| Original Article | ||
Open Vet J. 2021; 11(3): 385-389 Open Veterinary Journal, (2021), Vol. 11(3): 385–389 Original Research Electrochemotherapy for the treatment of cutaneous solid tumors in equids: A retrospective studyEnrico P. Spugnini1,2*, Licia Scacco2, Carlo Bolaffio2 and Alfonso Baldi1,31Biopulse Srl, Naples, Italy 2Equivet Roma Hospital, Rome, Italy 3Department of Environmental, Biological and Pharmaceutical Sciences and Technologies Campania University “Luigi Vanvitelli”, Caserta, Italy *Corresponding Author: Enrico P. Spugnini. Biopulse Srl, Naples, Italy. Email: info [at] enricospugnini.net Submitted: 06/04/2021 Accepted: 06/07/2021 Published: 29/07/2021 © 2021 Open Veterinary Journal
AbstractBackground: Electrochemotherapy (ECT) promotes the increased uptake of antitumor agents through the administration of permeabilizing electric pulses, thus enhancing chemotherapy effectiveness. Aim: Our study aimed to describe the tolerability and efficacy of ECT alone or in association with surgery to manage solid neoplasms in equids. Methods: Medical records of equids with a diagnosis of malignant tumors treated with ECT alone or in combination with surgery were retrospectively evaluated. Each equid received local treatment within the tumors or the tumors’ bed with cisplatin at the dose of 0.5 mg/cm2. Trains of permeabilizing biphasic electric pulses were then applied under spinal or general anesthesia. Results: Sixteen equids were enrolled in this study. There were nine melanoma cases, four fibrosarcoma, and three squamous cell carcinoma. Of those 16 equids, 7 received ECT for treatment of intraoperative local disease, while in 9 cases, ECT was the only treatment modality. The seven equids treated with the combination of ECT and surgery still have no evidence of disease at different times ranging from 9 to 60 months. The remaining nine had the following responses: two complete remissions, five partial responses, one stable disease, and one progressive disease. The treatment was well-tolerated, and local side effects were minimal. No systemic effects were documented. Conclusion: This retrospective study suggests that ECT may be beneficial for equids with solid neoplasms and could be a useful addition to the current therapeutic options considering its low cost, limited toxicity, and ease of administration. Keywords: Cisplatin, Fibrosarcoma, Horse, Melanoma, Squamous cell carcinoma. IntroductionCancer is a source of morbidity and mortality in equines although it is not as crucial as companion animals (Knottembelt et al., 2015a). The lack of proper central recording systems prevents researchers from knowing the tumor incidence in equines. Up until now, the general consensus, generated from Universities caseloads and slaughterhouses registries, is that the inter-tegumentary apparatus is the most common site of neoplasia in equines (Reid and Howie, 1992; Knottembelt et al., 2015b). The predominant tumor types of the skin in equines, accounting for 95% of cutaneous neoplasms, are sarcoids, melanomas, and squamous cell carcinomas (SCC), while mesenchymal tumors such as fibrosarcoma (FSA) are infrequently reported (Knottembelt et al., 2015b; Spugnini et al., 2016). Therapies for solid tumors in equines are generally centered on a surgical approach with wide resection (van den Top et al., 2008; Hewes and Sullins, 2009). Local control can be enhanced when surgery is incomplete or not feasible, by using laser ablation (McCauley et al., 2002), cryosurgery (Harling et al., 1983), immunotherapy with Bacillus Calmette Guerin (Martens et al., 2001), intralesional chemotherapy (Pucket and Gilmor, 2014), and radiotherapy (both brachytherapy and teletherapy) (Theón and Pascoe, 1994; Mosunic et al., 2004; Hollis, 2019). Electrochemotherapy (ECT) has recently been used in equine medicine to increase the chances of local control for cutaneous neoplasms of different nature, with good results (Spugnini et al., 2011, 2016, 2017; Scacco et al., 2013; Galant et al., 2019). ECT has been widely adopted over the past 15 years as a supplementary treatment modality for local control of solid tumors, especially in companion animals (Spugnini and Baldi, 2019). ECT combines the administration of chemotherapy agents (mostly lipophobic molecules) to permeabilize electric pulses that stimulate their capitation by cancer cells. In equine oncology, most applications of ECT have been aimed at controlling cutaneous sarcoids, with good response rates and cosmetic results (Tamzali et al., 2012; Tozon et al., 2016). Conversely, only scant case reports have been published on other histotypes (Spugnini et al., 2011, 2016, 2017; Scacco et al., 2013). This retrospective study aimed at evaluating the effectiveness of ECT in the treatment of non-viral solid tumors in equines. Material and MethodsStudy designMedical records of equids at an equine referral hospital were retrospectively searched for client-owned horses and ponies with a histopathologically confirmed diagnosis of solid neoplasms, such as FSA, melanoma, and SCC that underwent ECT treatment alone in association with surgeries between 2014 and 2020. Equid staging consisted of a complete blood cell count, serum biochemical profile, and ultrasonographic evaluation of the perianal and sub lumbar lymph nodes. The presence of lymph adenomegaly in the sub lumbar region (medial iliac and lumbar LNs) was recorded, and ultrasound guided LN fine needle aspirate was performed based on the feasibility of the procedure. In all cases, surgery and/or radiation therapy (RT) were discussed as primary treatment options with the owners and, if declined, ECT was offered. ECT was also offered as an adjuvant treatment in case of residual disease after surgery or as a single modality in case of inoperable disease. It was requisite for all equids to have received at least two sessions of ECT, performed 2 weeks apart with a minimum follow-up of 30 days, to be considered adequate to evaluate clinical measurable response and development of side effects. Informed consent was obtained by the equines’ owners before the ECT treatment was commenced. Patients were anesthetized with one of the two following protocols: Protocol one: premedication with a combination of acepromazine (Prequillan, Fatro, Ozzano Emilia, Italy), butorphanol (Nargesic, ACME, Cavriago, Italy), and detomidine (Domidine, Dechra, Milan, Italy) at the doses of 0.03, 0.04, and 0.005 mg/kg, respectively, additionally, followed by spinal anesthesia performed by injecting 2% lidocaine (Lidocaina 2%, Zoetis, Rome, Italy) (Doherty and Valverde, 2006). In one patient with eyelid melanoma, spinal anesthesia was replaced with local anesthesia with 2% lidocaine (See Figure 2). Protocol two: the horses were sedated with a combination of acepromazine (Prequillan, Fatro, Ozzano Emilia, Italy), butorphanol (Nargesic, ACME, Cavriago, Italy), and romifidine (Sedivet, Boehringer Ingelheim, Milan, Italy) at the doses of 0.03, 0.04, and 0.072 mg/kg, respectively. Premedication was followed by diazepam (Valium, Roche, Milan, Italy) at the dose of 0.05 mg/kg and ketamine (Ketavet 100, MSD, Aprilia, Italy) at the dose of 2.2 mg/kg. At this point, the patients were intubated and kept under general anesthesia with isoflurane (Forane, Abbott, Latina, Italy), as elsewhere described (Spugnini et al., 2017). At this point, the primary tumor or the tumor bed was infiltrated with cisplatin (Cisplatin Teva, Milan, Italy) with a dose of 0.5 mg/cm2 or until tumor saturation if the neoplasm was prone to leakage. After the chemotherapy injection, with animals under spinal or general anesthesia, as above described, sequences of eight biphasic pulses lasting 50 + 50 μsec each, were delivered to the primary tumor/tumor bed via clamp electrodes and/or via two-needle 45-S shielded electrodes at a voltage of 1,000–1,300 V/cm, depending on tumor electrical conduction as measured by the clinical electroporator (Onkodisruptor®). The selection of the electrode type was based on the extension and depth of the neoplasms undergoing ECT. The response to treatment was defined by the primary clinician’s assessment and based on the Veterinary Cooperative Oncology Group Response Evaluation Criteria in Solid Tumors (VCOG RECIST criteria) (Nguyen et al., 2015). Tumor response was classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) for equids treated with ECT alone based on tumor measurement by clinicians (Nguyen et al., 2015). The response was categorized as no evidence of disease (NED) or tumor recurrence for equids treated with the combination of surgery and ECT. Tolerability of the treatment was assessed based on the development of any local or systemic side effect, classified by Veterinary Cooperative Oncology Group Criteria (Veterinary Cooperative Oncology Group, 2016). Ethical approvalAll procedures were performed in accordance with institutional guidelines under the control of the Italian Ministry of Public Health (Italian Law D.lgs 26/2014). ResultsThe records of 16 equids held adequate information to allow the enrollment of these patients in this retrospective study. The treated cohort included 14 horses and 2 ponies. Patients’ characteristics and outcomes are summarized in Tables 1 and 2. Table 1 includes all the equids treated with ECT alone, while Table 2 summarizes the characteristics and outcome of equids treated with the combination of surgical debulking and ECT. Altogether, treated tumors included nine melanomas, four FSA, and three SCC. Seven equids were treated with tumor debulking combined with ECT (Table 2), while the remaining nine equids were treated with ECT alone (Table 1). The equids experienced minimal discomfort during the procedures, limited to transient inflammation, while systemic toxicoses were not observed. In terms of local tolerability, the therapy has been well endured by all the patients besides a horse with a large perianal melanoma that developed a fistula as a side effect of the first ECT session. The lesion spontaneously healed with just topical treatment (Figure 1). In terms of overall response, 13 patients had clinical benefit from ECT (see Tables 1 and 2) ranging from complete local control (nine subjects experiencing no recurrence or CR) to partial remission (five subjects) lasting for several months. The patients did not receive any additional treatment during the observation period. The remaining two patients had SD for 7 months and PD. In Figure 2, an exemplificative case of palpebral melanoma with long-term control (24 months) following combination therapy is shown. Overall, the owners expressed a high degree of satisfaction upon a post-therapy interview in terms of feasibility, results, and affordability. Table 1. Characteristics of equines with solid tumors treated with ECT single modality.
Table 2. Characteristics of equines with solid tumors treated with combination surgery (SX) and ECT.
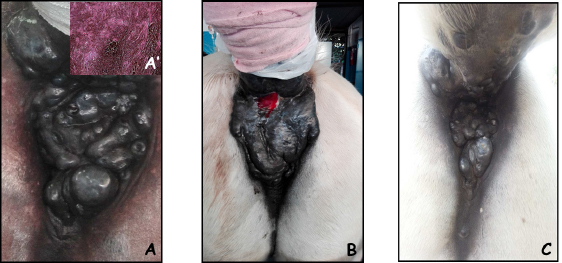
Fig. 1. A 16-year-old Gelding with perianal melanoma at presentation (A); after one session of ECT, of note, a fistula developed at the site of treatment that recovered without need of surgical debridement (B); the appearance of the lesion after two sessions of ECT (C). The insert in panel A’ shows the histological appearance of a melanotic melanoma.
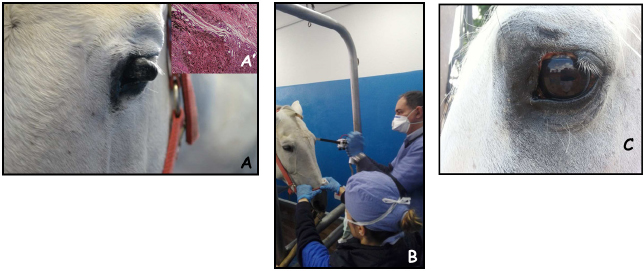
Fig. 2. A 17-year-old gelding with palpebral melanoma at presentation (A); during intraoperative ECT; the patient has been sedated as reported in materials and methods section and had local anesthesia around the neoplasm (B); the appearance of the lesion after two sessions of ECT, at 1-month control (C). The insert in panel A’ shows the histological appearance of a melanotic melanoma. DiscussionLocal control of solid tumors can be a challenge in certain body districts such as the head or the perianal region of the equines due to the presence of underlying structures that prevent wide surgical excision. Furthermore, the possibility of local control with the standard approach of surgery and/or cryotherapy carries a poor success rate (Knottembelt et al., 2015b). The use of adjuvant therapies after incomplete tumor resection is not yet a standard procedure in equine medicine; due to the lack of dedicated facilities such as RT, more frequently local chemotherapy or cryosurgery are adopted to enhance local control (Knottembelt et al., 2015b). ECT increases the uptake of lipophobic drugs such as cisplatin by tumor cells, obtaining a greater efficacy, and extended local control (Spugnini and Baldi, 2019). Its use in equine oncology is mostly still limited to the treatment of sarcoids (Tamzali et al., 2012; Tozon et al., 2016; Galant et al., 2019) although there are a few case reports of its adoption in the treatment of melanoma, FSA, and SCC (Spugnini et al., 2011, 2016, 2017; Scacco et al., 2013), frequently achieving extended local control. To the best of authors’ knowledge, this is the first retrospective report of ECT used to treat large solid tumors of non-viral origin in equids. The technique proved to be well tolerated and rather effective both as an adjuvant combined with surgery or single modality for unresectable neoplasms. Overall, 15 out of 16 equids had responses ranging from SD to greater (PR, CR, or NED), making for a response rate in excess of 93%. Of interest, all the patients in our cohort experiencing PR were treated with palliative ECT for unresectable melanomas. These responses lasted in excess of 12 months (see Table 1), potentially due to a stimulation of the immune system following reduction of tumor bulk. These results are similar to those reported for many solid tumors in companion animals (Spugnini and Baldi, 2019) and show promise. With all its limitation due to the retrospective nature and the low number of enrolled patients, this study suggests that ECT with cisplatin is well tolerated by equids treated for solid neoplasms. So far, the number of observed side effects has been minimal while maintaining treatment effectiveness. This warrants further investigations so that ECT could be added to the treatment options for such tumors, together with the other validated therapies, including cryosurgery, intralesional chemotherapy, and RT. AcknowledgmentsThe authors would like to thank Dr. Guy Beretich for reviewing the manuscript. Authors contributionsEPS and AB conceived and supervised the study, EPS performed the ECT sessions, LS and CB were involved in the clinical management of the patients, including anesthesia and surgical procedures and assisted during clinical outcome follow up and data collection, AB performed the histopathology analysis of most clinical cases. Conflict of interestEnrico P. Spugnini and Alfonso Baldi are the stockholders of Biopulse S.r.l. ReferencesDoherty, T. and Valverde, A. 2006. Manual of Equine Anesthesia and Analgesia. 1st edition. Blackwell Publishing, Ames, Iowa, USA. Galant, L., Delverdier, M., Lucas, M.N., Raymond-Letron, I., Teissie, J. and Tamzali, Y. 2019. Calcium electroporation: the bioelectrochemical treatment of spontaneous equine skin tumors results in a local necrosis. Bioelectrochemistry 129, 251–258. Harling, D.E., Peiffer, R.L. and Cook, C.S. 1983. Excision and cryosurgical treatment of five cases of squamous cell carcinoma in the horse. Equine Vet. J. 15, 105–109. Hewes, C.A. and Sullins, K.E. 2009. Review of the treatment of equine cutaneous neoplasia. AAEP Proc. 55, 386–393. Hollis, A.R. 2019. Radiotherapy for the treatment of periocular tumours in the horse. Equine Vet. Educ. 31, 647–652. Knottembelt, D.C., Patterson-Kane, J.C. and Snalune, K.L. 2015a. The challenges and problems of equine oncological practice. In: Clinical equine oncology. Eds., Knottenbelt, D.C., Snalune, K., Kane, J.P. Philadelphia, PA: Elsevier, pp: 7–10. Knottembelt, D.C., Patterson-Kane, J.C. and Snalune, K.L. 2015b. Tumors of the skin. In: Clinical equine oncology. Eds., Knottenbelt, D.C., Snalune, K., Kane, J.P. Philadelphia, PA: Elsevier, pp, 544–584. Martens, A., De Moor, A., Vlaminck, L., Pille, F. and Steenhaut, M. 2001. Evaluation of excision, cryosurgery and local BCG vaccination for the treatment of equine sarcoids. Vet. Rec. 149, 665–669. McCauley, C.T., Hawkins, J.F., Adams, S.B. and Fessler, J.F. 2002. Use of a carbon dioxide laser for surgical management of cutaneous masses in horses: 32 cases (1993–2000). J. Am. Vet. Med. Assoc. 220, 1192–1197. Mosunic, C.B., Moore, P.A., Carmicheal, K.P., Chandler, M.J., Vidyashankar, A., Zhao, Y., Roberts, R.E. and Dietrich, U.M. 2004. Effects of treatment with and without adjuvant radiation therapy on recurrence of ocular and adnexal squamous cell carcinoma in horses: 157 cases (1985–2002). J. Am. Vet. Med. Assoc. 225, 1733–1738. Nguyen, S.M., Thamm, D.H., Vail, D.M. and London, C.A. 2015. Response evaluation criteria for solid tumours in dogs (v1.0): a veterinary cooperative oncology group (VCOG) consensus document. Vet. Comp. Oncol. 13, 176–183. Pucket, J.D. and Gilmor, M.A. 2014. Intralesional 5-fluorouracil (5-FU) for the treatment of eyelid squamous cell carcinoma in 5 horses. Equine Vet. Educ. 26, 331–336. Reid, S.W. and Howie, F. 1992. Factors associated with neoplastic disease in the horse. Equine Vet. Educ. 4, 66–68. Scacco, L., Bolaffio, C., Romano, A., Fanciulli, M., Baldi, A. and Spugnini, E.P. 2013. Adjuvant electrochemotherapy increases local control in a recurring equine anal melanoma. J. Equine Vet. Sci. 33, 637–639. Spugnini, E.P. and Baldi, A. 2019. Electrochemotherapy in veterinary oncology: state-of-the-art and perspectives. Vet. Clin. North Am. Small Anim. Pract. 49, 967–979. Spugnini, E.P., Bolaffio, C., Scacco, L. and Baldi, A. 2016. Electrochemotherapy increases local control after incomplete excision of a recurring penile fibrosarcoma in a stallion. Open Vet. J. 6, 234–237. Spugnini, E.P., Bolaffio, C., Scacco, L. and Baldi, A. 2017. Isolated limb perfusion electrochemotherapy for the treatment of an advanced squamous cell carcinoma of the hoof in a mare. Open Vet. J. 7, 192–196. Spugnini, E.P., D’Alterio, G.L., Dotsinsky, I., Mudrov, T., Dragonetti, E., Murace, G., Citro, G. and Baldi, A. 2011. Electrochemotherapy for the treatment of multiple melanomas in a horse. J. Equine Vet. Sci. 31, 430–433. Tamzali, Y., Borde, L., Rols, M.P., Golzio, M., Lyazrhi, F. and Teissie, J. 2012. Successful treatment of equine sarcoids with cisplatin electrochemotherapy: a retrospective study of 48 cases. Equine Vet. J. 44, 214–220. Theón, A.P. and Pascoe, J.R. 1994. Iridium-192 interstitial brachytherapy for equine periocular tumours: treatment results and prognostic factors in 115 horses. Equine Vet. J. 27, 117–124. Tozon, N., Kramaric, P., Kos Kadunc, V., Sersa, G. and Cemazar, M. 2016. Electrochemotherapy as a single or adjuvant treatment to surgery of cutaneous sarcoid tumours in horses: a 31-case retrospective study. Vet. Rec. 179, 627. van den Top, J.G., de Heer, N., Klein, W.R. and Ensink, J.M. 2008. Penile and preputial squamous cell carcinoma in the horse: a retrospective study of treatment of 77 affected horses. Equine Vet. J. 40, 533–537. Veterinary Cooperative Oncology Group. 2016. Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet. Comp. Oncol. 14, 417–446. | ||
| How to Cite this Article |
| Pubmed Style Spugnini EP, Scacco L, Bolaffio C, Baldi A. Electrochemotherapy for the treatment of cutaneous solid tumors in Equids: A retrospective study. Open Vet J. 2021; 11(3): 385-389. doi:10.5455/OVJ.2021.v11.i3.8 Web Style Spugnini EP, Scacco L, Bolaffio C, Baldi A. Electrochemotherapy for the treatment of cutaneous solid tumors in Equids: A retrospective study. https://www.openveterinaryjournal.com/?mno=70842 [Access: July 01, 2025]. doi:10.5455/OVJ.2021.v11.i3.8 AMA (American Medical Association) Style Spugnini EP, Scacco L, Bolaffio C, Baldi A. Electrochemotherapy for the treatment of cutaneous solid tumors in Equids: A retrospective study. Open Vet J. 2021; 11(3): 385-389. doi:10.5455/OVJ.2021.v11.i3.8 Vancouver/ICMJE Style Spugnini EP, Scacco L, Bolaffio C, Baldi A. Electrochemotherapy for the treatment of cutaneous solid tumors in Equids: A retrospective study. Open Vet J. (2021), [cited July 01, 2025]; 11(3): 385-389. doi:10.5455/OVJ.2021.v11.i3.8 Harvard Style Spugnini, E. P., Scacco, . L., Bolaffio, . C. & Baldi, . A. (2021) Electrochemotherapy for the treatment of cutaneous solid tumors in Equids: A retrospective study. Open Vet J, 11 (3), 385-389. doi:10.5455/OVJ.2021.v11.i3.8 Turabian Style Spugnini, Enrico P, Licia Scacco, Carlo Bolaffio, and Alfonso Baldi. 2021. Electrochemotherapy for the treatment of cutaneous solid tumors in Equids: A retrospective study. Open Veterinary Journal, 11 (3), 385-389. doi:10.5455/OVJ.2021.v11.i3.8 Chicago Style Spugnini, Enrico P, Licia Scacco, Carlo Bolaffio, and Alfonso Baldi. "Electrochemotherapy for the treatment of cutaneous solid tumors in Equids: A retrospective study." Open Veterinary Journal 11 (2021), 385-389. doi:10.5455/OVJ.2021.v11.i3.8 MLA (The Modern Language Association) Style Spugnini, Enrico P, Licia Scacco, Carlo Bolaffio, and Alfonso Baldi. "Electrochemotherapy for the treatment of cutaneous solid tumors in Equids: A retrospective study." Open Veterinary Journal 11.3 (2021), 385-389. Print. doi:10.5455/OVJ.2021.v11.i3.8 APA (American Psychological Association) Style Spugnini, E. P., Scacco, . L., Bolaffio, . C. & Baldi, . A. (2021) Electrochemotherapy for the treatment of cutaneous solid tumors in Equids: A retrospective study. Open Veterinary Journal, 11 (3), 385-389. doi:10.5455/OVJ.2021.v11.i3.8 |