| Review Article | ||
Open Vet J. 2021; 11(4): 707-723 Open Veterinary Journal, (2021), Vol. 11(4): 707–723 Review Article Animal prion diseases: A review of intraspecies transmissionMauro Julián Gallardo1,2* and Fernando Oscar Delgado1,31Instituto de Patobiología Veterinaria, IPVet, UEDD INTA-CONICET, Hurlingham, Argentina 2Cátedra de Enfermedades Infecciosas, Facultad de Ciencias Veterinarias, Universidad de Buenos Aires, Buenos Aires, Argentina 3Facultad de Cs. Agrarias y Veterinarias, Universidad del Salvador, Pilar, Argentina *Corresponding Author: Mauro Julián Gallardo. Instituto de Patobiología Veterinaria, IPVet, UEDD INTA-CONICET, Hurlingham, Argentina. Email: gallardo.mauro [at] inta.gob.ar Submitted: 03/05/2021 Accepted: 16/11/2021 Published: 16/12/2021 © 2021 Open Veterinary Journal
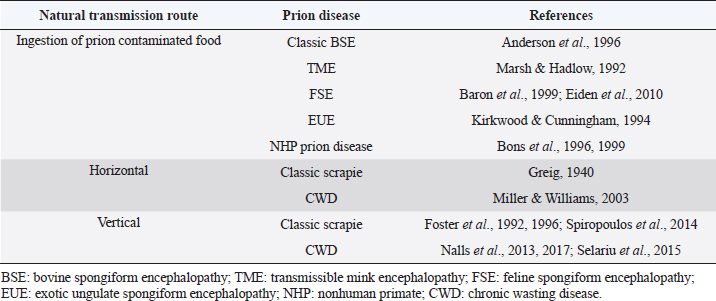
AbstractAnimal prion diseases are a group of neurodegenerative, transmissible, and fatal disorders that affect several animal species. The causative agent, prion, is a misfolded isoform of normal cellular prion protein, which is found in cells with higher concentration in the central nervous system. This review explored the sources of infection and different natural transmission routes of animal prion diseases in susceptible populations. Chronic wasting disease in cervids and scrapie in small ruminants are prion diseases capable of maintaining themselves in susceptible populations through horizontal and vertical transmission. The other prion animal diseases can only be transmitted through food contaminated with prions. Bovine spongiform encephalopathy (BSE) is the only animal prion disease considered zoonotic. However, due to its inability to transmit within a population, it could be controlled. The emergence of atypical cases of scrapie and BSE, even the recent report of prion disease in camels, demonstrates the importance of understanding the transmission routes of prion diseases to take measures to control them and to assess the risks to human and animal health. Keywords: Animal prion diseases, Scrapie, Bovine spongiform encephalopathy, Chronic wasting disease, Transmissible spongiform encephalopathies. IntroductionPrion diseases are a group of neurodegenerative disorders that affect humans and several animal species. These diseases are also known as transmissible spongiform encephalopathies (TSE) due to the microscopic changes they produce in the central nervous system (CNS) and their capability for transmission among susceptible individuals. Diseases within this group include Creutzfeldt Jakob disease (CJD) in humans, scrapie in sheep and goats, and bovine spongiform encephalopathy (BSE) in cattle, among others. All of them have a chronic course with neurological manifestations and culminate in the death of affected individuals since there is no curative treatment for these pathologies. The relevance of animal prion diseases has been changing over the years. BSE caused strong changes in animal production at the end of the 20th century, but its significance decreased with the implementation of measures to control it. However, BSE and other prion diseases are still being diagnosed worldwide, and a novel disorder designated as camel prion disease (CPD) was recently reported in dromedary camels from Algeria (Babelhadj et al., 2018). These findings indicated that prion diseases remain relevant for animal production and public health. Although the knowledge of animal prion diseases has increased drastically in the last years, many aspects remain poorly understood despite being studied in depth. Information about the spread of animal prion diseases became necessary for their control. This work aimed to compile and analyze information about the transmission routes of animal prion diseases within susceptible populations. Animal prion diseasesAnimal prion diseases have been known since 1732, when scrapie was described in a merino sheep in Spain, and later it was well documented in sheep from Great Britain (Liberski, 2012). During the 1960s, scrapie-like diseases were described in mink and deer in North America, later known as transmissible mink encephalopathy (TME) and chronic wasting disease (CWD) respectively. But it was in 1986 when animal prion diseases became more relevant. In that year, it was the first report about a disease similar to scrapie affecting cattle. That new disease was called BSE (Wells et al., 1987), and its impact was more significant than other prion diseases due to the economic importance of cattle. Interest in these pathologies increased significantly in 1996 when BSE was linked to a variant of the CJD in humans and was therefore considered a zoonosis (Will et al., 1996; Bruce et al., 1997). Because of the risk to animals and humans, strict measures to reduce the transmission of BSE were successfully implemented, and the number of new cases has decreased dramatically in recent years. Although prion diseases have been known for a long time, the etiological agent was characterized at the end of the 20th century when the prion hypothesis was postulated (Prusiner, 1982). It was proposed that scrapie is caused by a misfolded isoform of normal cellular prion protein (PrPC), which is found constitutively in nucleated cells of all superior species with a higher concentration in the CNS. The misfolded form was initially called protease resistant prion protein (PrPres) or scrapie prion protein (PrPSc). The conversion of PrPC to PrPres is a post-translational process that has not been completely elucidated yet. PrPres can be detected by several procedures. Immunoblotting, enzyme-linked immunosorbent assay, and immunohistochemistry are the most widely used methods in research and diagnosis (Gavier-Widén et al., 2005). New techniques have been developed, such as protein misfolding cyclic amplification (PMCA) and real time-quaking induced conversion (RT-QuIC). These consist of reproducing the conversion of PrPC to PrPres in vitro, using the PrPres present in infected samples. PMCA and RT-QuIC allowed them to advance in the knowledge of prion diseases since they have a higher sensitivity than other methods (Eraña et al., 2020). Considering all animal prion diseases, scrapie is the best known. It has been the prototype for studying prion diseases, and it is the most widespread, with the highest number of new cases reported. Additionally, although there has been no evidence of scrapie transmission to humans, it has been postulated as a potential risk for humans since it has been transmitted to primates and transgenic mice overexpressing human native PrPC (Cassard et al., 2014; Comoy et al., 2015). Beyond its hypothetical risk to other species, scrapie spread among small ruminants creates commercial barriers for sheep and goats, and their derived products. Nowadays, CWD has become the most worrisome animal prion disease because it has been shown to be maintained in wildlife animals and has spread across large areas. Initially confined to some states in the United States of America (USA) and Canada, CWD cases have been recently detected in other regions of both countries (Rivera et al., 2019) and more recently in Norway, Sweden, and Finland (Benestad et al., 2016; Koutsoumanis et al., 2019). Furthermore, it has been proposed that CWD could represent a zoonotic disease and, therefore, a potential risk for public health (Hannaoui et al., 2017). Prion proteinThe primary structure of PrPC consists of 256 amino acids in sheep, which varies slightly among animal species. The secondary structure of PrPC is mainly helical (40% α-helix and 3% β-sheet) when it is solubilized in detergents in the absence of cations. The formation of the PrPres leads to a modification of secondary structures (30% α-helix and 45% β-sheet), which modify its biochemical characteristics (Pan et al., 1993). While PrPC is completely degraded by proteases, PrPres is partially resistant. For this reason, PrPres accumulates in neurons and glial cells, causing vacuolization of gray matter with a microscopic “spongiform” change that characterizes prion diseases. PrPC is encoded in the PRNP gene, and it is expressed in high levels in the CNS, mainly associated with neurons and astrocytes, with lower levels present in oligodendrocytes and microglia. It can also be found in other tissues such as muscle, peripheral nervous system (PNS), or lymphoid tissue, but in less quantity than in the CNS (Watts et al., 2018). Although the function of PrPC is unknown, it has been shown that the absence of PrPC in knockout mice makes the animal resistant to prion diseases (Weissmann and Flechsig, 2003). Therefore, the expression of this protein in the cells of susceptible hosts is necessary for the development of prion diseases. Unlike other prion diseases, an association between the risk of scrapie infection and some sheep genotypes has been described. This genetic susceptibility to the disease is given by the polymorphism of PRNP gene and mainly to variations in the amino acids encoded in three codons: 136, 154, and 171. From these combinations arise 5 alleles (VRQ, ARQ, AHQ, ARH, and ARR) with 15 possible genotypes (Goldmann, 2008). Among these, the VRQ/VRQ genotype confers high susceptibility to scrapie, while ARR/ARR is associated with low susceptibility (Baylis et al., 2004). In this way, scrapie infection depends on exposure to the infectious agent and the host's genetic susceptibility. These findings led to the development of breeding programs in Europe intending to eliminate those individuals with genotypes susceptible to scrapie (Melchior et al., 2010). In goats, around 50 polymorphisms of the PRNP gene have been described. However, it has not been determined with the same precision as in sheep if there is a direct correlation between goat genotypes and the susceptibility of contracting scrapie (Greenlee, 2019). For this reason, no breeding programs have been developed in goats, as in the case of sheep. In other TSE, genetic predisposition has not been demonstrated. Some studies in cattle revealed that regions outside the coding region of the PRNP gene are associated with variations in disease susceptibility (Vernerova et al., 2014) Despite that, genetics is not an issue considered in current BSE prevention programs. In the case of CWD, it affects several species of cervids, so the genetic susceptibility or resistance will depend on each one. Studies have been conducted on some species, but no substantial results have been observed (Mead et al., 2019). Transmission routesAlthough there are some differences among these diseases, there is a consensus that the infection occurs mainly by oral route. Here, we describe aspects related to the transmission of the most relevant animal prion diseases (the natural transmission routes are summarized in Table 1). ScrapieThe transmissible nature of scrapie has been proposed since the 1800s. However, it was associated with a parasite of the genus Sarcosporidium. It was not until the 1930s when the transmissibility of the causative agent was demonstrated experimentally, even though at that time it was believed to be a “filterable agent” (i.e., a virus) (Liberski, 2012). Scrapie affects sheep, goats, and mouflons in natural conditions. But infection has been experimentally accomplished in rats (Chandler and Fisher, 1963), mice (Chandler, 1961), hamsters (Zlotnik and Rennie, 1965), and other species. Horizontal transmission of scrapieThe exact natural route of infection is uncertain, but it is agreed that there is horizontal transmission (Greig, 1940). Following the oral route, it was observed that after entering, PrPSc is deposited in lymphoid tissue, such as Peyer's patches, mesenteric lymph nodes, and gut-associated lymphoid tissue (GALT) (Andreoletti et al., 2000). From here, the agent spreads to the enteric nervous system, part of the PNS (McBride et al., 2001; Heggebø et al., 2003) (Fig. 1). For the infection, PrPSc must pass through the epithelium of the gastrointestinal tract to reach the lymphoid tissue, where it is found in the early stages of the disease. Several mechanisms have been proposed, and the most accepted includes transcytosis by M cells (Heppner et al., 2001; Miyazawa et al., 2010) and possibly capture and transport by migratory dendritic cells (Huang et al., 2002; Huang and MacPherson, 2004). At a cellular level, follicular dendritic cells (FDC) appear to play an essential role in scrapie pathogenesis. These cells are present in germinal centers of lymphoid follicles and express quite quantities of PrPC in normal conditions (McBride et al., 1992). The presence of mature FDC is essential for the development of the disease. Scrapie infection was ineffective in mice with mature FDC deficiency (Brown et al., 1999; Mabbott et al., 2000). B lymphocytes are also important, but probably because they are necessary for the maturation and maintenance of FDC (Bruce et al., 2000; Prinz et al., 2003). B lymphocytes may also be involved in the first stage of spread from the earliest accumulation sites to secondary lymphoid organs such as the spleen via blood and lymph (Edwards et al., 2010; Mok et al., 2012). Macrophages are other cells where PrPSc can be found early, especially tingible body macrophages (TBM). These cells are present in the germinal centers of lymphoid nodules, and their function is to phagocyte apoptotic lymphocytes and, in this case, prions (Heggebø et al., 2002; Herrmann et al., 2003). The nasal cavity was also proposed as a portal for PrPSc entry in horizontal transmission. This route of infection was confirmed by instilling a homogenate of the scrapie-infected brain into the nostrils of sheep that later developed scrapie (Hamir et al., 2008). However, there are discrepancies in how the agent accesses the CNS. In a study, authors suggested that the olfactory system is involved in the natural transmission of scrapie based on PrPSc deposition in the olfactory bulb and olfactory cortex of naturally infected sheep (Corona et al., 2009). On the other hand, assays in hamsters intranasally inoculated with PrPSc indicate that neuroinvasion occurs through unrelated olfactory pathways (Sbriccoli et al., 2009). Beyond the discrepancy, the nasal route could be considered a route of entry in case of PrPSc was present in contaminated forage, bed, or soil and could be inhaled by sheep and goats. Transepithelial transmission of scrapie has been studied since 1982, when infection through mucous membranes was first reported via gingival scarification in mice (Carp, 1982) and later percutaneously (Taylor et al., 1996). In the following years, several investigations tried to understand the mechanisms of transcutaneous infection and how prion neuroinvasion occurred by this route (Mohan et al., 2004, 2005). Although this form of transmission is possible, it does not seem as probable as the other proposed routes. Table 1. Summary of natural transmission routes in animal prion diseases.
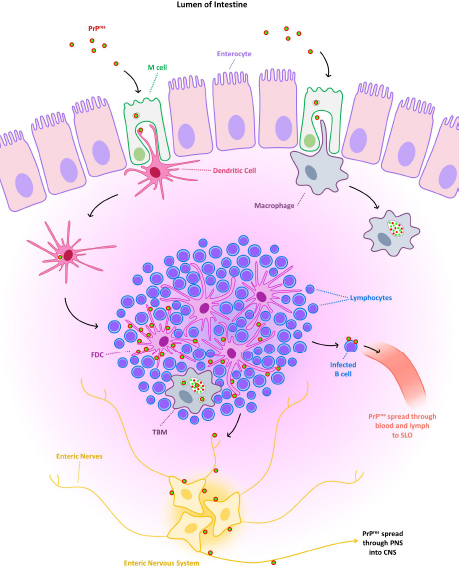
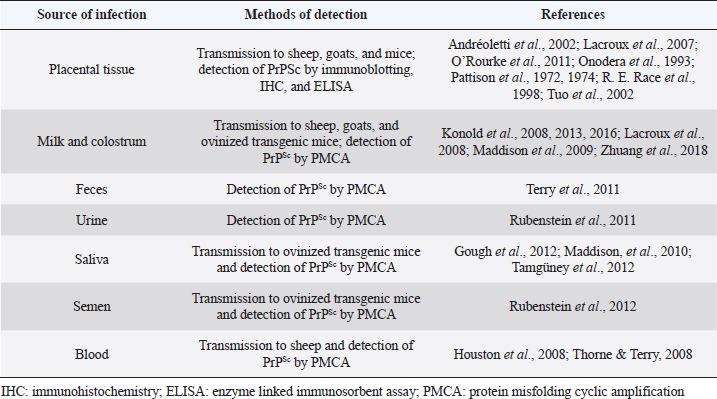
Fig. 1. Graphic representation of the possible spread of PrPres from the lumen of the intestine following oral route. TBM: tingible body macrophage; FDC: follicular dendritic cell; SLO: secondary lymphoid organs; PNS: peripheral nervous system; CNS: central nervous system. PrPSc has been detected in semen from scrapie-infected rams, and its infectivity has been proven in ovinized transgenic mice (Rubenstein et al., 2012). However, there is no evidence that scrapie can be transmitted by natural service or artificial insemination. Sources of environmental contaminationAs with other transmissible diseases, sources of environmental contamination could be involved in the spread of scrapie (Table 2). Secretions and excretions of affected individuals may contribute to horizontal transmission. PrPSc was detected in feces of sheep naturally infected with scrapie in preclinical and clinical stages (Terry et al., 2011). Studies done in Syrian hamsters suggest that it is possible to transmit scrapie through contaminated bedding of experimentally infected animals (Safar et al., 2008). In the first days after oral infection, the detection of PrPSc could be due to the passage of the inoculum through the gastrointestinal tract but, once replicated in GALT and Peyer's patches, PrPSc could be detected in low quantities in the feces of the hamsters (Krüger et al., 2009). These results could be influenced by the coprophagy that characterizes Syrian hamsters, which does not occur in the species naturally susceptible to scrapie. On the other hand, several studies have studied urine as a source of infection. In assays performed in mice and hamsters, it was demonstrated the infectivity and the presence of PrPSc in the urine of rodents infected with scrapie (Seeger, 2005; Kariv-Inbal et al., 2006; Murayama et al., 2007; Gonzalez-Romero et al., 2008; Gregori et al., 2008). In the case of species naturally affected by scrapie, PrPSc has also been detected in the urine of infected sheep by using PMCA (Rubenstein et al., 2011). Therefore, excretions of infected animals might be a risk to others. Table 2. Summary of possible sources of infection in the transmission of scrapie in sheep and goats.
Regarding secretions, saliva could play a role in horizontal transmission in two ways: environmental contamination and direct contact between animals. PrPSc was detected in oral secretions of sheep infected with scrapie in preclinical and clinical stages (Maddison, et al., 2010; Tamgüney et al., 2012). Infectivity could also be verified in transgenic mice expressing ovine PrPC (Gough et al., 2012; Tamgüney et al., 2012). In addition, it was suggested that when infected animals ingest the saliva contaminated with PrPSc, it may reinfect gastrointestinal tissues. This reinfection could contribute to eliminating the faecal prions since the PrPSc detected in saliva was similar to the PrPSc detected in the feces of scrapie-infected sheep (Tamgüney et al., 2012). The blood of animals naturally infected with scrapie was infectious in both preclinical and clinical stages. Transmissibility in sheep has been demonstrated through transfusion of whole blood and buffy coat from scrapie-infected donors to healthy recipients (Houston et al., 2008). Furthermore, the presence of PrPSc could be detected in the cell fractions of the blood of naturally infected sheep (Thorne and Terry, 2008). Blood transfusion is not a common practice in sheep and goats, but it is frequent in humans, and CJD could be a health hazard for the recipient. In addition to the contamination of the environment, secretions, or excretion can also contaminate inanimate objects, and these act as indirect transmitters of scrapie. For example, it was confirmed transmission through field furniture that had been in contact with an infected scrapie flock, indicating that these objects may act as a reservoir for PrPSc (Konold et al., 2015). Vertical transmission of scrapieVertical transmission seems to be another way of maintaining scrapie in goat and sheep herds. There is a higher incidence of scrapie in offspring of naturally affected ewes (Hoinville et al., 2010) although this could be for several transmission ways. The higher incidence could be explained by the transmission through milk or colostrum. PrPSc has been detected in sheep's milk infected with scrapie, even in preclinical stages of the disease (Lacroux et al., 2008; Maddison et al., 2009). Furthermore, it was possible to transmit scrapie to lambs using sheep and goat milk or colostrum from infected animals (Konold et al., 2008, 2013, 2016). This fact was also confirmed in goats in recent studies since scrapie was transmitted to lambs and kids using milk from goats clinically and preclinically affected with scrapie (Zhuang et al., 2018). Although some studies have shown that embryo transfer is a safe method to prevent scrapie infection from mothers to lambs (Foote et al., 1993; Wang et al., 2001; Low et al., 2009), other studies have reported the possibility of transmission of scrapie in utero (Foster et al., 1992, 1996; Spiropoulos et al., 2014). It was observed that experimentally infected ewes with a susceptible genotype could infect their offspring, and infection could not be prevented by embryo transfers or cesarean section and immediate separation from their mothers (Foster et al., 2013). Therefore, the genotype could play a role in the transmission in utero. Infectivity of placental tissues was confirmed by several reports. In the 1970s, scrapie transmission to sheep had already been proven through oral administration of fetal membranes (Pattison et al., 1972, 1974), even before the causative agent was known. It was not until the 1990s when PrPSc was detected in placental tissues of sheep by immunoblotting (Race et al., 1998). Subsequent studies evidenced that the accumulation of PrPSc depends on the PRNP genotype of the fetus. Scrapie-infected ewes can accumulate large amounts of PrPSc in placentomas only if the fetus possesses a genotype susceptible to contracting the disease (Andréoletti et al., 2002; Tuo et al., 2002; Lacroux et al., 2007; Garza et al., 2017). This discovery was essential for the development of sheep breeding programs that were implemented in different countries. In the case of goats with naturally acquired scrapie, the accumulation of PrPSc in placental tissues is low (O'Rourke et al., 2011). Despite the slight amount of PrPSc, goat placental tissues maintain their infective potential, and it has been possible to transmit scrapie to lambs and kids administering infected placenta orally (Schneider et al., 2015). These studies reveal that placental tissues are an important source of infection that could contaminate the environment in the lambing season. Several authors indicate that PrPSc accumulation increases in the presence of chronic inflammation in certain tissues. Considering transmission, this fact becomes relevant when chronic inflammation occurs in organs related to excretion or secretion. Some authors observed that scrapie transmission through milk can be enhanced by chronic lympho-follicular mastitis in sheep with lentiviral coinfection (Lacroux et al., 2008; Ligios et al., 2011). This enhancement is probably resulting from the action of small ruminant lentiviruses (SRLV). They produce an inflammatory reaction that favors the accumulation of PrPSc at the site of inflammation (Salazar et al., 2010). Increased prion aggregation also occurs in the mammary glands of goats affected with scrapie and coinfected with SRLV (González et al., 2010; Zhuang et al., 2018). This finding is important since SRLV infections are highly prevalent and global endemic, and they could act as enhancers in the transmission and perpetuation of scrapie in sheep and goat flocks. Bovine spongiform encephalopathyThe first case of classical BSE was reported in 1986 (Wells et al., 1987), and 2 years later, its transmissibility could be verified through inoculation in mice (Fraser et al., 1988). Although the origin of the outbreak remains uncertain, the transmission of BSE has been well studied in subsequent years due to the risk of transmission to humans. Many epidemiological studies were conducted to understand this novel disease. Thus, the incidence of BSE was found to be higher in dairy than in beef herds, and it could result from differences in feeding modes of both production systems (Bradley, 1991; Wilesmith et al., 1992). Based on these epidemiological data and the absence of direct horizontal transmission, it was established that the primary source of BSE infection was the food containing meat and bone meal with which the cattle were fed (Anderson et al., 1996). This fact led to the prohibition of feeding cattle with ruminant proteins in several countries. The pathogenesis of BSE, following the oral route, is similar to scrapie in the early stages. PrPBSE is initially deposited in Peyer's patches, GALT, and tonsils, and then it is distributed to the CNS through the PNS (Terry et al., 2003; Wells et al., 2005; Hoffmann et al., 2007, 2011). The main difference with scrapie is that individuals affected with BSE show slight or no distribution of prions in lymphoid tissue, except for those already mentioned (Birkett et al., 1997; Wells et al., 1998; Espinosa et al., 2007). Due to new cases of BSE in animals born after the feed ban in the United Kingdom (UK), some researchers suspected that vertical transmission was also possible (Lacey and Dealler, 1994; Curnow and Hau, 1996). Some cohort studies indicated an increased risk of developing BSE in calves born from dams that had already developed BSE clinical signs (Donnelly et al., 1997; Wilesmith et al., 1997). Years later, vertical transmission of BSE was achieved in a transgenic mouse model (Castilla et al., 2005). However, there is no clear evidence of vertical transmission in cattle. Also, it was shown that embryos would not be infectious even if they were collected from dams in the final stage of BSE (Wrathall et al., 2002). In fact, several authors proposed that the cause of the new cases of BSE in animals born after the feed ban was the cross-contamination with feed for other production animals such as pigs or poultry, which could legally contain meat and bone meal (Abrial et al., 2004; Stevenson et al., 2005; Pottgiesser et al., 2006; Allepuz et al., 2007; Jarrige et al., 2007; Paul et al., 2007; Schwermer et al., 2007). Milk as a source of PrPBSE was investigated because it could be a route of transmission between dam and calf, but mainly because of the risk to humans related to the consumption of dairy products. Many studies have been done on this subject, but to date, there is no evidence that BSE can be transmitted through milk or colostrum (Domingo, 2002; Vetrugno, 2004; Everest et al., 2006). Regarding other secretions, PrPBSE has been detected in concentrated samples of the saliva of cattle affected with BSE (Okada et al., 2012) although its infectivity was not established. More studies would be needed to expand the knowledge on the potential infectivity in secretions or excretions of BSE-infected animals, but the current low incidence of the disease might suggest that saliva is not an essential source for the infection. BSE in small ruminantsAs described, research on BSE indicated that feed was the main route of transmission of the disease. Small ruminants had been exposed to the same feed as cattle during the 1980s. Some investigations were carried out to discern the transmissibility of BSE in small ruminants and to determine the risk of the spread. It was shown that sheep and goats are susceptible to BSE by the oral or intracranial route and also that clinical signs produced by BSE remain indistinguishable from scrapie (Foster et al., 1993; Van Keulen et al., 2008). Therefore, the possibility that PrPBSE had been transmitted to sheep and goats constitutes another potential risk to public health. The distribution of PrPBSE in the tissues of experimentally infected sheep was similar to those observed in scrapie with a wide distribution in lymphoreticular tissue (Jeffrey et al., 2001; Bellworthy et al., 2005). It would be difficult to control if BSE in small ruminants were transmitted by the same routes in which scrapie is spread. Furthermore, scrapie-resistant genotypes are susceptible to contracting BSE. Thus, the applied breeding programs would not be helpful in preventing the spread of BSE in sheep herds (Foster et al., 1993; Jeffrey et al., 2001). Despite all this concern, only two cases have been detected in goats with a natural prion disease indistinguishable from BSE in experimentally infected goats (Eloit et al., 2005; Jeffrey et al., 2006). On the other hand, no case linked to BSE has been reported in sheep. Maternal or vertical transmission of BSE was demonstrated in 18% of lambs born from sheep experimentally infected (Jeffrey et al., 2015). The horizontal transmission was also observed in 0.5% of cases, although this value is lower than expected with scrapie. This poor transmission is probably due to the low presence of PrPBSE in placental tissues (Jeffrey et al., 2015). Despite these findings, the deficient transmission observed both vertically and horizontally suggests that it is unlikely that BSE could be maintained within a herd of sheep. Chronic wasting diseaseAs previously mentioned, CWD of cervids is considered as a concern because it is maintained in wildlife animals and has spread across large areas. CWD has been known since the late 1960s as a syndrome affecting mule deer (Odocoileus hemionus hemionus) and black-tailed deer (Odocoileus hemionus columbianus) in captivity. In 1980, CWD was clinically and pathologically characterized as a spongiform encephalopathy (Williams and Young, 1980). Over the years, CWD was also described in several species of captive and free-ranging cervids such as Rocky Mountain elk (Cervus elaphus nelsoni), white-tailed deer (Odocoileus virginianus), moose (Alces alces), elk (Cervus canadensis), red deer (Cervus elaphus elaphus), sika deer (Cervus nippon), and reindeer (Rangifer tarandus) (Schwabenlander et al., 2013; Benestad et al., 2016). Based on other animal prion diseases, the main route of entry for PrPCWD is presumed to be oral, which has been experimentally achieved in various species of cervids (Sigurdson et al., 1999; Kreeger et al., 2006; Balachandran et al., 2010; Mitchell et al., 2012). The pathogenesis through ingestion and tissue distribution of PrPCWD seems similar to that described in scrapie-infected sheep. There is an early distribution in lymphoid tissue, followed by spread to the CNS, PNS, and other organs (Sigurdson et al., 1999, 2001, 2002; Fox et al., 2006). But unlike what was observed in scrapie-infected sheep, PrPCWD accumulation was greater in retropharyngeal lymph nodes than in Peyer's patches of CWD-infected deer (Sigurdson et al., 1999; Fox et al., 2006). Similar to what happens in scrapie, direct, or indirect horizontal transmission has been observed in CWD (Miller and Williams, 2003), indicating that lymphoid tissue has a role in the transmissibility of the disease. In a study comparing PrPCDW levels between deer and elk affected with CWD, the distribution of prions in extraneural tissues was lower for elk (Race et al., 2007). The authors suggest that this low extraneural peripheral distribution of PrPCDW could explain two situations: first, the low incidence of CWD in elk; and second, insufficient horizontal transmission of CWD in elk compared to deer. In 2004, it was demonstrated that susceptible animals (mule deer) could contract CWD after the exposition to contaminated environments or infected animals (Miller et al., 2004). From this investigation, saliva, urine, and feces have been studied as possible sources of natural infection. The infectivity of saliva from infected animals was confirmed by oral administration to healthy deer that developed CWD after exposure (Mathiason et al., 2006, 2009). However, the infectivity of urine and feces was not confirmed in this assay. Years later, PrPCWD was detected in saliva, urine, and feces of affected animals, and their infectivity was demonstrated in the mice model (Haley et al., 2009; Pulford et al., 2012). Additionally, PrPCWD could even be detected in saliva (Henderson et al., 2013), urine (John et al., 2013), and feces (Tamgüney et al., 2009) of asymptomatic animals. These findings suggest that infected but healthy animals could be a source for infection of other animals. On the other hand, detection of PrPCWD in saliva, urine, and feces could be used as an alternative, non-invasive and preclinical diagnosis of animals. Since the numerous similarities between CWD and scrapie, several studies were conducted to investigate the possibility of vertical transmission in CWD. The first evidence of transmissibility between fawns and infected does was carried out on Reeves' muntjac deer, a species experimentally susceptible to the disease but with no reports of natural transmission to date (Nalls et al., 2013). This study demonstrated the presence of PrPCWD in harvested fetal tissues from infected pregnant does. In a subsequent investigation using the same cervid model, infective prions were detected in placentomes and amniotic fluid (Nalls et al., 2017). PrPCWD accumulation could also be evidenced in the female reproductive tract and fetal tissues of naturally exposed free-ranging elks (Selariu et al., 2015). All these findings suggest that transmission in utero of CWD is possible and that placental fluids and tissues could be another source of environmental contamination. Atypical prion diseasesIn 1998, some cases of scrapie were reported in Norway. These cases showed different clinical signs than typical scrapie (i.e., pruritus was absent), and the pathologic changes were unusually distributed (Benestad et al., 2003; Nentwig et al., 2007; Moore et al., 2008; Cook et al., 2016). PrPSc isolated from atypical scrapie cases also vary in the biochemical characteristics such as glycosylation and cleavage sites (Benestad et al., 2008). Regarding genetic susceptibility, atypical scrapie can affect sheep with genotypes resistant to the classical form (Buschmann et al., 2004; De Bosschere et al., 2007). In 2004, cases of BSE detected in Italy and France showed differences from the typical cases. The isolated prions showed fragments of peptides with different molecular masses after digestion with proteases. The one with fragments higher than previous cases of BSE was called H-BSE; the other with a fragment with lower molecular mass was called L-BSE. Then, the typical form of disease began to be called classical BSE (C-BSE), and H and L-BSE are recognized as “atypical forms of BSE.” Since its low prevalence, it is challenging to investigate atypical cases of BSE in its natural state, but the disease has been reproduced experimentally. Atypical scrapie and atypical BSE generally occur in elderly animals and have a sporadic distribution and low prevalence, unlike the classic forms of these diseases (Brown et al., 2006; Fediaevsky et al., 2008; Sala et al., 2012). In addition, atypical cases have been reported in countries or regions where classical prion diseases are exotic diseases, such as H-BSE cases in Norway, Sweden, and Brazil (OIE, 2020) or atypical scrapie cases in New Zealand (Kittelberger et al., 2010), Australia (Cook et al., 2016) and the Malvinas' (Falkland) Islands (Epstein et al., 2005). These findings suggest that the origin of the atypical form of prion diseases is completely different from the origin of the classical form. Although the first reported case of atypical scrapie was in 1998, retrospective studies showed that atypical cases have existed since at least 1987 (Webb et al., 2009). The potential transmissibility of atypical scrapie was experimentally verified intracranially and orally in sheep (Simmons et al., 2007, 2011) and ovinized transgenic mice (Le Dur et al., 2005). Nevertheless, there is no evidence that atypical scrapie is transmitted under natural conditions. The absence of horizontal transmission could be related to the lack of distribution and accumulation of PrPSc in the peripheral tissues of animals affected with atypical scrapie (Benestad et al., 2003; Buschmann et al., 2004; Nentwig et al., 2007). However, one study demonstrated that in natural and experimental cases of atypical scrapie in sheep, peripheral tissues can be infective for transgenic mice even though PrPSc is not detected through classical techniques such as immunohistochemistry, enzyme immunoassay, or immunoblot (Andréoletti et al., 2011). PrPSc could be present in small quantities, probably under the analytic sensitivity of those techniques. In that case, the epidemiologic impact of instances of atypical scrapie should be evaluated in further studies. In the case of atypical BSE, both H-type and L-type infectivity could be demonstrated in transgenic mice overexpressing bovine PrPC (Buschmann et al., 2006), and then via intracranial in cattle (Lombardi et al., 2008; Balkema-Buschmann et al., 2011). The infectivity of peripheral nervous tissue and skeletal muscle has been demonstrated in cattle experimentally infected with L-BSE and H-BSE (Sawada et al., 2019). However, the oral route would not be an efficient route of transmission based on the results in cattle challenged with L-BSE (Okada et al., 2017). Only 1 of 16 orally inoculated calves developed mild clinical signs after 88 months of incubation, and this cow had been inoculated with a high dose of L-BSE infected brain homogenate. Based on these results and the epidemiological data, it is believed that the atypical forms of prion diseases may have a spontaneous origin and their transmission could be very low or null in natural conditions. Other animal prion diseasesIn addition to the aforementioned species, prion diseases have been reported in minks, felids, several species of antelopes, and nonhuman primates (Hartsough and Burger, 1965; Bons et al., 1996, 1999; DEFRA, 2019). These reports are believed to be the result of ingestion of food contaminated with ruminant prions. TME was associated with scrapie and more recently with L-BSE (Hanson et al., 1971; Comoy et al., 2013). Reports of prion diseases in felids, nonhuman primates, and antelopes appeared from 1990, mainly in the UK during the BSE outbreak, and were associated with the consumption of PrPBSE (Jeffrey et al., 1992; Kirkwood and Cunningham, 1994; Baron et al., 1999; Eiden et al., 2010). In fact, after the measures are taken to control BSE, no more cases of prion diseases were reported in the mentioned species. Novel prion disease was recently reported in dromedary camels (Camelus dromedarius) from Algeria, designated as CPD (Babelhadj et al., 2018). Three affected animals showed neurological signs during a routine ante-mortem inspection in a slaughterhouse. Then, the presence of PrPCPD was detected in nervous tissues by immunohistochemistry and immunoblotting along with the characteristic spongiform lesions of prion disease. PrPCPD was also detected in peripheral lymph nodes in one of the affected animals. This is important since the distribution of prions in peripheral lymphoid tissue is associated with horizontal transmission in scrapie and CWD. More studies should be developed to advance the knowledge of this disease. ConclusionsSince scrapie was described in 1732, knowledge of animal prion diseases has increased, particularly after the prion hypothesis was proposed. The appearance of BSE in the late 1980s and its subsequent association with the variant of CJD put prion diseases in the spotlight. The transmission route and infection sources of prion diseases were studied in depth to take epidemiological measures to control them. Direct and indirect horizontal transmission has been observed in scrapie and CWD due to the excretion of prions into the environment. In both diseases, PrPres can be detected in non-nervous tissues. The extraneural distribution of PrPres appears to be necessary for horizontal transmission of animal prion diseases. Besides BSE, there are currently no other prion diseases considered a risk to Public Health. However, the wide distribution of CWD, the transmission of scrapie to humanized mouse and the recently described CPD are issues to consider. Thus, further research is important to understanding the potential risks of prion diseases to other animals or humans. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAbrial, D., Calavas, D., Jarrige, N. and Ducrot, C. 2004. Poultry, pig and the risk of BSE following the feed ban in France—a spatial analysis. Vet. Res. 36(4), 615–628. Allepuz, A., López-Quílez, A., Forte, A., Fernández, G. and Casal, J. 2007. Spatial analysis of bovine spongiform encephalopathy in Galicia, Spain (2000–2005). Prev. Vet. Med. 79(2–4), 174–185. Anderson, R.M., Donnelly, C.A., Ferguson, N.M., Woolhouse, M.E.J., Watt, C.J., Udy, H.J., MaWhinney, S., Dunstan, S.P., Southwood, T.R.E., Wilesmith, J.W., Ryan, J.B.M., Hoinville, L.J., Hillerton, J.E., Austin, A.R. and Wells, G.A.H. 1996. Transmission dynamics and epidemiology of BSE in British cattle. Nature 382(6594), 779–788. Andreoletti, O., Berthon, P., Marc, D., Sarradin, P., Grosclaude, J., van Keulen, L., Schelcher, F., Elsen, J.M. and Lantier, F. 2000. Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 81(12), 3115–3126. Andréoletti, O., Lacroux, C., Chabert, A., Monnereau, L., Tabouret, G., Lantier, F., Berthon, P., Eychenne, F., Lafond-Benestad, S., Elsen, J.M. and Schelcher, F. 2002. PrPSc accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J. Gen. Virol. 83(10), 2607–2616. Andréoletti, O., Orge, L., Benestad, S.L., Beringue, V., Litaise, C., Simon, S., Le Dur, A., Laude, H., Simmons, H., Lugan, S., Corbière, F., Costes, P., Morel, N., Schelcher, F. and Lacroux, C. 2011. Atypical/Nor98 scrapie infectivity in sheep peripheral tissues. PLoS Pathog. 7(2), 1–14. Babelhadj, B., Di Bari, M.A., Pirisinu, L., Chiappini, B., Gaouar, S.B.S., Riccardi, G., Marcon, S., Agrimi, U., Nonno, R. and Vaccari, G. 2018. Prion disease in dromedary camels, Algeria. Emerg. Infect. Dis. 24(6), 1029–1036. Balachandran, A., Harrington, N.P., Algire, J., Soutyrine, A., Spraker, T.R., Jeffrey, M., González, L. and O’Rourke, K.I. 2010. Experimental oral transmission of chronic wasting disease to red deer (Census elaphus elaphus):early detection and late stage distribution of protease-resistant prion protein. Can. Vet. J. 51(2), 169–178. Balkema-Buschmann, A., Ziegler, U., McIntyre, L., Keller, M., Hoffmann, C., Rogers, R., Hills, B. and Groschup, M.H. 2011. Experimental challenge of cattle with German atypical bovine spongiform encephalopathy (BSE) isolates. J. Toxicol. Environ. Health Part A. 74(2–4), 103–109. Baron, T.G.M., Madec, J.-Y. and Calavas, D. 1999. Similar signature of the prion protein in natural sheep scrapie and bovine spongiform encephalopathy-linked diseases. J. Clin. Microbiol. 37(11), 3701–3704. Baylis, M., Chihota, C., Stevenson, E., Goldmann, W., Smith, A., Sivam, K., Tongue, S. and Gravenor, M.B. 2004. Risk of scrapie in British sheep of different prion protein genotype. J. Gen. Virol. 85(9), 2735–2740. Bellworthy, S.J., Hawkins, S.A.C., Green, R.B., Blamire, I., Dexter, G., Dexter, I., Lockey, R., Ryder, S., Berthelin-Baker, C., Simmons, M.M. and Jeffrey, M. 2005. Tissue distribution of bovine spongiform encephalopathy infectivity in Romney sheep up to the onset of clinical disease after oral challenge. Vet. Rec. 156(7), 197–202. Benestad, S.L., Arsac, J.N., Goldmann, W. and Nöremark, M. 2008. Atypical/Nor98 scrapie: properties of the agent, genetics and epidemiology. Vet. Res. 39(4), 1–14. Benestad, S.L., Mitchell, G., Simmons, M.M., Ytrehus, B. and Vikøren, T. 2016. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet. Res. 47(1), 1–7. Benestad, S.L., Sarradin, P., Thu, B., Schönheit, J., Tranulis, M.A. and Bratberg, B. 2003. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet. Rec. 153(7), 202–208. Birkett, C.R., Foster, J.D., Hunter, N., Dornan, J., Farquhar, C.F., Jeffrey, M., Hennion, R.M., Goldmann, W., Grover, D., Somerville, R.A. and Percy, C. 1997. Immunodetection of PrPSc in spleens of some scrapie-infected sheep but not BSE-infected cows. J. Gen. Virol. 78(9), 2389–2396. Bons, N., Mestre-Frances, N., Belli, P., Cathala, F., Gajdusek, D.C. and Brown, P. 1999. Natural and experimental oral infection of nonhuman primates by bovine spongiform encephalopathy agents. Proc. Natl. Acad. Sci. 96(7), 4046–4051. Bons, N., Mestre-Francés, N., Charnay, Y. and Tagliavini, F. 1996. Spontaneous spongiform encephalopathy in a young adult rhesus monkey. Lancet 348(9019), 55; doi:10.1016/s0140-6736(96)24027-9 Bradley, R. 1991. Bovine spongiform encephalopathy (BSE): the current situation and research. Eur. J. Epidemiol. 7(5), 532–544. Brown, K.F.D., Stewart, K., Ritchie, D.L., Mabbott, N.A., Williams, A., Fraser, H., Morrison, W.I. and Bruce, M.E. 1999. Scrapie replication in lymphoid tissues depends on prion protein- expressing follicular dendritic cells. Nat. Med. 5(11), 1308–1312. Brown, P., McShane, L., Zanusso, G. and Detwiler, L. 2006. On the question of sporadic or atypical bovine spongiform encephalopathy and Creutzfeldt-Jakob disease. Emerg. Infect. Dis. 12(12), 1816–1821. Bruce, M.E., Brown, K.F.D., Mabbott, N.A., Farquhar, C.F. and Jeffrey, M. 2000. Follicular dendritic cells in TSE pathogenesis. Immunol. Today 21(9), 442–446. Bruce, M.E., Will, R.G., Ironside, J.W., McConnell, I., Drummond, D., Suttie, A., McCardle, L., Chree, A., Hope, J., Birkett, C., Cousens, S., Fraser, H. and Bostock, C.J. 1997. Transmissions to mice indicate that “new variant” CJD is caused by the BSE agent. Nature 389(6650), 498–501. Buschmann, A., Gretzschel, A., Biacabe, A.-G., Schiebel, K., Corona, C., Hoffmann, C., Eiden, M., Baron, T., Casalone, C. and Groschup, M.H. 2006. Atypical BSE in Germany—proof of transmissibility and biochemical characterization. Vet. Microbiol. 117(2–4), 103–116. Buschmann, A., Lühken, G., Schultz, J., Erhardt, G. and Groschup, M.H. 2004. Neuronal accumulation of abnormal prion protein in sheep carrying a scrapie-resistant genotype (PrPARR/ARR). J. Gen. Virol. 85(9), 2727–2733; doi:10.1099/vir.0.79997-0 Carp, R. 1982. Transmission of scrapie by oral route effect of gingival scarification. Lancet 319(8264), 170–171. Cassard, H., Torres, J.M., Lacroux, C., Douet, J.Y., Benestad, S.L., Lantier, F., Lugan, S., Lantier, I., Costes, P., Aron, N., Reine, F., Herzog, L., Espinosa, J.C., Beringue, V. and Andreóletti, O. 2014. Evidence for zoonotic potential of ovine scrapie prions. Nat. Commun. 5; doi:10.1038/ncomms6821 Castilla, J., Brun, A., Díaz-San Segundo, F., Salguero, F.J., Gutiérrez-Adán, A., Pintado, B., Ramírez, M.A., del Riego, L. and Torres, J.M. 2005. Vertical transmission of bovine spongiform encephalopathy prions evaluated in a transgenic mouse model. J. Virol. 79(13), 8665–8668. Chandler, R.L. 1961. Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet 277(7191), 1378–1379. Chandler, R.L. and Fisher, J. 1963. Experimental transmission of scrapie to rats. Lancet 282(7318), 1165; doi:10.1016/S0140-6736(63)90820-1 Comoy, E., Mikol, J., Luccantoni-Freire, S., Correia, E., Lescoutra-Etchegaray, N., Durand, V., Dehen, C. andreoletti, O., Casalone, C., Richt, J.A., Greenlee, J.J., Baron, T., Benestad, S.L., Brown, P. and Deslys, J.P. 2015. Transmission of scrapie prions to primate after an extended silent incubation period. Sci. Rep. 5, 1–11. Comoy, E., Mikol, J., Ruchoux, M.-M., Durand, V., Luccantoni-Freire, S., Dehen, C., Correia, E., Casalone, C., Richt, J., Greenlee, J., Torres, J., Brown, P. and Deslys, J.-P. 2013. Evaluation of the zoonotic potential of transmissible mink encephalopathy. Pathogens 2(3), 520–532. Cook, R.W., Bingham, J., Besier, A.S., Bayley, C.L., Hawes, M., Shearer, P.L., Yamada, M., Bergfeld, J., Williams, D.T. and Middleton, D.J. 2016. Atypical scrapie in Australia. Aust. Vet. J. 94(12), 452–455. Corona, C., Porcario, C., Martucci, F., Iulini, B., Manea, B., Gallo, M., Palmitessa, C., Maurella, C., Mazza, M., Pezzolato, M., Acutis, P. and Casalone, C. 2009. Olfactory system involvement in natural scrapie disease. J. Virol. 83(8), 3657–3667. Curnow, R.N. and Hau, C.M. 1996. The incidence of bovine spongiform encephalopathy in the progeny of affected sires and dams. Vet. Rec. 138(17), 407–408. De Bosschere, H., Roels, S., Dechamps, P. and Vanopdenbosch, E. 2007. TSE detected in a Belgian ARR-homozygous sheep via active surveillance. Vet. J. 173(2), 449–451. DEFRA. 2019. TSE surveillance statistics: exotic species and domestic cats. Available via https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/840096/pub-tse-stats-exotic.pdf Domingo, J.L. 2002. Lack of experimental studies on human transmission of BSE in relation with the consumption of specified risk materials (SRM): the case of the milk. Prev. Med. (Baltim). 34(6), 655–656. Donnelly, C.A., Ferguson, N.M., Ghani, A.C., Wilesmith, J.W. and Anderson, R.M. 1997. Analysis of dam-calf pairs of BSE cases: confirmation of a maternal risk enhancement. Proc. R. Soc. B Biol. Sci. 264(1388), 1647–1656. Edwards, J.C., Moore, S.J., Hawthorn, J.A., Neale, M.H. and Terry, L.A. 2010. PrPSc is associated with B cells in the blood of scrapie-infected sheep. Virology 405(1), 110–119. Eiden, M., Hoffmann, C., Balkema-Buschmann, A., Muller, M., Baumgartner, K. and Groschup, M.H. 2010. Biochemical and immunohistochemical characterization of feline spongiform encephalopathy in a German captive cheetah. J. Gen. Virol. 91(11), 2874–2883. Eloit, M., Adjou, K., Coulpier, M., Fontaine, J.J., Hamel, R., Lilin, T., Messiaen, S. andreoletti, O., Baron, T., Bencsik, A., Gaelle Biacabe, A., Beringue, V., Laude, H., Le Dur, A., Vilotte, J.L., Comoy, E., Deslys, J.P., Grassi, J., Simon, S. and Sarradin, P. 2005. BSE agent signatures in a goat. Vet. Rec. 156(16), 523–524. Epstein, V., Pointing, S. and Halfacre, S. 2005. Atypical scrapie in the Falkland Islands. Vet. Rec. 157(21), 667–668. Eraña, H., Charco, J.M., González-Miranda, E., García-Martínez, S., López-Moreno, R., Pérez-Castro, M.A., Díaz-Domínguez, C.M., García-Salvador, A. and Castilla, J. 2020. Detection of pathognomonic biomarker PrPsc and the contribution of cell free-amplification techniques to the diagnosis of prion diseases. Biomolecules 10(3), 1–44. Espinosa, J.C., Morales, M., Castilla, J., Rogers, M. and Torres, J.M. 2007. Progression of prion infectivity in asymptomatic cattle after oral bovine spongiform encephalopathy challenge. J. Gen. Virol. 88(4), 1379–1383. Everest, S.J., Thorne, L.T., Hawthorn, J.A., Jenkins, R., Hammersley, C., Ramsay, A.M., Hawkins, S.A., Venables, L., Flynn, L., Sayers, R., Kilpatrick, J., Sach, A., Hope, J. and Jackman, R. 2006. No abnormal prion protein detected in the milk of cattle infected with the bovine spongiform encephalopathy agent. J. Gen. Virol. 87(8), 2433–2441. Fediaevsky, A., Tongue, S.C., Nöremark, M., Calavas, D., Ru, G. and Hopp, P. 2008. A descriptive study of the prevalence of atypical and classical scrapie in sheep in 20 European countries. BMC Vet. Res. 4, 1–24. Foote, W.C., Clark, W., Maciulis, A., Call, J.W., Hourrigan, J., Evans, R.C., Marshall, M.R. and de Camp, M. 1993. Prevention of scrapie transmission in sheep, using embryo transfer. Am. J. Vet. Res. 54(11), 1863–1868. Foster, J.D., Goldmann, W. and Hunter, N. 2013. Evidence in sheep for pre-natal transmission of scrapie to lambs from infected mothers. PLoS One 8(11); doi:10.1371/journal.pone.0079433 Foster, J.D., Hope, J. and Fraser, H. 1993. Transmission of bovine spongiform encephalopathy to sheep and goats. Vet. Rec. 133(14), 339–341. Foster, J.D., Hunter, N., Williams, A., Mylne, M.J.A., McKelvey, W.A.C., Hope, J., Fraser, H. and Bostock, C. 1996. Observations on the transmission of scrapie in experiments using embryo transfer. Vet. Rec. 138(23), 559–562. Foster, J.D., McKelvey, W.A., Mylne, M.J., Williams, A., Hunter, N., Hope, J. and Fraser, H. 1992. Studies on maternal transmission of scrapie in sheep by embryo transfer. Vet. Rec. 130(16), 341–343. Fox, K.A., Jewell, J.E., Williams, E.S. and Miller, M.W. 2006. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). J. Gen. Virol. 87(11), 3451–3461. Fraser, H., McConnell, I., Wells, G.A.H. and Dawson, M. 1988. Transmission of bovine spongiform encephalopathy to mice. Vet. Rec. 123(18), 472. Garza, M.C., Eraña, H., Castilla, J., Acín, C., Vargas, A., Badiola, J.J. and Monleón, E. 2017. Protein misfolding cyclic amplification corroborates the absence of PrP Sc accumulation in placenta from foetuses with the ARR/ARQ genotype in natural scrapie. Vet. Microbiol. 203, 294–300. Gavier-Widén, D., Stack, M.J., Baron, T., Balachandran, A. and Simmons, M. 2005. Diagnosis of transmissible spongiform encephalopathies in animals: a review. J. Vet. Diagn. Investig. 17(6), 509–527. Glaysher, B.R. and Mabbott, N.A. 2007. Role of the draining lymph node in scrapie agent transmission from the skin. Immunol. Lett. 109(1), 64–71. Goldmann, W. 2008. PrP genetics in ruminant transmissible spongiform encephalopathies. Vet. Res. 39(4); doi:10.1051/vetres:2008010 Gonzalez-Romero, D., Barria, M.A., Leon, P., Morales, R. and Soto, C. 2008. Detection of infectious prions in urine. FEBS Lett. 582(21–22), 3161–3166. González, L., Martin, S., Hawkins, S.A.C., Goldmann, W., Jeffrey, M. and Sisó, S. 2010. Pathogenesis of natural goat scrapie: modulation by host PRNP genotype and effect of co-existent conditions. Vet. Res. 41(4), 48; doi:10.1051/vetres/2010020 Gossner, A., Hunter, N. and Hopkins, J. 2006. Role of lymph-borne cells in the early stages of scrapie agent dissemination from the skin. Vet. Immunol. Immunopathol. 109(3–4), 267–278. Gough, K.C., Baker, C.A., Rees, H.C., Terry, L.A., Spiropoulos, J., Thorne, L. and Maddison, B.C. 2012. The oral secretion of infectious scrapie prions occurs in preclinical sheep with a range of PRNP genotypes. J. Virol. 86(1), 566–571. Greenlee, J.J. 2019. Review: update on classical and atypical scrapie in sheep and goats. Vet. Pathol. 56(1), 6–16. Gregori, L., Kovacs, G.G., Alexeeva, I., Budka, H. and Rohwer, R.G. 2008. Excretion of transmissible spongiform encephalopathy infectivity in urine. Emerg. Infect. Dis. 14(9), 1406–1412. Greig, J.R. 1940. Observations on the transmission of the disease by mediate contact. Vet. J. 96(5), 203–206. Haley, N.J., Seelig, D.M., Zabel, M.D., Telling, G.C. and Hoover, E.A. 2009. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One 4(3); doi:10.1371/journal.pone.0004848 Hamir, A.N., Kunkle, R.A., Richt, J.A., Miller, J.M. and Greenlee, J.J. 2008. Experimental transmission of US Scrapie Agent by nasal, peritoneal and conjunctival routes to genetically susceptible sheep. Vet. Pathol. 45(1), 7–11. Hannaoui, S., Schatzl, H.M. and Gilch, S. 2017. Chronic wasting disease: emerging prions and their potential risk. PLoS Pathog. 13(11), 1–5. Hanson, R.P., Eckroade, R.J., Marsh, R.F., Zu Rhein, G.M., Kanitz, C.L. and Gustafson, D.P. 1971. Susceptibility of mink to sheep scrapie. Science 172(3985), 859–861. Hartsough, G.R. and Burger, D. 1965. Encephalopathy of mink: I. Epizootiologic and clinical observations. J. Infect. Dis. 115(4), 387–392. Heggebø, R., González, L., Press, C.M.L., Gunnes, G., Espenes, A. and Jeffrey, M. 2003. Disease-associated PrP in the enteric nervous system of scrapie-affected Suffolk sheep. J. Gen. Virol. 84(5), 1327–1338. Heggebø, R., Press, C.M.L., Gunnes, G., González, L. and Jeffrey, M. 2002. Distribution and accumulation of PrP in gut-associated and peripheral lymphoid tissue of scrapie-affected Suffolk sheep. J. Gen. Virol. 83(2), 479–489. Henderson, D.M., Manca, M., Haley, N.J., Denkers, N.D., Nalls, A.V., Mathiason, C.K., Caughey, B. and Hoover, E.A. 2013. Rapid antemortem detection of CWD prions in deer saliva. PLoS One 8(9), e74377; doi:10.1371/journal.pone.0074377 Heppner, F.L., Christ, A.D., Klein, M.A., Prinz, M., Fried, M., Kraehenbuhl, J.-P. and Aguzzi, A. 2001. Transepithelial prion transport by M cells. Nat. Med. 7(9), 976–977. Herrmann, L.M., Cheevers, W.P., Davis, W.C., Knowles, D.P. and O’Rourke, K.I. 2003. Cd21-positive follicular dendritic cells: a possible source of PrPSc in lymph node macrophages of scrapie-infected sheep. Am. J. Pathol. 162(4), 1075–1081. Hoffmann, C., Eiden, M., Kaatz, M., Keller, M., Ziegler, U., Rogers, R., Hills, B., Balkema-Buschmann, A., Van Keulen, L., Jacobs, J.G. and Groschup, M.H. 2011. BSE infectivity in jejunum, ileum and ileocaecal junction of incubating cattle. Vet. Res. 42(1), 1–12. Hoffmann, C., Ziegler, U., Buschmann, A., Weber, A., Kupfer, L., Oelschlegel, A., Hammerschmidt, B. and Groschup, M.H. 2007. Prions spread via the autonomic nervous system from the gut to the central nervous system in cattle incubating bovine spongiform encephalopathy. J. Gen. Virol. 88(3), 1048–1055. Hoinville, L.J., Tongue, S.C. and Wilesmith, J.W. 2010. Evidence for maternal transmission of scrapie in naturally affected flocks. Prev. Vet. Med. 93, 121–128. Houston, F., McCutcheon, S., Goldmann, W., Chong, A., Foster, J.D., Sisó, S., González, L., Jeffrey, M. and Hunter, N. 2008. Prion diseases are efficiently transmitted by blood transfusion in sheep. Blood 112(12), 4739–4745. Huang, F.P., Farquhar, C.F., Mabbott, N.A., Bruce, M.E. and Macpherson, G.G. 2002. Migrating intestinal dendritic cells transport PrP Sc from the gut. J. Gen Virol. 83, 267–271. Huang, F.P. and MacPherson, G.G. 2004. Dendritic cells and oral transmission of prion diseases. Adv. study on feed risk factors for BSE cases born after the feed ban in France. Vet. Res. 38(3), 505–516. Jeffrey, M., Martin, S., González, L., Foster, J.D., Langeveld, J.P.M., van Zijderveld, F.G., Grassi, J. and Hunter, N. 2006. Immunohistochemical features of PrPd accumulation in natural and experimental goat transmissible spongiform encephalopathies. J. Comp. Pathol. 134(2–3), 171–181. Jeffrey, M., Ryder, S., Martin, S., Hawkins, S.A.C., Terry, L., Berthelin-Baker, C. and Bellworthy, S.J. 2001. Oral inoculation of sheep with the agent of bovine spongiform encephalopathy (BSE). 1. Onset and distribution of disease-specific PrP accumulation in brain and viscera. J. Comp. Pathol. species of bovidae. Acta Neuropathol. 84(5), 559–569. Jeffrey, M., Witz, J.P., Martin, S., Hawkins, S.A.C., Bellworthy, S.J., Dexter, G.E., Thurston, L. and González, L. 2015. Dynamics of the natural transmission of bovine spongiform encephalopathy within an intensively managed sheep flock. Vet. Res. 46(1), 1–14. John, T.R., Schätzl, H.M. and Gilch, S. 2013. Early detection of chronic wasting disease prions in urine of pre-symptomatic deer by real-time quaking-induced conversion assay. Prion 7(3), 253–258. Kariv-Inbal, Z., Ben-Hur, T., Grigoriadis, N.C., Engelstein, R. and Gabizon, R. 2006. Urine from scrapie-infected hamsters comprises low levels of prion infectivity. Neurodegener. Dis. 3(3), 123–128. Kirkwood, J.K. and Cunningham, A.A. 1994. Epidemiological observations on spongiform encephalopathies in captive wild animals in the British Isles. Vet. Rec. 135(13), 296–303. Kittelberger, R., Chaplin, M.J., Simmons, M.M., Ramirez-Villaescusa, A., McIntyre, L., MacDiarmid, S.C., Hannah, M.J., Jenner, J., Bueno, R., Bayliss, D., Black, H., Pigott, C.J. and O’Keefe, J.S. 2010. Atypical scrapie/nor98 in a sheep from New Zealand. J. Vet. Diagn. Investig. 22(6), 863–875. Konold, T., Hawkins, S.A.C., Thurston, L.C., Maddison, B.C., Gough, K.C., Duarte, A. and Simmons, H.A. 2015. Objects in contact with classical scrapie sheep act as a reservoir for scrapie transmission. Front. Vet. Sci. 2, 1–7. Konold, T., Moore, S.J., Bellworthy, S.J. and Simmons, H.A. 2008. Evidence of scrapie transmission via milk. BMC Vet. Res. 4, 1–10. Konold, T., Moore, S.J., Bellworthy, S.J., Terry, L.A., Thorne, L., Ramsay, A., Salguero, F.J., Simmons, M.M. and Simmons, H.A. 2013. Evidence of effective scrapie transmission via colostrum and milk in sheep. BMC Vet. Res. 9(1), 1; doi:10.1186/1746-6148-9-99 Konold, T., Thorne, L., Simmons, H.A., Hawkins, S.A.C., Simmons, M.M. and González, L. 2016. Evidence of scrapie transmission to sheep via goat milk. BMC Vet. Res. 12(1), 1–10. Koutsoumanis, K., Allende, A., Alvarez-Ordoňez, A., Bolton, D., Bover-Cid, S., Chemaly, M., Davies, R., De Cesare, A., Herman, L., Hilbert, F., Lindqvist, R., Nauta, M., Peixe, L., Ru, G., Skandamis, P., Suffredini, E. andreoletti, O., Benestad, S.L., Comoy, E. and Simmons, M.M. 2019. Update on chronic wasting disease (CWD) III. EFSA J. 17(11); doi:10.2903/j.efsa.2019.5863 Kreeger, T.J., Montgomery, D.L., Jewell, J.E., Schultz, W. and Williams, E.S. 2006. Oral transmission of chronic wasting disease in captive Shira’s moose. J. Wildl. Dis. 42(3), 640–645. Krüger, D., Thomzig, A., Lenz, G., Kampf, K., McBride, P.A. and Beekes, M. 2009. Faecal shedding, alimentary clearance and intestinal spread of prions in hamsters fed with scrapie. Vet. Res. 40(1), 04; doi:10.1051/vetres:2008042 Lacey, R.W. and Dealler, S.F. 1994. The transmission of prion disease: vertical transmission of prion disease. Hum. Reprod. 9(10), 1796–1797. Lacroux, C., Corbière, F., Tabouret, G., Lugan, S., Costes, P., Mathey, J., Delamas, J.M., Weisbecker, J.L., Foucras, G., Cassard, H., Elsen, J.M., Schelcher, F. and Andréoletti, O. 2007. Dynamics and genetics of PrPSc placental accumulation in sheep. J. Gen. Virol. 88(3), 1056–1061. Lacroux, C., Simon, S., Benestad, S.L., Maillet, S., Mathey, J., Lugan, S., Corbière, F., Cassard, H., Costes, P., Bergonier, D., Weisbecker, J.L., Moldal, T., Simmons, H., Lantier, F., Feraudet-Tarisse, C., Morel, N., Schelcher, F., Grassi, J. and Andréoletti, O. 2008. Prions in milk from ewes incubating natural scrapie. PLoS Pathog. 4(12); doi:10.1371/journal.ppat.1000238 Le Dur, A., Béringue, V. Andréoletti, O., Reine, F., Laï, T.L., Baron, T., Bratberg, B., Vilotte, J.L., Sarradin, P., Benestad, S.L. and Laude, H. 2005. A newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotypes. Proc. Natl. Acad. Sci. U. S. A. 102(44), 16031–16036. Liberski, P.P. 2012. Historical overview of prion diseases: a view from afar. Folia Neuropathol. 50(1), 1–12. Ligios, C., Cancedda, M.G., Carta, A., Santucciu, C., Maestrale, C., Demontis, F., Saba, M., Patta, C., DeMartini, J.C., Aguzzi, A. and Sigurdson, C.J. 2011. Sheep with scrapie and mastitis transmit infectious prions through the milk. J. Virol. 85(2), 1136–1139. Lombardi, G., Casalone, C., D’Angelo, A., Gelmetti, D., Torcoli, G., Barbieri, I., Corona, C., Fasoli, E., Farinazzo, A., Fiorini, M., Gelati, M., Iulini, B., Tagliavini, F., Ferrari, S., Caramelli, M., Monaco, S., Capucci, L. and Zanusso, G. 2008. Intraspecies transmission of BASE induces clinical dullness and amyotrophic changes. PLoS Pathog. 4(5), e1000075; doi:10.1371/journal.ppat.1000075 Low, J.C., Chambers, J., McKelvey, W.A.C., McKendrick, I.J. and Jeffrey, M. 2009. Failure to transmit scrapie infection by transferring preimplantation embryos from naturally infected donor sheep. Theriogenology 72(6), 809–816. Mabbott, N.A., Williams, A., Farquhar, C.F., Pasparakis, M., Kollias, G. and Bruce, M.E. 2000. Tumor necrosis factor alpha-deficient, but not interleukin-6-deficient, mice resist peripheral infection with scrapie. J. Virol. 74(7), 3338–3344. Maddison, B.C., Baker, C.A., Rees, H.C., Terry, L.A., Thorne, L., Bellworthy, S.J., Whitelam, G.C. and Gough, K.C. 2009. Prions are secreted in milk from clinically Normal scrapie-exposed sheep. J. Virol. 83(16), 8293–8296. Maddison, B.C., Rees, H.C., Baker, C.A., Taema, M., Bellworthy, S.J., Thorne, L., Terry, L.A. and Gough, K.C. 2010. Prions are secreted into the oral cavity in sheep with preclinical scrapie. J. Infect. Dis. 201(11), 1672–1676. Marsh, R.F. and Hadlow, W.J. 1992. Transmissible mink encephalopathy. Rev. Sci. Tech. 11(2), 539–550. Mathiason, C.K., Hays, S.A., Powers, J., Hayes-Klug, J., Langenberg, J., Dahmes, S.J., Osborn, D.A., Miller, K.V., Warren, R.J., Mason, G.L. and Hoover, E.A. 2009. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS One 4(6), e5916; doi:10.1371/journal.pone.0005916 Mathiason, C.K., Powers, J.G., Dahmes, S.J., Osborn, D.A., Miller, K.V., Warren, R.J., Mason, G.L., Hays, S.A., Hayes-Klug, J., Seelig, D.M., Wild, M.A., Wolfe, L.L., Spraker, T.R., Miller, M.W., Sigurdson, C.J., Telling, G.C. and Hoover, E.A. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314(5796), 133–136. McBride, P.A., Eikelenboom, P., Kraal, G., Fraser, H. and Bruce, M.E. 1992. PrP protein is associated with follicular dendritic cells of spleens and lymph nodes in uninfected and scrapie-infected mice. J. Pathol. 168(4), 413–418. McBride, P.A., Schulz-Schaeffer, W.J., Donaldson, M., Bruce, M., Diringer, H., Kretzschmar, H.A. and Beekes, M. 2001. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J. Virol. 75(19), 9320–9327. Mead, S., Lloyd, S. and Collinge, J. 2019. Genetic factors in mammalian prion diseases. Annu. Rev. Genet. 53(1), 117–147. Melchior, M.B., Windig, J.J., Hagenaars, T.J., Bossers, A., Davidse, A. and van Zijderveld, F.G. 2010. Eradication of scrapie with selective breeding: are we nearly there? BMC Vet. Res. 6; doi:10.1186/1746-6148-6-24 Miller, M.W. and Williams, E.S. 2003. Horizontal prion transmission in mule deer. Nature 425(6953), 35–36. Miller, M.W., Williams, E.S., Hobbs, N.T. and Wolfe, L.L. 2004. Environmental sources of prion transmission in mule deer. Emerg. Infect. Dis. 10(6), 1003–1006. Mitchell, G.B., Sigurdson, C.J., O’Rourke, K.I., Algire, J., Harrington, N.P., Walther, I., Spraker, T.R. and Balachandran, A. 2012. Experimental oral transmission of chronic wasting disease to reindeer (Rangifer tarandus tarandus). PLoS One 7(6); doi:10.1371/journal.pone.0039055 Miyazawa, K., Kanaya, T., Takakura, I., Tanaka, S., Hondo, T., Watanabe, H., Rose, M.T., Kitazawa, H., Yamaguchi, T., Katamine, S., Nishida, N. and Aso, H. 2010. Transcytosis of murine-adapted bovine spongiform encephalopathy agents in an in vitro bovine M cell model. J. Virol. 84(23), 12285–12291. Mohan, J., Brown, K.F.D., Farquhar, C.F., Bruce, M.E. and Mabbott, N.A. 2004. Scrapie transmission following exposure through the skin is dependent on follicular dendritic cells in lymphoid tissues. J. Dermatol. Sci. 35(2), 101–111. Mohan, J., Bruce, M.E. and Mabbott, N.A. 2005. Neuroinvasion by scrapie following inoculation via the skin is independent of migratory langerhans cells. J. Virol. 79(3), 1888–1897. Mok, S.W.F., Proia, R.L., Brinkmann, V. and Mabbott, N.A. 2012. B Cell-specific S1PR1 deficiency blocks prion dissemination between secondary lymphoid organs. J. Immunol. 188(10), 5032–5040. Moore, S.J., Simmons, M.M., Chaplin, M. and Spiropoulos, J. 2008. Neuroanatomical distribution of abnormal prion protein in naturally occurring atypical scrapie cases in Great Britain. Acta Neuropathol. 116(5), 547–559. Murayama, Y., Yoshioka, M., Okada, H., Takata, M., Yokoyama, T. and Mohri, S. 2007. Urinary excretion and blood level of prions in scrapie-infected hamsters. J. Gen. Virol. 88(10), 2890–2898. Nalls, A.V., McNulty, E., Hoover, C.E., Pulscher, L.A., Hoover, E.A. and Mathiason, C.K. 2017. Infectious prions in the pregnancy microenvironment of chronic wasting disease-infected Reeves’ Muntjac deer. J. Virol. 91(15), 1–15. Nalls, A.V., McNulty, E., Powers, J., Seelig, D.M., Hoover, C., Haley, N.J., Hayes-Klug, J. anderson, K., Stewart, P., Goldmann, W., Hoover, E.A. and Mathiason, C.K. 2013. Mother to offspring transmission of chronic wasting disease in Reeves’ Muntjac deer. PLoS One 8(8); doi:10.1371/journal.pone.0071844 Nentwig, A., Oevermann, A., Heim, D., Botteron, C., Zellweger, K., Drögemüller, C., Zurbriggen, A. and Seuberlich, T. 2007. Diversity in neuroanatomical distribution of abnormal prion protein in atypical scrapie. PLoS Pathog. 3(6), 743–751. O’Rourke, K.I., Zhuang, D., Truscott, T.C., Yan, H. and Schneider, D.A. 2011. Sparse PrP(Sc) accumulation in the placentas of goats with naturally acquired scrapie. BMC Vet. Res. 7(1), 7; doi:10.1186/1746-6148-7-7 OIE. 2020. BSE situation in the world and annual incidence rate. Available via https://www.oie.int/en/animal-health-in-the-world/test-bse-situation-in-the-world-and-annual-incidence-rate-copy-1/ Okada, H., Iwamaru, Y., Imamura, M., Miyazawa, K., Matsuura, Y., Masujin, K., Murayama, Y. and Yokoyama, T. 2017. Oral transmission of L-type bovine spongiform encephalopathy agent among cattle. Emerg. Infect. Dis. 23(2), 284–287. Okada, H., Murayama, Y., Shimozaki, N., Yoshioka, M., Masujin, K., Imamura, M., Iwamaru, Y., Matsuura, Y., Miyazawa, K., Fukuda, S., Yokoyama, T. and Mohri, S. 2012. Prion in saliva of bovine spongiform encephalopathy- infected cattle. Emerg. Infect. Dis. 18(12), 2091–2092. Onodera, T., Ikeda, T., Muramatsu, Y. and Shinagawa, M. 1993. Isolation of scrapie agent from the placenta of sheep with natural scrapie in Japan. Microbiol. Immunol. 37(4), 311–316. Pan, K.M., Baldwin, M., Nguyen, J., Gasset, M., Serban, A., Groth, D., Mehlhorn, I., Huang, Z., Fletterick, R.J., Cohen, F.E. and Prusiner, S.B. 1993. Conversion of α-helices into β-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. U. S. A. 90(23), 10962–10966. Pattison, I.H., Hoare, M., Jebbett, J. and Watson, W. 1972. Spread of scrapie to sheep and goats by oral dosing with foetal membranes from scrapie-affected sheep. Vet. Rec. 90(17), 465–468. Pattison, I.H., Hoare, M.N., Jebbett, J.N. and Watson, W.A. 1974. Further observations on the production of scrapie in sheep by oral dosing with foetal membranes from scrapie affected sheep. Br. Vet. J. 130(4); doi:10.1016/S0007-1935(17)35851-7 Paul, M., Abrial, D., Jarrige, N., Rican, S., Garrido, M., Calavas, D. and Ducrot, C. 2007. Bovine spongiform encephalopathy and spatial analysis of the feed industry. Emerg. Infect. Dis. 13(6), 867–872. Pottgiesser, C., Ovelhey, A., Ziller, M., Kramer, M., Selhorst, T. and Conraths, F.J. 2006. Potential risk factors associated with bovine spongiform encephalopathy in cattle from Schleswig-Holstein, Germany. J. Vet. Med. Ser. B 53(7), 306–311. Prinz, M., Huber, G., Macpherson, A.J.S., Heppner, F.L., Glatzel, M., Eugster, H. Pietro, Wagner, N. and Aguzzi, A. 2003. Oral prion infection requires normal numbers of Peyer’s patches but not of enteric lymphocytes. Am. J. Pathol. 162(4), 1103–1111. Prusiner, S.B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216(4542), 136–144. Pulford, B., Spraker, T.R., Christy Wyckoff, A., Meyerett, C., Bender, H., Ferguson, A., Wyatt, B., Lockwood, K., Powers, J., Telling, G.C., Wild, M.A. and Zabel, M.D. 2012. Detection of PrPCWD in feces from naturally exposed Rocky Mountain elk (Cervus elaphus nelsoni) using protein misfolding cyclic amplification. J. Wildl. Dis. 48(2), 425–434. Race, B.L., Meade-White, K.D., Ward, A., Jewell, J., Miller, M.W., Williams, E.S., Chesebro, B. and Race, R.E. 2007. Levels of abnormal prion protein in deer and elk with chronic wasting disease. Emerg. Infect. Dis. 13(6), 824–830. Race, R.E., Jenny, A. and Sutton, D. 1998. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen and lymph node: implications for transmission and antemortem diagnosis. J. Infect. Dis. 178(4), 949–953 Rivera, N.A., Brandt, A.L., Novakofski, J.E. and Mateus-Pinilla, N.E. 2019. Chronic wasting disease in cervids: prevalence, impact and management strategies. Vet. Med. Res. Rep. 10, 123–139. Rubenstein, R., Bulgin, M.S., Chang, B., Sorensen-Melson, S., Petersen, R.B. and LaFauci, G. 2012. PrPSc detection and infectivity in semen from scrapie-infected sheep. J. Gen. Virol. 93(6), 1375–1383. Rubenstein, R., Chang, B., Gray, P., Piltch, M., Bulgin, M.S., Sorensen-Melson, S. and Miller, M.W. 2011. Prion disease detection, PMCA kinetics and IgG in urine from sheep naturally/experimentally infected with scrapie and deer with preclinical/clinical chronic wasting disease. J. Virol. 85(17), 9031–9038. Safar, J.G., Lessard, P., Tamgüney, G., Freyman, Y., Deering, C., Letessier, F., DeArmond, S.J. and Prusiner, S.B. 2008. Transmission and detection of prions in feces. J. Infect. Dis. 198(1), 81–89. Sala, C., Morignat, E., Oussaïd, N., Gay, E., Abrial, D., Ducrot, C. and Calavas, D. 2012. Individual factors associated with L- and H-type bovine spongiform encephalopathy in France. BMC Vet. Res. 8; doi:10.1186/1746-6148-8-74 Salazar, E., Monleón, E., Bolea, R., Acín, C., Pérez, M., Álvarez, N., Leginagoikoa, I., Juste, R., Minguijón, E., Reina, R., Glaria, I., Berriatua, E., de Andrés, D., Badiola, J.J., Amorena, B. and Luján, L. 2010. Detection of PrP Sc in lung and mammary gland is favored by the presence of Visna/maedi virus lesions in naturally coinfected sheep. Vet. Res. 41(5), 58; doi:10.1051/vetres/2010030 Sawada, K., Suzuki, A., Yamasaki, T., Iwamaru, Y., Matsuura, Y., Miyazawa, K., Masujin, K., Atarashi, R. and Horiuchi, M. 2019. Estimation of prion infectivity in tissues of cattle infected with atypical BSE by real time-quaking induced conversion assay. J. Vet. Med. Sci. 81(6), 846–850. Sbriccoli, M., Cardone, F., Valanzano, A., Lu, M., Graziano, S., De Pascalis, A., Ingrosso, L., Zanusso, G., Monaco, S., Bentivoglio, M. and Pocchiari, M. 2009. Neuroinvasion of the 263K scrapie strain after intranasal administration occurs through olfactory-unrelated pathways. Acta Neuropathol. 117(2), 175–184. Schneider, D.A., Madsen-Bouterse, S.A., Zhuang, D., Truscott, T.C., Dassanayake, R.P. and O’Rourke, K.I. 2015. The placenta shed from goats with classical scrapie is infectious to goat kids and lambs. J. Gen. Virol. 96(8), 2464–2469. Schwabenlander, M.D., Culhane, M.R., Hall, S.M., Goyal, S.M. anderson, P.L., Carstensen, M., Wells, S.J., Slade, W.B. and Armién, A.G. 2013. A case of chronic wasting disease in a captive red deer (Cervus elaphus). J. Vet. Diagnostic Investig. 25(5), 573–576. Schwermer, H., Forster, K., Brülisauer, F., Chaubert, C. and Heim, D. 2007. BSE, feed and cattle in Switzerland: is there a spatial relation? Vet. Res. 38(3), 409–418. Seeger, H. 2005. Coincident scrapie infection and nephritis lead to urinary prion excretion. Sci. 310(5746), 324–326. Selariu, A., Powers, J.G., Nalls, A., Brandhuber, M., Mayfield, A., Fullaway, S., Wyckoff, C.A., Goldmann, W., Zabel, M.M., Wild, M.A., Hoover, E.A. and Mathiason, C.K. 2015. In utero transmission and tissue distribution of chronic wasting disease-associated prions in free-ranging Rocky Mountain elk. J. Gen. Virol. 96(11), 3444–3455. Sigurdson, C.J., Barillas-Mury, C., Miller, M.W., Oesch, B., van Keulen, L.J.M., Langeveld, J.P.M. and Hoover, E.A. 2002. PrPCWD lymphoid cell targets in early and advanced chronic wasting disease of mule deer. J. Gen. Virol. 83(10), 2617–2628. Sigurdson, C.J., Spraker, T.R., Miller, M.W., Oesch, B. and Hoover, E.A. 2001. PrPCWD in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J. Gen. Virol. 82(10), 2327–2334. Sigurdson, C.J., Williams, E.S., Miller, M.W., Spraker, T.R., O’Rourke, K.I. and Hoover, E.A. 1999. Oral transmission and early lymphoid tropism of chronic wasting disease PrP(res) in mule deer fawns (Odocoileus hemionus). J. Gen. Virol. 80(10), 2757–2764. Simmons, M.M., Jo Moore, S., Konold, T., Thurston, L., Terry, L.A., Thorne, L., Lockey, R., Vickery, C., Hawkins, S.A.C., Chaplin, M.J. and Spiropoulos, J. 2011. Experimental oral transmission of atypical scrapie to sheep. Emerg. Infect. Dis. 17(5), 848–854. Simmons, M.M., Konold, T., Simmons, H.A., Spencer, Y.I., Lockey, R., Spiropoulos, J., Everitt, S. and Clifford, D. 2007. Experimental transmission of atypical scrapie to sheep. BMC Vet. Res. 3, 1–7. Spiropoulos, J., Hawkins, S.A.C., Simmons, M.M. and Bellworthy, S.J. 2014. Evidence of in utero transmission of classical scrapie in sheep. J. Virol. 88(8), 4591–4594. Stevenson, M.A., Morris, R.S., Lawson, A.B., Wilesmith, J.W., Ryan, J.B.M. and Jackson, R. 2005. Area-level risks for BSE in British cattle before and after the July 1988 meat and bone meal feed ban. Prev. Vet. Med. 69(1–2), 129–144. Tamgüney, G., Miller, M.W., Wolfe, L.L., Sirochman, T.M., Glidden, D.V., Palmer, C., Lemus, A., DeArmond, S.J. and Prusiner, S.B. 2009. Asymptomatic deer excrete infectious prions in faeces. Nature 461(7263), 529–532. Tamgüney, G., Richt, J.A., Hamir, A.N., Greenlee, J.J., Miller, M.W., Wolfe, L.L., Sirochman, T.M., Young, A.J., Glidden, D.V, Johnson, N.L., Giles, K., DeArmond, S.J. and Prusiner, S.B. 2012. Salivary prions in sheep and deer. Prion 6(1), 52–61. Taylor, D.M., McConnell, I. and Fraser, H. 1996. Scrapie infection can be established readily through skin scarification in immunocompetent but not immunodeficient mice. J. Gen. Virol. 77(7), 1595–1599. Terry, L.A., Howells, L., Bishop, K., Baker, C.A., Everest, S., Thorne, L., Maddison, B.C. and Gough, K.C. 2011. Detection of prions in the faeces of sheep naturally infected with classical scrapie. Vet. Res. 42(1), 65; doi:10.1186/1297-9716-42-65 Terry, L.A., Marsh, S., Ryder, S.J., Hawkins, S.A.C., Wells, G.A.H. and Spencer, Y.I. 2003. Detection of disease-specific PrP in the distal ileum of cattle exposed orally to the agent of bovine spongiform encephalopathy. Vet. Rec. 152(13), 387–392. Thorne, L. and Terry, L.A. 2008. In vitro amplification of PrPSc derived from the brain and blood of sheep infected with scrapie. J. Gen. Virol. 89(12), 3177–3184. Tuo, W., O’Rourke, K.I., Zhuang, D., Cheevers, W.P., Spraker, T.R. and Knowles, D.P. 2002. Pregnancy status and fetal prion genetics determine PrPSc in placentomes of scrapie-infected sheep. Proc. Natl. Acad. Sci. U. S. A. 99(9), 6310–6315. Van Keulen, L.J.M., Vromans, M.E.W., Dolstra, C.H., Bossers, A. and Van Zijderveld, F.G. 2008. Pathogenesis of bovine spongiform encephalopathy in sheep. Arch. Virol. 153(3), 445–453. Vernerova, K., Tothova, L., Mikova, A., Vodrazka, P., Simek, B., Hanusova, L. and Citek, J. 2014. BSE-associated polymorphisms in the prion protein gene: an investigation. J. Anim. Breed. Genet. 131(5), 403–408. Vetrugno, V. 2004. Safety of milk and milk derivatives in relation to BSE: the lactoferrin example. BioMetals 17(3), 353–356. Wang, S., Foote, W.C., Sutton, D.L., Maciulis, A., Miller, J.M., Evans, R.C., Holyoak, G.R., Call, J.W., Bunch, T.D., Taylor, W.D. and Marshall, M.R. 2001. Preventing experimental vertical transmission of scrapie by embryo transfer. Theriogenology 56(2), 315–327. Watts, J.C., Bourkas, M.E.C. and Arshad, H. 2018. The function of the cellular prion protein in health and disease. Acta Neuropathol. 135(2), 159–178. Webb, P.R., Powell, L., Denyer, M., Marsh, S., Weaver, C., Simmons, M.M., Johns, E., Sheehan, J., Horsfield, P., Lyth, C., Wilson, C., Long, A., Cawthraw, S., Saunders, G.C. and Spencer, Y.I. 2009. A retrospective immunohistochemical study reveals atypical scrapie has existed in the United Kingdom since at least 1987. J. Vet. Diagn. Investig. 21(6), 826–829. Weissmann, C. and Flechsig, E. 2003. PrP knock-out and PrP transgenic mice in prion research. Br. Med. Bul. 2003, 1–18; doi:10.1093/bmb/dg66.043 Wells, G.A.H., Hawkins, S.A.C., Green, R.B., Austin, A.R., Dexter, I., Spencer, Y.I., Chaplin, M.J., Stack, M.J. and Dawson, M. 1998. Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): an update. Vet. Rec. 142(5), 103–106. Wells, G.A.H., Scott, A., Johnson, C., Gunning, R., Hancock, R., Jeffrey, M., Dawson, M. and Bradley, R. 1987. A novel progressive spongiform encephalopathy in cattle. Vet. Rec. 121(18), 419–420. Wells, G.A.H., Spiropoulos, J., Hawkins, S.A.C. and Ryder, S.J. 2005. Pathogenesis of experimental bovine spongiform encephalopathy: preclinical infectivity in tonsil and observations on the distribution of lingual tonsil in slaughtered cattle. Vet. Rec. 156(13), 401–407. Wilesmith, J.W., Ryan, J., Hueston, W. and Hoinville, L.J. 1992. Bovine spongiform encephalopathy: epidemiological features 1985 to 1990. Vet. Rec. 130(5), 90–94. Wilesmith, J.W., Wells, G.A.H., Ryan, J.B.M., Gavier-Widén, D. and Simmons, M.M. 1997. A cohort study to examine maternally-associated risk factors for bovine spongiform encephalopathy. Vet. Rec. 141(10), 239–243. Will, R.G., Ironside, J.W., Zeidler, M., Cousens, S.N., Estibeiro, K., Alperovitch, A., Poser, S., Pocchiari, M., Hofmar, A. and Smith, P.G. 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347(9006), 921–925. Williams, E.S. and Young, S. 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J. Wildl. Dis. 16(1), 89–98. Wrathall, A.E., Brown, K.F.D., Sayers, A.R., Wells, G.A.H., Simmons, M.M., Farrelly, S.S.J., Bellerby, P., Squirrell, J., Spencer, Y.I., Wells, M., Stack, M.J., Bastiman, B., Pullar, D., Scatcherd, J., Heasman, L., Parker, J., Hannam, D.A.R., Helliwell, D.W., Chree, A. and Fraser, H. 2002. Studies of embryo transfer from cattle clinically affected by bovine spongiform encephalopathy (BSE). Vet. Rec. 150(12), 365–378. Zhuang, D., Schneider, D.A., Madsen-Bouterse, S.A., Highland, M.A. and Dassanayake, R.P. 2018. Low-volume goat milk transmission of classical scrapie to lambs and goat kids. PLoS One 13(/9), 1–16; doi:10.1371/journal.pone.0204281 Zlotnik, I. and Rennie, J.C. 1965. Experimental transmission of mouse passaged scrapie to goats, sheep, rats and hamsters. J. Comp. Pathol. 75(2); doi:10.1016/0021-9975(65)90005-8 | ||
| How to Cite this Article |
| Pubmed Style Gallardo MJ, Delgado FO. Animal prion diseases: a review of intraspecies transmission. Open Vet J. 2021; 11(4): 707-723. doi:10.5455/OVJ.2021.v11.i4.23 Web Style Gallardo MJ, Delgado FO. Animal prion diseases: a review of intraspecies transmission. https://www.openveterinaryjournal.com/?mno=78626 [Access: July 01, 2025]. doi:10.5455/OVJ.2021.v11.i4.23 AMA (American Medical Association) Style Gallardo MJ, Delgado FO. Animal prion diseases: a review of intraspecies transmission. Open Vet J. 2021; 11(4): 707-723. doi:10.5455/OVJ.2021.v11.i4.23 Vancouver/ICMJE Style Gallardo MJ, Delgado FO. Animal prion diseases: a review of intraspecies transmission. Open Vet J. (2021), [cited July 01, 2025]; 11(4): 707-723. doi:10.5455/OVJ.2021.v11.i4.23 Harvard Style Gallardo, M. J. & Delgado, . F. O. (2021) Animal prion diseases: a review of intraspecies transmission. Open Vet J, 11 (4), 707-723. doi:10.5455/OVJ.2021.v11.i4.23 Turabian Style Gallardo, Mauro Julian, and Fernando Oscar Delgado. 2021. Animal prion diseases: a review of intraspecies transmission. Open Veterinary Journal, 11 (4), 707-723. doi:10.5455/OVJ.2021.v11.i4.23 Chicago Style Gallardo, Mauro Julian, and Fernando Oscar Delgado. "Animal prion diseases: a review of intraspecies transmission." Open Veterinary Journal 11 (2021), 707-723. doi:10.5455/OVJ.2021.v11.i4.23 MLA (The Modern Language Association) Style Gallardo, Mauro Julian, and Fernando Oscar Delgado. "Animal prion diseases: a review of intraspecies transmission." Open Veterinary Journal 11.4 (2021), 707-723. Print. doi:10.5455/OVJ.2021.v11.i4.23 APA (American Psychological Association) Style Gallardo, M. J. & Delgado, . F. O. (2021) Animal prion diseases: a review of intraspecies transmission. Open Veterinary Journal, 11 (4), 707-723. doi:10.5455/OVJ.2021.v11.i4.23 |