| Short Communication | ||
Open Vet J. 2022; 12(6): 782-786 Open Veterinary Journal, (2022), Vol. 12(6): 782–786 Short Communication The presence of adhesion factors NOX, α-enolase, TrmFO, P27, and VpmaX in Mycoplasma bovis wild isolates in JapanFumitaka Shitamori, Ryoko Uemura*, Takuya Kanda and Masuo SueyoshiDepartment of Veterinary Sciences, Faculty of Agriculture, University of Miyazaki, 1-1 GakuenKibanadai-Nishi, Miyazaki 889-2192, Japan Submitted: 15/06/2022 Accepted: 05/10/2022 Published: 03/11/2022 *Corresponding Author: Ryoko Uemura. Department of Veterinary Sciences, Faculty of Agriculture, University of Miyazaki, Japan. Email: uemurary [at] cc.miyazaki-u.ac.jp © 2022 Open Veterinary Journal
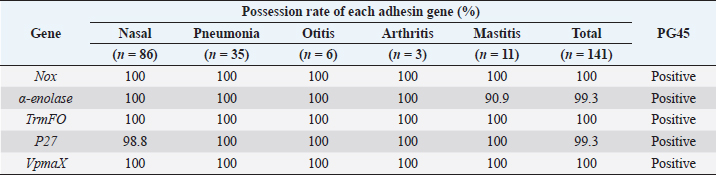
AbstractBackground: Mycoplasma bovis causes various diseases such as bronchopneumonia, otitis media, arthritis, and mastitis in cattle. Mycoplasma bovis is often isolated from the deep pharynges of healthy cattle and is generally considered not to cause clinical symptoms while in the upper respiratory tract. In mycoplasma infections, adhesion to the host cells is a crucial step. In recent years, five new adhesins, NOX, α-enolase, TrmFO, P27, and VpmaX, have been reported in M. bovis strains from pneumonia cases. However, the presence of these adhesins in wild isolates has not been established. Aim: This study aimed to investigate the presence of these adhesin genes in wild isolates isolated from cattle nasal cavities and lesion sites (pneumonia, otitis media, arthritis, and mastitis) in various regions in Japan and clarify the relationship between adhesion and the symptoms caused by M. bovis infection. Methods: A total of 141 M. bovis wild isolates isolated from nasal cavities (healthy or sick cattle), lungs with pneumonia, ears with otitis media, joint fluids of arthritic animals, and milk of mastitic animals. Mycoplasma bovis type strain PG45 was also used. Specific polymerase chain reaction reactions were performed to detect nox, α-enolase, trmFO, P27, and vpmaX, which are adhesins of M. bovis. Results: This study reports 139 M. bovis wild isolates were positive for nox, α-enolase, trmFO, P27, and vpmaX, while two isolates each lacked α-enolase or P27 genes. Mycoplasma bovis PG45 also had all five adherens genes. Conclusion: Almost all M. bovis wild isolates possessed all nox, α-enolase, trmFO, P27, and vpmaX genes regardless of the lesion site or region of origin. This means no relationship was found between the presence of the five adhesins and lesion sites in M. bovis and M. bovis isolated from the nasal cavities of asymptomatic cattle have the same numbers and types of adhesins as isolates from symptomatic lesion sites (pneumonia, otitis media, arthritis, and mastitis). This suggests that not only M. bovis isolates from pulmonary lesions, but also M. bovis existing in the nasal cavity has the potential to causes symptoms in the host. Keywords: Adhesin, Cattle, Mycoplasma bovis. IntroductionMycoplasma bovis belongs to the Mollicutes and lacks a cell wall, and it can cause bronchopneumonia, otitis media, arthritis, and mastitis in cattle (Bürki et al., 2015). However, M. bovis is often isolated from the deep pharynges of healthy cattle (Lima et al., 2016) and is generally considered not to cause clinical symptoms while in the upper respiratory tract (Hananeh et al., 2018). When M. bovis reaches the lung airways though it causes pneumonia. Otitis media is caused following pneumonia and arthritis, and pneumonia or mastitis is often observed during arthritis onset (Maunsell et al., 2011). Clinical signs of arthritis and pneumonia are often present at the same time (Caswell and Archambault, 2007). Mycoplasma bovis antigen has also been detected in the liver and kidney by immunohistochemistry (Maeda et al., 2003). In addition, it was reported that M. bovis is involved in bovine endocarditis (Kanda et al., 2019). These studies strongly suggest that M. bovis can hematogenously spread to the several organs and tissues and cause lesions at various sites. One of the first steps in mycoplasma infection is adhesion to host cells (Bürki et al., 2015). Adhesins expressed on the cell membrane of M. bovis, which lacks a cell wall, are very important factors for the microorganism. P26 and variable surface lipoproteins (Vsps) are well known as typical M. bovis adhesins (Sachse et al, 2000; Bürki et al., 2015). In recent years, with progress in M. bovis genomics, NOX, α-enolase, TrmFO, P27, and VpmaX have been reported as M. bovis adhesins in strains Hubei-1 and HB0801 (Song et al., 2012; Zou et al., 2013; Guo et al, 2017; Zhao et al., 2017; Chen et al, 2018). NOX, α-enolase, TrmFO, and P27 are fibronectin- or plasminogen-binding proteins (Song et al., 2012; Guo et al, 2017; Zhao et al., 2017; Chen et al, 2018), and VpmaX is a lipoprotein expressed on the cell membrane (Zou et al., 2013). Unlike Hubei-1 and HB0801 strains, which were isolated from pneumonia cases (Li et al., 2011; Qi et al., 2012), it is unknown whether isolates isolated from other lesion sites such as those of otitis media, arthritis, and mastitis possess these adhesins. In addition, the number of isolates whose genomes have been analyzed is very few, and the presence of these adhesins in wild isolates has not been established. Therefore, we investigated the presence of NOX, α-enolase, TrmFO, P27, and VpmaX genes by polymerase chain reaction (PCR) in wild M. bovis isolates from various lesion sites in various regions of Japan. This will clarify the relationship between adhesion and the symptoms caused by M. bovis infection and help elucidate its pathology. Materials and MethodsSample used in this studyA total of 141 M. bovis wild isolates from 134 cattle in 10 prefectures in Japan from 2008 to 2013 were used in this study. The number of isolates from nasal cavities (healthy or sick cattle), lungs with pneumonia, ears with otitis media, joint fluids of arthritic animals, and milk of mastitic animals was 86, 35, 6, 3, and 11, respectively. To confirm the identity of the isolates, PCR with specific primers for the OppD/F gene (Hotzel et al., 1996) or loop-mediated isothermal amplification (Higa et al., 2016) of M. bovis were performed. Mycoplasma bovis type strain PG45 was also used, and all isolates were stored at −80°C until use. DNA of each isolate was extracted by the hot-shot method after incubation for 4 or 5 days at 37 °C in NK broth (Kanto Chemistry, Tokyo, Japan) and stored at −20°C until use. PCR to detect adhesinsSpecific PCR reactions were performed to detect nox, α-enolase, trmFO, P27, and vpmaX, which are adhesins of M. bovis. Primers for α-enolase and trmFO were designed according to the conserved sequences of M. bovis provided by GeneBank (accession numbers: CP002513 and CP002058). Primers for nox, P27, and vpmaX were designed based on previous reports (Zou et al., 2013; Zhao et al., 2017; Chen et al., 2018). The primer information is shown in Table 1. Amplification of these genes was performed using KAPA2G Fast HS ReadyMix + dye (2×) (Nippon Genetics, Tokyo, Japan). Each primer was added at 0.5 µM final concentration, and the template DNA was added at 1.0 l. The reaction mixture was subjected to the following conditions: 2 minutes at 94°C, 30 cycles of 30 seconds at 94°C, 30 seconds at 44°C–53°C, and 1–2 minutes at 72°C, with a final cycle of 4 minutes at 72°C, followed by a hold at 4°C (Table 2). Samples were subjected to horizontal gel electrophoresis in 1.5% agarose and photographed using ChemiDocTM Touch (Bio-Rad, Hercules, CA). Ethical approvalNot required for this study. Results and DiscussionThe results are shown in Table 3. Of the M. bovis wild isolates, 139 were positive for nox, α-enolase, trmFO, P27, and vpmaX, while two isolates each lacked α-enolase or P27 genes. Mycoplasma bovis PG45 also had all five adherens genes. Mycoplasma bovis tested in this study were wild isolates from nasal cavities and infection sites with pneumonia (lung), otitis media (ear), arthritis (joint fluid), and mastitis (milk) of beef cattle and dairy cows from various regions in Japan. We found that 139 wild isolates possessed all nox, α-enolase, trmFO, P27, and vpmaX genes regardless of the lesion site or region of origin. This suggests that the M. bovis in Japan may have these genes universally. Mycoplasma is a host-dependent parasite with a small genome and poor metabolic systems (Caswell et al., 2007; Wise et al, 2011). Therefore, adhesion to the host cells is considered to be very important for survival. Although M. bovis has one of the smallest genomes among bacteria, at approximately 1,080 kb (Wise et al, 2011), it has abundant adhesins. Mycoplasma bovis NOX is an enzyme that is similar to Streptococcus pneumoniae and Streptococcus pyogenes NADH oxidase and catalyzes the reduction of oxygen to hydrogen peroxide (Zhao et al., 2017). That is, M. bovis NOX may be able to adapt to oxygen to allow pathogen growth under oxidative stress and promote growth in the oxygen-rich bovine respiratory tract (Zhao et al., 2017). Hydrogen peroxide is a major virulence factor in mycoplasma, in that it has cytotoxic effects and inhibits ciliary clearance (Bürki et al., 2015). Therefore, M. bovis NOX is considered to be an important virulence factor not only for adhesion but also for adaptability to oxygen. α-Enolase is expressed on the cell surfaces of various bacteria such as Streptococcus pnemoniae, Streptococcus mutans, and Mycoplasma fermentas, and it binds to plasminogen (Bergmann et al., 2003; Jones and Holt, 2007; Yavlovich et al., 2007). Because plasminogen produced from the liver is in systemic circulation, the binding of those pathogens to plasminogen may facilitate dissemination of the infection in the host (Song et al., 2012). Streptococcus pneumoniae is the most common causative pathogen of community-acquired pneumonia and causes otitis media and sinusitis in humans (Bergmann et al., 2003). However, sometimes the bacteria cause fatal diseases such as bacteremia and meningitis, leading to high mortality (Bergmann et al., 2003). Streptococcus mutans enters the bloodstream and may be associated with infective endocarditis (Jones and Holt, 2007), and M. bovis has also been reported to be involved in endocarditis (Kanda et al., 2019). Arthritis caused by M. bovis is secondary to pneumonia and mastitis, and the clinical symptoms of arthritis and pneumonia are often present at the same time (Caswell and Archambault, 2007). In addition, M. bovis antigen has been detected in the liver and kidney (Maeda et al., 2003). These findings suggest that M. bovis also enters the bloodstream and is disseminated throughout the whole body. In this study, almost all isolates possessed α-enolase. This suggests that the presence of M. bovis α-enolase may be involved in dissemination to the whole body. TrmFO and P27 bind to fibronectin (Guo et al., 2017; Chen et al., 2018), which exists in soluble form and in the extracellular matrix in various bodily fluids and tissues (Guo et al., 2017; Chen et al., 2018), so M. bovis TrmFO and P27 may be associated with its spread to various host tissues. VpmaX is a lipoprotein expressed on the cell membrane surface and is thought to play an important role in M. bovis infection (Zou et al., 2013), but the details are unknown. Table 1. Primer set for each adhesin gene specific PCR used in this study.
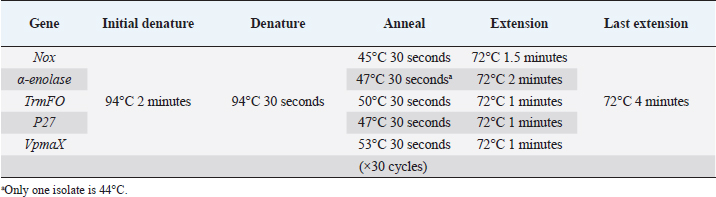
Table 2. PCR conditions for each adhesin gene in this study.
Table 3. Possession rate (%) of each adhesin genes in Mycoplasma bovis wild isolates from each site, addition to M. bovis PG45.
In this study, no relationship was found between the presence of the five adhesins and lesion sites in M. bovis. However, this means that M. bovis isolated from the nasal cavities of asymptomatic cattle have the same numbers and types of adhesins as isolates from symptomatic lesion sites (pneumonia, otitis media, arthritis, and mastitis). Mycoplasma bovis has also been isolated from the deep pharynges of healthy cows (Lima et al., 2016), and it may not cause clinical symptoms as long as it is in the upper respiratory tract (Hananeh et al., 2018). However, it is thought that suppression of the host immune system caused by stress or viral infection results in the rapid growth of pathogenic bacteria in the upper respiratory tract and reduced clearance in the lower respiratory tract, and these pathogenic bacteria reach the lungs (Griffin et al., 2010). After reaching the lung airways, this microorganism causes pneumonia, spreads throughout the body via the bloodstream, and causes various symptoms in infected hosts. This suggests that even M. bovis existing in the nasal cavity of healthy cows may colonize various tissues and result in lesions. The period during which M. bovis exists in the bloodstream in the host is short (Caswell and Archambault, 2007). Nevertheless, during this time, it spreads to various tissues and causes myriad symptoms. This may be due to the abundance of adhesins as shown in this study. In conclusion, we found that M. bovis wild isolates in Japan universally possess nox, α-enolase, trmFO, P27, and vpmaX. The presence of this variety of adhesins may be involved in the pathogenicity and pathology of M. bovis. AcknowledgmentsThe authors would like to thank Drs. Higuchi and Gondaira of Rakuno Gakuen University who provided some M. bovis wild isolates. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsConceptualization, F. Shitamori, T. Kanda, and R. Uemura; methodology, F. Shitamori and T. Kanda; validation, F. Shitamori and R. Uemura; investigation, F. Shitamori and T. Kanda; resources, R. Uemura; writing—original draft preparation, F. Shitamori; writing—review and editing, F. Shitamori, R. Uemura and M. Sueyoshi; supervision, R. Uemura and M. Sueyoshi; project administration, R. Uemura; funding acquisition, R. Uemura. All authors have read and agreed to the published version of the manuscript. ReferencesBergmann, S., Wild, D., Diekmann, O., Frank, R., Bracht, D., Chhatwal, G.S. and Hammerschmidt, S. 2003. Identification of a novel plasmin (ogen)-binding motif in surface displayed α-enolase of Streptococcus pneumoniae. Mol. Microbiol. 49, 411–423. Bürki, S., Frey, J. and Pilo, P. 2015. Virulence, persistence and dissemination of Mycoplasma bovis. Vet. Microbiol. 179, 15–22. Caswell, J. L. and Archambault, M. 2007. Mycoplasma bovis pneumonia in cattle. Anim. Health Res. Rev. 8, 161–186. Chen, X., Huang, J., Zhu, H., Guo, Y., Khan, F.A., Menghwar, H., Zhao, G. and Guo, A. 2018. P27 (MBOV_RS03440) is a novel fibronectin binding adhesin of Mycoplasma bovis. Int. J. Med. Microbiol. 308, 848–857. Griffin, D., Chengappa, M.M., Kuszak, J. and McVey, D.S. 2010. Bacterial pathogens of the bovine respiratory disease complex. Vet Clin North Am Food Anim Pract. 26, 381–394. Guo, Y., Zhu, H., Wang, J., Huang, J., Khan, F.A., Zhang, J., Guo, A. and Chen, X. 2017. TrmFO, a fibronectin-binding adhesin of Mycoplasma bovis. Int. J. Mol. Sci. 18, 1732. Hananeh, W.M., Momani, W., Ababneh, M.M. and Abutarbush, S.M. 2018. Mycoplasma bovis arthritis and pneumonia in calves in Jordan: An emerging disease. Vet. World. 11, 1663–1668. Higa, Y., Uemura, R., Yamazaki, W., Goto, S., Goto, Y. and Sueyoshi, M. 2016. An improved loop-mediated isothermal amplification assay for the detection of Mycoplasma bovis. J Vet. Med. Sci. 78, 1343–1346. Hotzel, H., Saches, K. and Pfutzner, H. 1996. Rapid detection of Mycoplasma bovis in milk samples and nasal swabs using the polymerase chain reaction. J. Appl. Bacteriol. 80, 505–510. Jones, M.N. and Holt, R.G. 2007. Cloning and characterization of an α-enolase of the oral pathogen Streptococcus mutans that binds human plasminogen. Biochem. Biophys. Res. Commun. 364, 924–929. Kanda, T., Tanaka, S., Suwanruengsri, M., Sukmawinata, E., Uemura, R., Yamaguchi, R. and Sueyoshi, M. 2019. Bovine endocarditis associated with Mycoplasma bovis. J. Comp. Pathol. 171, 53–58. Li, Y., Zheng, H., Liu, Y., Jiang, Y., Xin, J., Chen, W. and Song, Z. 2011. The complete genome sequence of Mycoplasma bovis strain Hubei-1. PLoS One 6, e20999. Lima, S.F., Teixeira, A.G., Higgins, C.H., Lima, F.S. and Bicalho, R.C. 2016. The upper respiratory tract microbiome and its potential role in bovine respiratory disease and otitis media. Sci. Rep. 6, 29050. Maeda, T., Shibahara, T., Kimura, K., Wada, Y., Sato, K., Imada, Y., Ishikawa, Y. and Kadota, K. 2003. Mycoplasma bovis-associated suppurative otitis media and pneumonia in bull calves. J. Comp. Pathol. 129, 100–110. Maunsell, F.P., Woolums, A.R., Francoz, D., Rosenbusch, R.F., Step, D.L., Wilson, D.J. and Janzen, E.D. 2011. Mycoplasma bovis infections in cattle. J. Vet. Intern. Med. 25, 772–783. Qi, J., Guo, A., Cui, P., Chen, Y., Mustafa, R., Ba, X., Hu, C., Bai, Z., Chen, X., Shi, L. and Chen, H. 2012. Comparative geno-plasticity analysis of Mycoplasma bovis HB0801 (Chinese isolate). PLoS One 7, e38239. Sachse, K., Helbig, J.H., Lysnyansky, I., Grajetzki, C., Müller, W., Jacobs, E. and Yogev, D. 2000. Epitope mapping of immunogenic and adhesive structures in repetitive domains of Mycoplasma bovis variable surface lipoproteins. Infect. Immun. 68, 680–687. Song, Z., Li, Y., Liu, Y., Xin, J., Zou, X. and Sun, W. 2012. α-enolase, an adhesion-related factor of Mycoplasma bovis. PLoS One 7, e38836. Wise, K.S., Calcutt, M.J., Foecking, M.F., Röske, K., Madupu, R. and Methé, B.A. 2011. Complete genome sequence of Mycoplasma bovis type strain PG45 (ATCC 25523). Infect. Immun. 79, 982–983. Yavlovich, A., Rechnitzer, H. and Rottem, S. 2007. α-enolase resides on the cell surface of Mycoplasma fermentans and binds plasminogen. Infect. Immun. 75, 5716–5719. Zhao, G., Zhang, H., Chen, X., Zhu, X., Guo, Y., He, C., Anwar Khan, F., Chen, Y., Hu, C., Chen, H. and Guo, A. 2017. Mycoplasma bovis NADH oxidase functions as both a NADH oxidizing and O2 reducing enzyme and an adhesin. Sci. Rep. 7, 44. Zou, X., Li, Y., Wang, Y., Zhou, Y., Liu, Y. and Xin, J. 2013. Molecular cloning and characterization of a surface-localized adhesion protein in Mycoplasma bovis Hubei-1 strain. PloS One 8, e69644. | ||
| How to Cite this Article |
| Pubmed Style Shitamori F, Uemura R, Kanda T, Sueyoshi M. The presence of adhesion factors NOX, α-enolase, TrmFO, P27, and VpmaX in Mycoplasma bovis wild isolates in Japan. Open Vet J. 2022; 12(6): 782-786. doi:10.5455/OVJ.2022.v12.i6.1 Web Style Shitamori F, Uemura R, Kanda T, Sueyoshi M. The presence of adhesion factors NOX, α-enolase, TrmFO, P27, and VpmaX in Mycoplasma bovis wild isolates in Japan. https://www.openveterinaryjournal.com/?mno=55171 [Access: July 01, 2025]. doi:10.5455/OVJ.2022.v12.i6.1 AMA (American Medical Association) Style Shitamori F, Uemura R, Kanda T, Sueyoshi M. The presence of adhesion factors NOX, α-enolase, TrmFO, P27, and VpmaX in Mycoplasma bovis wild isolates in Japan. Open Vet J. 2022; 12(6): 782-786. doi:10.5455/OVJ.2022.v12.i6.1 Vancouver/ICMJE Style Shitamori F, Uemura R, Kanda T, Sueyoshi M. The presence of adhesion factors NOX, α-enolase, TrmFO, P27, and VpmaX in Mycoplasma bovis wild isolates in Japan. Open Vet J. (2022), [cited July 01, 2025]; 12(6): 782-786. doi:10.5455/OVJ.2022.v12.i6.1 Harvard Style Shitamori, F., Uemura, . R., Kanda, . T. & Sueyoshi, . M. (2022) The presence of adhesion factors NOX, α-enolase, TrmFO, P27, and VpmaX in Mycoplasma bovis wild isolates in Japan. Open Vet J, 12 (6), 782-786. doi:10.5455/OVJ.2022.v12.i6.1 Turabian Style Shitamori, Fumitaka, Ryoko Uemura, Takuya Kanda, and Masuo Sueyoshi. 2022. The presence of adhesion factors NOX, α-enolase, TrmFO, P27, and VpmaX in Mycoplasma bovis wild isolates in Japan. Open Veterinary Journal, 12 (6), 782-786. doi:10.5455/OVJ.2022.v12.i6.1 Chicago Style Shitamori, Fumitaka, Ryoko Uemura, Takuya Kanda, and Masuo Sueyoshi. "The presence of adhesion factors NOX, α-enolase, TrmFO, P27, and VpmaX in Mycoplasma bovis wild isolates in Japan." Open Veterinary Journal 12 (2022), 782-786. doi:10.5455/OVJ.2022.v12.i6.1 MLA (The Modern Language Association) Style Shitamori, Fumitaka, Ryoko Uemura, Takuya Kanda, and Masuo Sueyoshi. "The presence of adhesion factors NOX, α-enolase, TrmFO, P27, and VpmaX in Mycoplasma bovis wild isolates in Japan." Open Veterinary Journal 12.6 (2022), 782-786. Print. doi:10.5455/OVJ.2022.v12.i6.1 APA (American Psychological Association) Style Shitamori, F., Uemura, . R., Kanda, . T. & Sueyoshi, . M. (2022) The presence of adhesion factors NOX, α-enolase, TrmFO, P27, and VpmaX in Mycoplasma bovis wild isolates in Japan. Open Veterinary Journal, 12 (6), 782-786. doi:10.5455/OVJ.2022.v12.i6.1 |