022) Impact of vitamin AD3E treatment before application of controlled intra-vaginal drug releaser (CIDR) on clinical and hematologic findings, ovarian hormones and calving rates in she camels (Camelus dromedarius). Open Vet J, 12 (6), 965-974. doi:10.5455/OVJ.2022.v12.i6.24 Turabian Style Mohamed, Ragab H., Arafat Khalphallah, Fatma Ali, Ebtsam S. Abdel-lah, Abdulrahman Abdulkarim, and Enas Elmeligy. 2022. Impact of vitamin AD3E treatment before application of controlled intra-vaginal drug releaser (CIDR) on clinical and hematologic findings, ovarian hormones and calving rates in she camels (Camelus dromedarius). Open Veterinary Journal, 12 (6), 965-974. doi:10.5455/OVJ.2022.v12.i6.24 Chicago Style Mohamed, Ragab H., Arafat Khalphallah, Fatma Ali, Ebtsam S. Abdel-lah, Abdulrahman Abdulkarim, and Enas Elmeligy. "Impact of vitamin AD3E treatment before application of controlled intra-vaginal drug releaser (CIDR) on clinical and hematologic findings, ovarian hormones and calving rates in she camels (Camelus dromedarius)." Open Veterinary Journal 12 (2022), 965-974. doi:10.5455/OVJ.2022.v12.i6.24 MLA (The Modern Language Association) Style Mohamed, Ragab H., Arafat Khalphallah, Fatma Ali, Ebtsam S. Abdel-lah, Abdulrahman Abdulkarim, and Enas Elmeligy. "Impact of vitamin AD3E treatment before application of controlled intra-vaginal drug releaser (CIDR) on clinical and hematologic findings, ovarian hormones and calving rates in she camels (Camelus dromedarius)." Open Veterinary Journal 12.6 (2022), 965-974. Print. doi:10.5455/OVJ.2022.v12.i6.24 APA (American Psychological Association) Style Mohamed, R. H., Khalphallah, . A., Ali, . F., Abdel-lah, . E. S., Abdulkarim, . A. & Elmeligy, . E. (2022) Impact of vitamin AD3E treatment before application of controlled intra-vaginal drug releaser (CIDR) on clinical and hematologic findings, ovarian hormones and calving rates in she camels (Camelus dromedarius). Open Veterinary Journal, 12 (6), 965-974. doi:10.5455/OVJ.2022.v12.i6.24 |