Open Veterinary Journal, (2021), Vol. 11(3): 508–516
Original Article
10.5455/OVJ.2021.v11.i3.24
Is proteinuria a rare condition in apparently healthy and sick cats? A feline practice experience (2007–2018)
Maria Cristina López1* , Valentina Aybar2, Andrea Zatelli3, Anna Vila4, Juan Jose Vega2, Eduard Hernando4, Alejandro Jiménez5 and Xavier Roura1
, Valentina Aybar2, Andrea Zatelli3, Anna Vila4, Juan Jose Vega2, Eduard Hernando4, Alejandro Jiménez5 and Xavier Roura1
1Hospital Clínic Veterinari, Universitat Autònoma de Barcelona, Bellaterra, Spain
2Hospital Felino Madrid, Madrid, Spain
3Department of Veterinary Medicine, University of Bari, Valenzano, Italy
4Hospital Veterinario San Vicente Mártir, Universidad Católica de Valencia, Valencia, Spain
5Facultat de Matemàtiques, Universitat Autònoma de Barcelona, Bellaterra, Spain
*Corresponding Author: Maria Cristina López. Hospital Clínic Veterinari, Universitat Autònoma de Barcelona, Bellaterra, Spain. Email: mariavirgolopez [at] hotmail.com
Submitted: 14/07/2021 Accepted: 01/09/2021 Published: 20/09/2021
© 2021 Open Veterinary Journal
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited, and is not altered, transformed, or built upon in any way.
Abstract
Background: Proteinuria is assumed to be less frequent in cats than in dogs and is mainly associated with chronic kidney disease (CKD).
Aim: The current study aimed to evaluate and compare urine protein-to-creatinine (UPC) values retrospectively in cats visited for comprehensive annual health check or for presenting systemic clinical signs related to CKD.
Methods: UPC ratio was retrospectively evaluated in 112 owned cats, out of which 51 (45.5%) were apparently healthy cats according to their owners who visited for comprehensive annual health checks and 61 (54.5%) sick cats, presenting systemic clinical signs suggesting CKD, such as weight loss or polyuria/polydipsia, among others.
Results: Based on UPC, the present study found that 54.5% of all cats included were borderline proteinuric or proteinuric, having increased UPC (UPC ≥ 0.2), with 35.7% included in the sick group and 18.7% in the health-check group. Increased UPC was also statistically associated with azotemia and isosthenuria (urinary-specific gravity between 1,008 and 1,035) in both sick and health-check groups of cats.
Conclusion: Independent of the reason for their medical visit, it could be concluded that borderline proteinuria and proteinuria were statistically mainly related to CKD in cats. Furthermore, the measurement of UPC could be very useful in the detection and management of CKD in apparently healthy cats during a medical visit for annual health check irrespective of the age.
Keywords: Annual health check, Kidney, Urine protein-to-creatinine ratio, UPC, Urinalysis.
Introduction
Proteinuria is defined as the presence of an excessive amount of proteins or abnormal proteins in the urine (Lees et al., 2005; Harley and Langston, 2012). Studies conducted in the past decade have shown that proteinuria is highly related to reduced survival in both azotemic and non-azotemic cats and dogs (Lees et al., 2005; Syme et al., 2006; King et al., 2007; Wehner et al., 2008; Jepson et al., 2009; Littman, 2011; Bartges, 2012).
Proteinuria in cats is less frequent than in dogs due to the different pathophysiology and predisposing causes of kidney disease (Harley and Langston, 2012; Roura et al., 2017; Rayhel et al., 2020), and in the absence of active urinary sediment, it is considered a marker of renal damage or dysfunction (Lees et al., 2005; Harley and Langston, 2012). Furthermore, unlike what happens to cats, many other potential causes of proteinuria have been described in dogs, such as infections, neoplasms, endocrinopathies, familial or aging (Herring et al., 2014; Lavoué et al., 2015; Marynissen et al., 2017; Smith et al., 2017; Prudic et al., 2018; Purswell et al., 2020).
Chronic kidney disease (CKD) is one of the most common diseases in adult domestic cats (Marino et al., 2014; Brown et al., 2016; KuKanich et al., 2021), and the main pathological feature of feline CKD consists of chronic tubular interstitial nephritis (Lawson et al., 2015; McLeland et al., 2015; Rayhel et al., 2020). In this setting, proteinuria is mainly secondary to tubular damage, and its increase seems associated with CKD progression and usually correlates with interstitial fibrosis, although the exact mechanisms of injury are not clearly established (Syme et al., 2006; Chakrabarti et al., 2012; Brown et al., 2016; Lawson et al., 2018). Furthermore, it seems that there is a strong association between systolic hypertension and the magnitude of proteinuria in cats with CKD (Syme et al., 2006). This link could be very important because proteinuria is strongly associated with survival in cats with CKD or hypertension, and managing systolic hypertension could reduce proteinuria (Syme et al., 2006; Jepson et al., 2007; King et al., 2007).
Therefore, renal proteinuria is diagnostic and, especially, a prognostic factor of CKD in cats proportionally related to the risk of renal disease progression and mortality (Lees et al., 2005; Syme et al., 2006; Chakrabarti et al., 2012; IRIS, 2019; Giraldi et al., 2020). These findings have led the International Renal Interest Society (IRIS) to propose proteinuria as relevant in staging cats with CKD to properly manage the progression of this disease (IRIS, 2019). Cats with urine protein-to-creatinine ratio (UPC) < 0.2 are classified as non-proteinuric, with UPC > 0.4 as proteinuric, and with UPC between 0.2 and 0.4 to be borderline proteinuric (Lees et al., 2005; IRIS, 2019). The current IRIS recommendation is the treatment of either borderline and proteinuric cats to reduce the progression of kidney disease (IRIS, 2019).
As descendants of solitary animals, where hiding illness is important for survival, our domestic cats display minimal behavioral signs of disease (Horwitz and Rodan, 2018). Furthermore, owners are reticent to bring cats to the veterinarian to avoid them getting stressed, or relate that it is unnecessary given the lack of evident clinical signs (Diez et al., 2015; Gates et al., 2019). Based on the above, cats should benefit from annual health checks for early detection of diseases, such as kidney disease, especially when they are getting older and urine evaluation could be a useful test to accomplish this objective (Chakrabarti et al., 2012; Piech and Wycislo, 2019; Mösch et al., 2020; Pérez-Accino et al., 2020).
Although some original published studies have evaluated the presence of proteinuria in cats (Syme et al., 2006; King et al., 2007; Jepson et al., 2009; Paepe et al., 2013; Rayhel et al., 2020), information about proteinuria in cats with and without CKD is mostly based on reviews and expert opinions (Lees et al., 2005; Littman, 2011; Bartges, 2012; Harley and Langston, 2012; Sparkes et al., 2016; Roura et al., 2017; IRIS, 2019; Piech and Wycislo, 2019) in contrast with the information available in dogs (Jacob et al., 2005; Herring et al., 2014; Marynissen et al., 2017; Willems et al., 2017; Prudic et al., 2018; Gizzarelli et al., 2019; Purswell et al., 2020).
We hypothesized that the prevalence of proteinuria in cats considered healthy, based on owners’ criteria at home evaluation, was higher than in previous studies and mainly associated with CKD. So, the present study aimed to evaluate and compare UPC values retrospectively in cats visiting for comprehensive annual health checks or for presenting systemic clinical signs related to CKD.
Materials and Methods
Study design
This was a retrospective, observational, cross-sectional study.
Case selection
Clinical records, where urinalysis and UPC measured by IDEXX Laboratories should be available, of owned cats visiting a feline practice between April 2007 and December 2018 for comprehensive annual health check or for presenting systemic clinical signs suggesting CKD were selected. After this first selection, for each case to be eligible for final inclusion in the study, a complete medical record including clinical history, signalment (age, sex, neuter status, body weight, and breed), data of physical examination, total blood count, serum biochemistry, complete urinalysis with inactive sediment, UPC and systolic blood pressure (SBP), and final diagnosis, should be available for review. Moreover, if available, other additional diagnostic investigations such as feline immunodeficiency virus (FIV) and feline leukemia virus tests, serum thyroid levels, or abdomen ultrasound were also evaluated.
Data handling and statistical analysis
Cats were subdivided into two groups for statistical analysis to evaluate UPC: health-check group, which included healthy cats according to their owners visiting for comprehensive annual health check; and sick group, which included cats visiting for presenting one or more of these systemic clinical signs suggesting underlying CKD, such as weight loss, anorexia, polyuria/polydipsia (PU/PD), depression, lethargy, vomiting, diarrhea, or blindness, among others.
After carefully evaluating previous comprehensive medical information, all cats were also divided into two additional groups, CKD group and non-CKD group, depending on the final diagnosis. IRIS CKD staging guidelines (IRIS, 2019) were followed in the CKD group for statistical analyses. Finally, to evaluate an age effect, all cats were also assigned to one of these groups: 0–2 years (junior), >2–10 years (mature), and >10 years (geriatric) (Vogt et al., 2010).
All cats were also classified as non-proteinuric (UPC < 0.2), borderline proteinuric (UPC 0.2–0.4) or proteinuric (UPC > 0.4); increased UPC was considered when cats had values of UPC ≥ 0.2. Finally, systemic hypertension was considered when SBP measurement was 160 mmHg or higher.
Statistical analysis was conducted in R (R Core Team, 2014), and figures were generated using the package ggplot2 (Wickham, 2009). The level of significance was set at p < 0.05. If not normally distributed data were expressed as median (range). The relationship between continuous variables has been studied by the non-parametric approach of calculating the Spearman’s rank correlation coefficient. Pearson’s chi-squared test was used to examine the relationship between the presence of clinical signs and proteinuria classification. Wilcoxon signed-rank test was also used to test associations between the presence of clinical signs and UPC.
Results
During the study period, a total of 2,391 cats visited the feline practice. Of those, 403 had a complete urinalysis and UPC identified through the clinical record database search, and 112 cats finally met the eligibility criteria. Reasons for exclusion were incomplete clinical records and active urinary sediment in 239 and 52 cats, respectively (Fig. 1).
51 out of 112 (45.5%) cats were included in the health-check group and 61/112 (54.5%) in the sick group. Furthermore, 82/112 (73.2%) cats had CKD as a final diagnosis following the IRIS guidelines, 37/51 (72.5%) in the health-check group, and 45/61 (73.7%) in the sick group. Inside the health-check group, 2/37 (5.4%) were classified as IRIS stage 1, 24/37 (64.9%) as IRIS stage 2, 11/37 (29.7%) as IRIS stage 3, and none as IRIS stage 4. Instead, within the sick group, 3/45 (6.7%) were classified as IRIS stage 1, 15/45 (33.3%) as IRIS stage 2, 12/45 (26.7%) as IRIS stage 3, and 15/45 (33.3%) as IRIS stage 4 (Fig. 1).
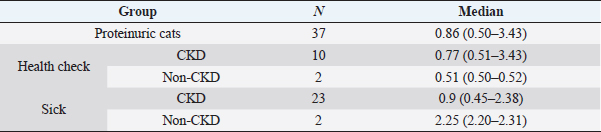
Based on UPC values of all cats (Fig. 2), 51/112 (45.5%) were non-proteinuric, 24/112 (21.4%) were borderline proteinuric, and 37/112 (33.1%) were proteinuric. A UPC ≥ 0.2, defined previously as increased UPC, was statistically associated with cats belonging to the sick (p =0.026) and CKD (p =0.014) groups. Among the 61/112 (54.5%) cats with increased UPC, 48/61 (78.6%) were finally diagnosed with CKD. Furthermore, all cats diagnosed with CKD had an increased UPC (p =0.014). However, four cats had proteinuria without increased creatinine, although the final diagnosis was hyperthyroidism in two cats of the health-check group and FIV in the other two of the sick group. UPC values of all cats with proteinuria are described in Table 1.
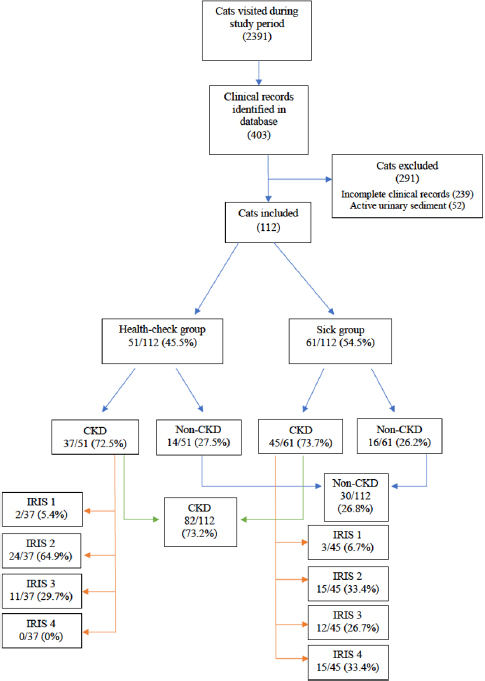
Fig. 1. Flow chart of all cats included in this study.
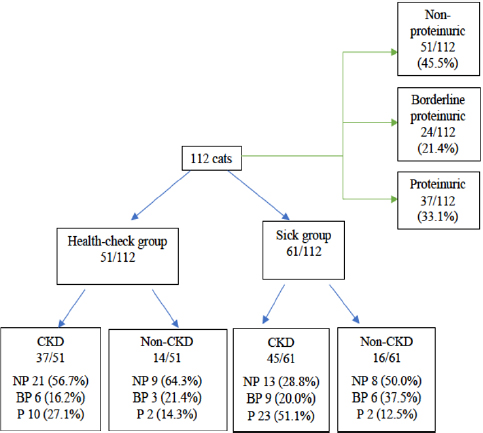
Fig. 2. Flow chart of UPC values of all cats included in this study. NP: non-proteinuric; BP: borderline proteinuric; P: proteinuric.
Table 1. UPC results in proteinuric cats included in this study.
The signalment of the enrolled cats is summarized in Table 2. In the health-check group, neither body weight (p =0.081), age (p =0.385) and sex (p =0.580) were statistically associated with proteinuria. In the sick group, lower body weight (p =0.049) was associated with proteinuria. No other statistical associations were found with age (p =0.176) or sex (p =0.321). Breed data were insufficient to draw conclusions.
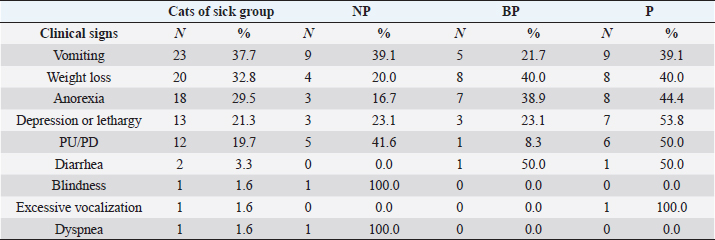
Systemic clinical signs suggestive of CKD described in the sick group were vomiting (23, 37.7%), weight loss (20, 32.8%), anorexia (18, 29.5%), depression or lethargy (13, 21.3%), PU/PD (12, 19.7%), diarrhea (2, 3.3%), excessive vocalization (1, 1.6%), blindness (1, 1.6%), and dyspnea (1, 1.6%). Proteinuric cats from the sick group presented the highest frequencies of all clinical signs: vomiting, lethargy, and PU/PD compared to the NP and BP cats of the sick group (Table 3). However, no statistical association was found between any specific systemic clinical sign and grade of proteinuria.
When clinicopathological alterations were evaluated in the health-check group, increased UPC was associated with higher values of creatinine (p =0.037) and urea (p =0.009) and lower values of urinary-specific gravity (USG) (p =0.018) but did not show any statistical association with packed cell volume (PCV), potassium, phosphorous, albumin, or total protein (p > 0.05). Whereas in the sick group, increased UPC was associated with higher values of creatinine (p =0.046), urea (p =0.009), and phosphorous (p =0.049), and with lower values of potassium (p =0.008) and USG (p =0.018), but did not show any statistical association with PCV, albumin, or total protein (p > 0.05).
Table 2. Signalment of the 112 cats included in this study.
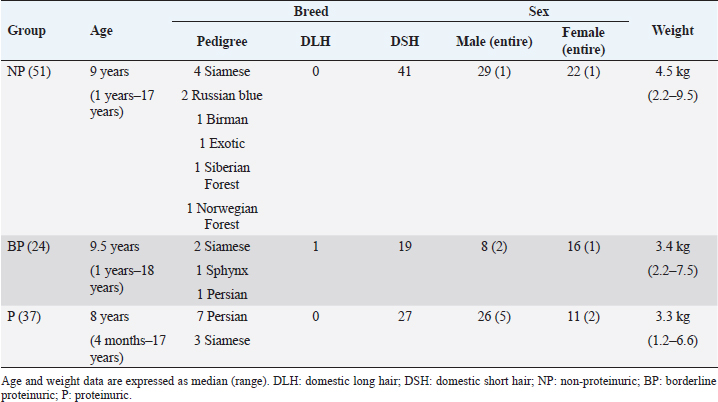
Table 3. Clinical signs described in cats of sick group.
15 out of 112 (13.4%) cats had hypertension (SBP ≥ 160 mmHg). Cats with hypertension had increased UPC compared with those without hypertension, and this difference was statistically significant (p =0.029). 13 out of these 15 (86.6%) cats were finally diagnosed with CKD, and the other 2 cats were diagnosed with hyperthyroidism.
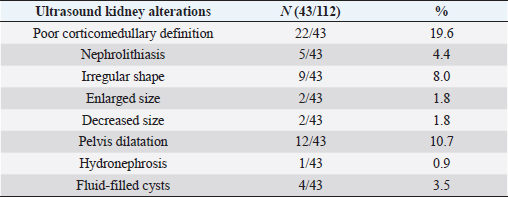
To finalize the results, ultrasound alterations were found in 43 out of all cats (38.4%), but there was no statistical association between the presence of abnormalities and proteinuria (p =0.63) (Table 4).
Discussion
The present study showed a high prevalence of proteinuria in apparently healthy cats according to their owners visited for a comprehensive annual health check. CKD was highly correlated with borderline proteinuria and proteinuria in cats.
This increased UPC (UPC ≥ 0.2) was associated with CKD in 78.6% of cats included, similar to the results recently published (Fidalgo et al., 2020). Moreover, 43.3% of the cats visited during the study period as part of annual health check were diagnosed with CKD and increased UPC. Finally, the majority of cats (86.7%) included in the present study had proteinuria with UPC < 1.0, which is compatible with previously published data, suggesting that the main disease process occurring in the majority of these cats is chronic tubulointerstitial fibrosis rather than a primary glomerular disease (Lees et al., 2005; Syme et al., 2006). All this together points out the utility of UPC measurements in routine health check to evaluate the presence of CKD in cats without evident clinical signs according to their owners.
Table 4. Kidney alterations revealed by abdominal ultrasound.
Furthermore, the present study found that 54.5% of cats included had an increased UPC, with 35.7% included in the sick group and 18.7% in the health-check group. These interesting results were similar to a previous feline report where 34% of sick cats and 18% of healthy cats were proteinuric (Al-Ghazlat et al., 2011). However, a more recent study reported that 72.7% of healthy cats analyzed were non-proteinuric, higher than the 45.5% found in the present study (Paepe et al., 2013). This fact could be explained because 27.5% of cats included in the healthy group, according to their owners, visited for comprehensive annual health check were finally diagnosed with CKD. The results presented here point out the importance of the urinalysis with UPC measurement in annual health check for the evaluation of chronic diseases, such as kidney disease (Chakrabarti et al., 2012; Piech and Wycislo, 2019). This could be important in cats due to the limitation of owners to detect subtle or even evident signs of illness in cats (Diez et al., 2015; Horwitz and Rodan, 2018; Gates et al., 2019). Furthermore, this could be especially determinant due to mild clinical signs displayed by cats with early stages of CKD such as IRIS stages 1, 2, and early 3 that were the majority in the health-check group (IRIS, 2019).
Proteinuria in cats has been associated with aging, suggesting its monitoring in geriatric cats (Paepe et al., 2013). However, the present study did not show this association in accordance with other previous studies (Syme et al., 2006). Moreover, lower weight was associated with increased UPC in the sick group, but this was not found in the health-check group. Thus, weight loss could probably be associated with the illness found in these cats, the majority with CKD, instead of the increased UPC. Accordingly, lower body weight has been previously associated with azotemic cats (Lamb et al., 2017).
Increased UPC was also associated with higher creatinine values in both sick and health-check groups. This association has been previously reported in cats and can be explained by the hemodynamic consequences of declining renal function, with afferent arteriolar vasodilation, resulting in increased glomerular capillary pressure (Lees et al., 2005; Syme et al., 2006; King et al., 2007; Jepson et al., 2009; Littman, 2011). As renal function declines, the decreased number of functioning tubules is less able to reabsorb and degrade filtered protein, resulting in more intact protein molecules present in urine (Lees et al., 2005; Syme et al., 2006; King et al., 2007; Jepson et al., 2009; Littman, 2011). Moreover, in the present study, higher urea and lower USG values were associated with increased UPC in both groups, which supports the relationship between the reduction in renal function with an increased UPC. In the sick group, increased UPC was also associated with higher phosphorous and lower potassium values that could also be related to more advanced CKD IRIS stages.
The present study found that hypertension was also associated with increased UPC, similarly to previously published data (Syme et al., 2006; Chakrabarti et al., 2013). However, this study just found that 15.8% of cats with CKD had hypertension, although several studies previously reported a very variable but higher prevalence of hypertension in cats with CKD, ranging from 19% to 61% (Kobayashi et al., 1990; Syme et al., 2002; Bijsmans et al., 2015). The lack of uniform measurement techniques, variable inclusion criteria, and inconsistent thresholds for establishing a diagnosis of hypertension in veterinary medicine could have made prevalence data difficult to interpret. However, in the present study, most of the cats were CKD IRIS stages 1 and 2, so it could explain this low prevalence of hypertension. It was previously described that the frequency of hypertension increased with an increase in the CKD IRIS stage (Hori et al., 2018).
Kidney ultrasound alterations such as perinephric fluid, small kidneys, hyperechoic renal cortex, loss of corticomedullary differentiation, renal calculi, and dilated renal pelvis have been statistically associated with azotemia in cats similar to previously published study (Lamb et al., 2017). Furthermore, loss of corticomedullary differentiation (19.6%) and dilated renal pelvis (10.7%) were the most common ultrasound findings in the present study. However, no significant association was found between increased UPC and ultrasound alterations. A possible explanation could be that ultrasonography was recommended for the investigation of individual cats with CKD but was not recommended as a screening test for proteinuria or azotemia, probably because renal ultrasound alterations have low sensitivity and specificity for an early diagnosis of kidney disease (Sparkes et al., 2016; Lamb et al., 2017).
This study has several limitations that could produce information and selection biases. First, data were collected retrospectively, as a consequence, the authors were reliant upon the history taken at the time of presentation. Second, lack of homogeneous population and missing data for treatment applied or cause of death was also present. Finally, serum symmetric dimethylarginine was not carried out in all the cats included in this study, so it is possible that some cats had already CKD even without other markers of CKD such as an increased creatinine or lower USG.
Conclusion
The present study pointed out that proteinuria was a frequent condition in cats considered healthy and without overt clinical signs suggestive of CKD. Furthermore, UPC ≥ 0.2 was mainly only related to CKD in cats. Nevertheless, the results of this study were suggestive that urinalysis and UPC measurement could be warranted in the early detection and management of CKD in apparently healthy cats, irrespective of their age, during the medical visits for annual health check.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
All authors contributed to making the completion of this manuscript possible. Valentina Aybar and Juanjo Vega obtained all data and review inclusion criteria for analyses and review the paper; Alejandro Jiménez analyzed the data; Eduard Hernando and Anna Vila analyzed the data and review the paper; María C. López wrote the paper and analyzed the data; Xavier Roura and Andrea Zatelli designed the study, supervised the data, and reviewed the paper.
References
Al-Ghazlat, S.A., Langston, C.E., Greco, D.S., Reine, N.J., May, S.N. and Shofer, F.S. 2011. The prevalence of microalbuminuria and proteinuria in cats with diabetes mellitus. Top. Companion Anim. Med. 26, 154–157.
Bartges, J.W. 2012. Chronic kidney disease in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 42, 669–692.
Bijsmans, E.S., Jepson, R.E., Chang, Y.M., Syme, H.M. and Elliott, J. 2015. Changes in systolic blood pressure over time in healthy cats and cats with chronic kidney disease. J. Vet. Intern. Med. 29, 855–861.
Brown, C.A., Elliott, J., Schmiedt, C.W. and Brown, S.A. 2016. Chronic kidney disease in aged cats: clinical features, morphology, and proposed pathogeneses. Vet. Pathol. 53, 309–326.
Chakrabarti, S., Syme, H.M., Brown, C.A. and Elliott, J. 2013. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet. Pathol. 50, 147–155.
Chakrabarti, S., Syme, H.M. and Elliott, J. 2012. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J. Vet. Intern. Med. 26, 275–281.
Diez, M., Picavet, P., Ricci, R., Dequenne, M., Renard, M., Bongartz, A. and Farnir, F. 2015. Health screening to identify opportunities to improve preventive medicine in cats and dogs. J. Small Anim. Pract. 56, 463–469.
Fidalgo, M.A., Leal, R.O. and Correia, J.H.D. 2020. Significant feline proteinuria: a retrospective study of its aetiology in 61 cats from Lisbon research. In Communications of the 29th ECVIM-CA Congress. J. Vet. Intern. Med. 34, 339–445.
Gates, M.C., Walker, J., Zito, S. and Dale, A. 2019. Cross-sectional survey of pet ownership, veterinary service utilisation, and pet-related expenditures in New Zealand. N. Z. Vet. J. 67, 306–314.
Giraldi, M., Paltrinieri, S. and Scarpa, P. 2020. Electrophoretic patterns of proteinuria in feline spontaneous chronic kidney disease. J. Feline Med. Surg. 22, 114–121.
Gizzarelli, M., Roura, X., Scarpa, P., D’Ippolito, P., Foglia Manzillo, V., Oliva, G., Tarducci, A., Borrelli, A., Melis, G., Quintavalla, F., Uva, A., Guarraci, A. and Zatelli, A. 2019. Prevalence of proteinuria in owned dogs from Italy: a multicentric study. Vet. Med. Int. 2019, 5.
Harley, L. and Langston, C. 2012. Proteinuria in dogs and cats. Can. Vet. J. 53, 631–638.
Herring, I.P., Panciera, D.L. and Werre, S.R. 2014. Longitudinal prevalence of hypertension, proteinuria, and retinopathy in dogs with spontaneous diabetes mellitus. J. Vet. Intern. Med. 28, 488–495.
Hori, Y., Heishima, Y., Yamashita, Y., Isayama, N., Kanno, N., Nakamura, K., Iguchi, M., Ibaragi, T., Onodera, H., Aramaki, Y., Hirakawa, A., Yamano, S., Katagi, M., Kitade, A. and Sawada, T. 2018. Relationship between indirect blood pressure and various stages of chronic kidney disease in cats. J. Vet. Med. Sci. 80, 447–452.
Horwitz, D.F. and Rodan, I. 2018. Behavioral awareness in the feline consultation: understanding physical and emotional health. J. Feline Med. Surg. 20, 423–436.
IRIS. 2019. IRIS (International Renal Interest Society). Available via http://www.iris-kidney.com (Accessed 15 June 2020).
Jacob, F., Polzin, D.J., Osborne, C.A., Neaton, J.D., Kirk, C.A., Allen, T.A. and Swanson, L.L. 2005. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J. Am. Vet. Med. Assoc. 226, 393–400.
Jepson, R.E., Brodbelt, D., Vallance, C., Syme, H.M. and Elliott, J. 2009. Evaluation of predictors of the development of azotemia in cats. J. Vet. Intern. Med. 23, 806–813.
Jepson, R.E., Elliott, J., Brodbelt, D. and Syme, H.M. 2007. Effect of control of systolic blood pressure on survival in cats with systemic hypertension. J. Vet. Intern. Med. 21, 402–409.
King, J.N., Tasker, S., Gunn-Moore, D.A., Strehlau, G., Gunn-Moore, A., Strehlau, G. and BENRIC Study Group. 2007. Prognostic factors in cats with chronic kidney disease. J. Vet. Intern. Med. 21, 906–916.
Kobayashi, D.L., Peterson, M.E., Graves, T.K., Nichols, C.E. and Lesser, M. 1990. Hypertension in cats with chronic renal failure or hyperthyroidism. J. Vet. Intern. Med. 4, 58–62.
KuKanich, K., George, C., Roush, J.K., Sharp, S., Farace, G., Yerramilli, M., Peterson, S. and Grauer, G.F. 2021. Effects of low-dose meloxicam in cats with chronic kidney disease. J. Feline Med. Surg. 23, 138–148.
Lamb, C.R., Dirrig, H. and Cortellini, S. 2017. Comparison of ultrasonographic findings in cats with and without azotaemia. J. Feline Med. Surg. 20, 948–954.
Lavoué, R., Trumel, C., Smets, P.M.Y., Braun, J.P., Aresu, L., Daminet, S., Concordet, D., Palanché, F. and Peeters, D. 2015. Characterization of proteinuria in Dogue de Bordeaux dogs, a breed predisposed to a familial glomerulonephropathy: a retrospective study. PLoS One 10, e0133311.
Lawson, J., Elliott, J., Wheeler-Jones, C., Syme, H. and Jepson, R. 2015. Renal fibrosis in feline chronic kidney disease: known mediators and mechanisms of injury. Vet. J. 203, 18–26.
Lawson, J.S., Liu, H.H., Syme, H.M., Purcell, R., Wheeler-Jones, C.P.D. and Elliott, J. 2018. The cat as a naturally occurring model of renal interstitial fibrosis: characterisation of primary feline proximal tubular epithelial cells and comparative pro-fibrotic effects of TGF-β1. PLoS One 13, e0202577.
Lees, G.E., Brown, S.A., Elliott, J., Grauer, G.F. and Vaden, S.L. 2005. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement (small animal). J. Vet. Intern. Med. 19, 377–385.
Littman, M.P. 2011. Protein-losing nephropathy in small animals. Vet. Clin. North Am. Small Anim. Pract. 41, 31–62.
Marino, C.L., Lascelles, B.D.X., Vaden, S.L., Gruen, M.E. and Marks, S.L. 2014. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J. Feline Med. Surg. 16, 465–472.
Marynissen, S.J.J., Willems, A.L., Paepe, D., Smets, P.M.Y., Picavet, P., Duchateau, L. and Daminet, S. 2017. Proteinuria in apparently healthy elderly dogs: persistency and comparison between free catch and cystocentesis urine. J. Vet. Intern. Med. 31, 93–101.
McLeland, S.M., Cianciolo, R.E., Duncan, C.G. and Quimby, J.M. 2015. A comparison of biochemical and histopathologic staging in cats with chronic kidney disease. Vet. Pathol. 52, 524–534.
Mösch, M., Reese, S., Hartmann, K. and Dorsch, R. 2020. Influence of preanalytic and analytic variables in canine and feline urine specific gravity measurement by refractometer. J. Vet. Diagn. Investig. 32, 36–43.
Paepe, D., Verjans, G., Duchateau, L., Piron, K., Ghys, L. and Daminet, S. 2013. Routine health screening: findings in apparently healthy middle-aged and old cats. J. Feline Med. Surg. 15, 8–19.
Pérez-Accino, J., Feo Bernabe, L., Manzanilla, E.G. and Puig, J. 2020. The utility of combined urine dipstick analysis and specific gravity measurement to determine feline proteinuria. J. Small Anim. Pract. 61, 541–546.
Piech, T.L. and Wycislo, K.L. 2019. Importance of urinalysis. Vet. Clin. North Am. Small Anim. Pract. 49, 233–245.
Prudic, R.A., Saba, C.F., Lourenço, B.N. and Bugbee, A.C. 2018. Prevalence of proteinuria in a canine oncology population. J. Small Anim. Pract. 59, 496–500.
Purswell, E.K., Lashnits, E.W., Breitschwerdt, E.B. and Vaden, S.L. 2020. A retrospective study of vector-borne disease prevalence in dogs with proteinuria: southeastern United States. J. Vet. Intern. Med. 34, 742–753.
R Core Team. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Rayhel, L.H., Quimby, J.M., Cianciolo, R.E., Cléroux, A., McLeland, S.M. and Franken, T. 2020. Clinicopathologic and pathologic characteristics of feline proteinuric kidney disease. J. Feline Med. Surg. 22, 1219–1229.
Roura, X., Elliot, J. and Grauer, G., 2017. Proteinuria. In: BSAVA manual of canine and feline nephrology and urology, 3rd ed. Eds., Elliot, J. and Grauer, G.F. Gloucester, UK: British Small Animal Veterinary Association, pp: 50–59.
Smith, R.E., Granick, J.L., Stauthammer, C.D., Polzin, D.J., Heinrich, D.A. and Furrow, E. 2017. Clinical consequences of hypertriglyceridemia-associated proteinuria in miniature schnauzers. J. Vet. Intern. Med. 31, 1–9.
Sparkes, A.H., Caney, S., Chalhoub, S., Elliott, J., Finch, N., Gajanayake, I., Langston, C., Lefebvre, H.P., White, J. and Quimby, J. 2016. ISFM consensus guidelines on the diagnosis and management of feline chronic kidney disease. J. Feline Med. Surg. 18, 219–239.
Syme, H., Markwell, P.J., Pfeiffer, D. and Elliott, J. 2006. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J. Vet. Intern. Med. 20, 528–535.
Syme, H.M., Barber, P.J., Markwell, P.J. and Elliott, J. 2002. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J. Am. Vet. Med. Assoc. 220, 1799–1804.
Vogt, A.H., Rodan, I., Brown, M., Brown, S., Buffington, C.A.T., Forman, M.J., Neilson, J. and Sparkes, A. 2010. Feline life stage guidelines for all cats. J. Feline Med. Surg. 12, 43–54.
Wehner, A., Hartmann, K. and Hirschberger, J. 2008. Associations between proteinuria, systemic hypertension and glomerular filtration rate in dogs with renal and non-renal diseases. Vet. Rec. 162, 141–147.
Wickham, H. 2009. Ggplot2: Elegant Graphics for Data Analysis. 2nd Edition, Springer, New York. https://doi.org/10.1007/978-0-387-98141-3.
Willems, A., Paepe, D., Marynissen, S., Smets, P., Van de Maele, I., Picavet, P., Duchateau, L. and Daminet, S. 2017. Results of screening of apparently healthy senior and geriatric dogs. J. Vet. Intern. Med. 31, 81–92.














