| Original Article | ||
Open Vet J. 2022; 12(6): 888-902 Open Veterinary Journal, (2022), Vol. 12(6): 888–902 Original Research Insecticidal efficacy and safety of Phoxim and influence on hematological, biochemical, and antioxidant profiles in German Shepherd dogsMamdouh M. El-Maghraby1, Ahmed E. Mahmoud1, Dina A. Abdelkhalek1, Nahla H. Sallam2, Abdelfattah M. Ali3, Aliaa A. Abd-Elaziz3 and Essam S. Soliman4*1Internal Medicine Division, Department of Animal Medicine, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt 2Department of Parasitology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt 3Department of Pharmacology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt 4Department of Veterinary Public Health, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt Submitted: 02/08/2022 Accepted: 16/10/2022 Published: 21/11/2022 *Corresponding Author: Essam S. Soliman. Department of Veterinary Public Health, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt. Email: soliman.essam [at] vet.suez.eu.eg © 2022 Open Veterinary Journal
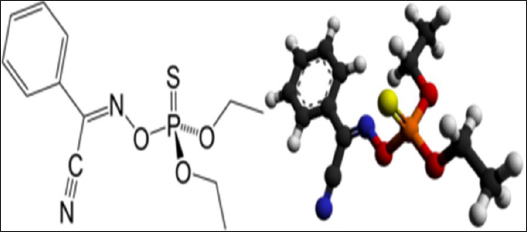
AbstractBackground: Dogs’ health and welfare enhancement can be achieved using some prophylactics and immunization go with strict hygienic and optimum biosecurity measures. Aim: Exploration of the insecticidal action of Phoxim® for combating Rhipicephalus sanguineus infestation in dogs and its prophylactic influences on the blood indices, biochemistry, antioxidant enzymes, and cortisol hormone in healthy and infested dogs. Methods: Twenty German Shepherd male dogs at 1 year old and 44.0 kg were divided randomly into four groups of five dogs in four separate Kennels with optimum biosecurity measures. The 1st group (G1) was artificially infested with R. sanguineus and treated with Phoxim®, the 2nd (G2) was non-infested and treated with Phoxim®, the 3rd (G3) was infested with R. sanguineus and not treated (positive control), and the 4th (G4) was accounted as negative control (non-infested and non-treated). A total of 160 (80 whole blood and 80 sera) samples were collected. Results: Parasitological examination revealed prominent characteristic features of R. sanguineus such as a distinct anal groove, the basis capitulum is hexagonal and lateral, the palpi are short, the second segment of the palpi as long as wide and not produced laterally, and the spiracular plate is comma-shaped and consists of stigma, peritreme, and tail. The results conveyed highly significant (p < 0.01) enhancement in erythrocytes, leukocytes, hematohiston, hematocrit, hemoglobin centering, granulocytes, alanine aminotransferase, random blood sugars, triglycerides, and total cholesterol, and highly significant (p < 0.01) declines of all measured antioxidant enzymes in treated non-infested dogs. Conclusion: Phoxim® proved efficient insecticidal activity with optimum safety and can be brought into play in the prophylactic biosecurity measures installed to eradicate external parasitism in dogs. Keywords: Biosecurity, Dogs, Phoxim®, Prophylactic efficacy, Rhipicephalus sanguineus. IntroductionDogs have been exposed to many disease attacks even with their owner’s protection (De Leeuw, 2003). The maximum safety of dogs on small and large scales is a must for maintaining their health and welfare. Many breeders are less aware of the proper management and biosecurity measures that must be taken for maintaining the prosperity of dogs (Moore, 2003). A good keeping record has to be fulfilled with all treatments, diseases control measures, and cleaning routines took. Advancement from using some prophylactics and immunization can be achieved if accompanied by strict hygienic and optimum biosecurity measures. Biosecurity is the total measures enforced to prevent exposure to disease-causing agents and it was divided into external biosecurity (bio-extinction) to limit the entrance of the infective agents (Villarroel et al., 2007) and internal biosecurity (biocontainment) to prevent distribution and reoccurrence of diseases (Dendoncker et al., 2018). The biosecurity measures to be taken in dog houses to combat external parasitism are adapting proper housing design, sealing the cracks in walls and floors with cement, optimum in-door practices, rotational positioning to discourage the larval microclimate, routine animal cleaning, and applying prophylactic/treating agent, i.e., chemical, biological, or herbal (Cochi et al., 1998). Ectoparasites are organisms that populate the external body surface of other organisms contributing to extensive harmful influences (Zendehfili et al., 2015), great economic losses (Tong et al., 2019), and transmission of bacterial, protozoal, and viral microorganisms (Mansour et al., 2017). The transmission of these microorganisms is carried out by mechanical means as in Corynebacteria, Staphylococcus, or Streptococcus (Kwak et al., 2021), or by biological means as in Babesia, Thileria, Trypanosoma, and Pasteurella pestis (Apanaskevich et al., 2020). Ticks are hematophagous ectoparasites that have an extended host range and wide geographic habitats, they transmit protozoan, bacterial, rickettsia, and viral pathogens contributing to debilitating/fatal diseases (Adenubi et al., 2016). Ticks are subclassified into Argasidae or soft ticks and Ixodidae or hard ticks. Hard ticks are the most dangerous as they feed on their hosts for days to weeks depending on the growing stage, animal species, and types of ticks (Rajput et al., 2006). Ticks can transmit some animal infectious and human zoonotic diseases. Insecticides are chemical, biological, or herbal substances that are designed to kill ectoparasites by interfering with the nervous system via exerting several actions such as acetylcholinesterase inhibitors, sodium channel modulators or blockers, inhibitors of mitochondrial ATP synthase, and inhibitors of chitin biosynthesis (Metcalf, 2002). Phoxim® is a spray-effective and widely used organophosphate insecticide against sorbent and sucking lice, sheep and cattle ticks, flies, ticks, and fly larvae present in wounds, and three scabies insects in a single dose of 10 ml/10 l of water (WHO, 2000). WHO committee established a dose of 0–4 µg/kg body weight from Phoxim® concerning their influence on the liver and brain. Phoxim® of a 0.1% aqueous has proven high efficiency in reducing the prevalence of fleas (Larsen et al., 2018) and chicken mite infestations (Meyer-Kühling et al., 2007). We aimed in the current experimental design to determine the insecticidal action of Phoxim® for combating and eradicating Rhipicephalus sanguineus canine infestation. The study also investigated the prophylactic and treatment influences of Phoxim® on the blood indices, biochemistry, antioxidant enzymes, and cortisol hormone in healthy and infested dogs. Material and MethodsStudy location and durationThe experiment was conducted in dog kennels installed at the educational farm in the Faculty of Veterinary Medicine, Suez Canal University. The study was structured to last for 3 months from December 1st, 2021 to February 28th, 2022. The experimental duration was partitioned into three consecutive stages of four weeks, the 1st stage for dog housing, accommodation, and habituation, the 2nd stage for experimental infestation with R. sanguineus ticks, and the 3rd stage was assigned for insecticidal treatment, sampling, and animals’ recovery under observation. The biochemistry assessment was conveyed in the Veterinary Public Health Laboratory at the Faculty of Veterinary Medicine, Suez Canal University. Blood indices and antioxidant enzymes were assessed in the Clinical Pathology Laboratories at the University Hospital. Phoxim® insecticideA commercial form of Phoxim® was acquired from a clinical veterinary pharmacy in Ismailia governorate, Egypt. It is recommended to treat ectoparasitic infestations of cattle, sheep, goats, and pigs. Phoxim® is an organophosphate insecticide that is composed of Diethoxy-trio-phosphoryl-oxyimino-phenyl-acetor (C10H15N2O3PS, Fig. 1) with a molecular weight up to 298.30 and melting point of 6.1°C. It was designed to act as a cholinesterases inhibitor causing cessation of the acetylcholine at the cholinergic synapses (Wang et al., 2010). Phoxim® can contribute to the pooling of acetylcholine up to toxic levels contributing to the overexcitation of nerve cells, parasympathetic, and neuromuscular systems. It also might contribute to neuropathy by inhibiting target esterase and aging of the esterase enzyme. Usually, after exerting its action, it is excreted in urine and feces. Phoxim® can act as contact, stomach, and fumigant poison (Taktak et al., 2021).
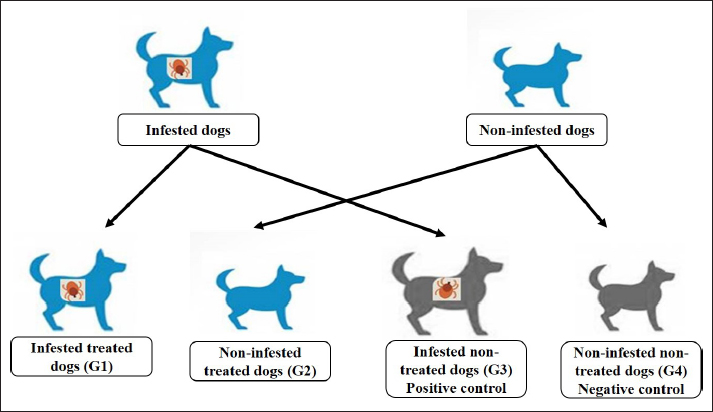
Fig. 1. The molecular structure and 3D shape of the commercial Phoxim®. Experimental dogs’ housing microclimateKennels were provided with as much as biosecurity measures to control and minimize the entrance and spread of pathogens after Soliman and Abdallah (2020). The external and internal biosecurity measures were foot dips of crude carbolic acid 5%, fly-proof nets, clean feeding porcelain dishes, clean watering bowls, secured feed storage area, mechanical control of rodents using baited wire traps, and a proper drainage system. The Kennels were supplied with white LED lights following Soliman and Hassan (2019). The floors were regularly washed and disinfected in sequence with hypochlorite 3%, quaternary ammonium compounds, and glutaraldehyde. The gutter was sprinkled with slaked lime from time to time to maintain the optimum microclimatic conditions after Soliman et al. (2018). The Kennels were ventilated with a mixed design of natural (V-shaped side wall windows) and artificial means (ceiling fans and sidewall suction fans) to encourage cross-sectional air convection (Stack effect). Experimental animals’ managementTwenty German Shepherd male dogs at one year old and 44.0 kg body weight were picked up from a pet shop in Ismailia governorate, Egypt. All the picked-up dogs were nearly similar to overcome the individual biological variation in response to the Phoxim® treatment (Taktak et al., 2021). Dogs were divided randomly on their arrival into four groups of five dogs in four separate Kennels. The Kennels were adjusted at 37°C with oil heaters (Oil—11 blades—2,500 W heater) after Soliman et al. (2021). Dogs were provided ad libitum access to dechlorinated tap water previously tested for residual chlorine using an orthotolidine test. Dogs were fed twice (in the morning and evening) daily to eliminate the influence of overwhelming stress. The morning meal consisted of a homogenous mix of well-done chicken, bread, liver, rice, minced meat, and pasta, while the evening meal contained ½ to ¾ kg of dry food with 23% crude protein. Dogs were dewormed on their first arrival with praziquantel and fenbendazole to ensure the complete freedom of internal parasites that might contribute to overwhelming stressful conditions. Dogs were also vaccinated subcutaneously in the back of the neck with two doses of Vanguard® Plus 5 DHLPPC viral vaccine (Zeotis® US) with an interval of 21 days between the two shots against canine adenovirus type I and II, canine distemper, parainfluenza, canine hepatitis, leptospirosis, canine parvovirus, and coronavirus, and rabies. Study designExperimentally challenged animals were divided in a completely randomized pattern into four groups of five dogs in four separate Kennels. The groups were assigned for treatments as (Fig. 2) as follows: the 1st group (G1) was artificially infested with R. sanguineus and treated with Phoxim®, the 2nd group (G2) was non-infested and treated with Phoxim®, the 3rd group (G3) was infested with R. sanguineus and did not receive any treatment (positive control), and the 4th group (G4) was kept non-infested and non-treated (negative control). Experimental infestation of dogsRhipicephalus sanguineus ticks were aseptically handpicked from field cases admitted to veterinary clinics in Ismailia-Egypt by anticlockwise orientation technique until the capitulum was detached undamaged from the hosts with forceps into sterilized screw-capped bottles and transferred to the laboratory. The female ticks were identified by the morphological characteristics key after Walker et al. (2003) including scutum formation, capitulum, and leg coloration using light microscopy (Barska® AY13180 Binocular Stereo Microscope, 10x), and incubated at microclimatic temperature and humidity of up to 25°C and 75%, respectively. Rhipicephalus sanguineus ticks were used as prescribed by Aboelela et al. (2022b) to produce artificial infestation using 6 females per dog in two experimental groups (G1; infested and treated with Phoxim® and G3; infested and did not receive any treatment i.e. positive control) at the beginning of the 2nd stage. The infested dogs were regularly observed until ensuring the manifestation signs through the development of new stages on the animal’s skin. The infesting ticks were sampled in sterile screw-capped bottles for light microscopical (10x) confirmation. Parasitological examinationTicks were collected by brushing over the different parts of the dog’s body into screw-capped bottles containing an appropriate amount of 70% alcohol. Sample preparations were conveyed as recommended by Farid et al. (2021a,b). Ticks were subjected to installments such as being immersed into sodium hydroxide (NaOH 10%), washed in double distilled water, and dehydrated using ethyl alcohol. The processed tick samples were immersed into xylene, mounted onto the slides, and covered. The slides were dried at 40-50°C for 24 h in a hot oven (Daihan® LabTech® Hot air Oven, LDO-080N, Indonesia) and examined using a light microscope (Barska® AY13180 Binocular Stereo Microscope 10x magnification). Tick identification was carried out according to Walker et al. (2003). SamplingA total of 160 samples (4 sampling times × 20 dogs × 2 types of samples) including 80 whole blood on EDTA and 80 sera were collected by the end of the experiment. The samples were aseptically transferred to the laboratory into a dry-ice box. Whole blood samples (n=80) were collected on EDTA-vacutainers and used for the blood indices examinations. Sera samples (n=80) on plain serum tubes were held at 25°C for 30 minutes in a water bath (Thermo® water bath Precision series Standard, 20°C, 30°C to 100°C, 392 mm, GP20) and centrifuged (Fisher®Thermo Scientific CL10 Centrifuge w/ F-G3 Rotor with a max RPM of 4000) at 3,000 rpm for 20 minutes. Clear non-hemolytic sera were pipetted using an automatic pipette (Fisherbrand®, variable 200: 1,000 micron) into 2.5 ml Eppendorf tubes and stored at −20°C for biochemical and antioxidant assessment as recommended by Soliman et al. (2017).
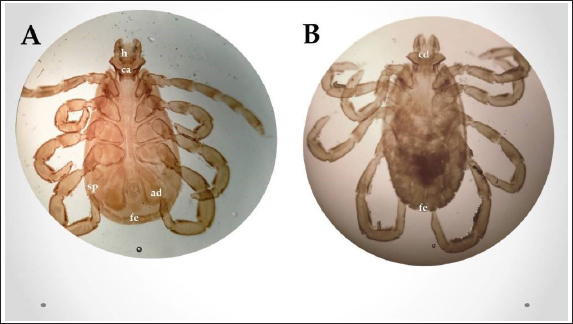
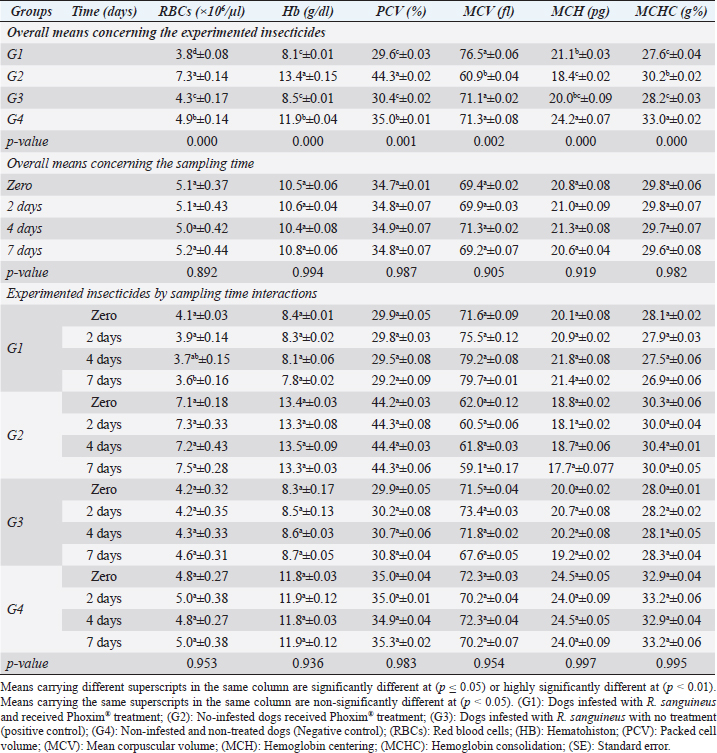
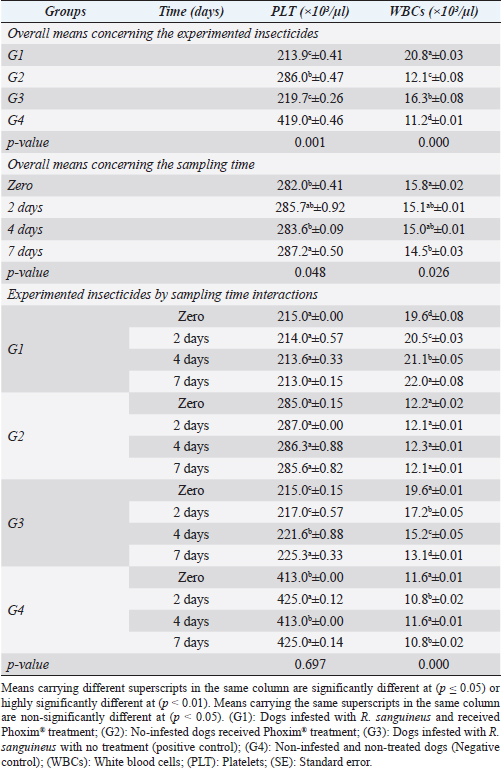
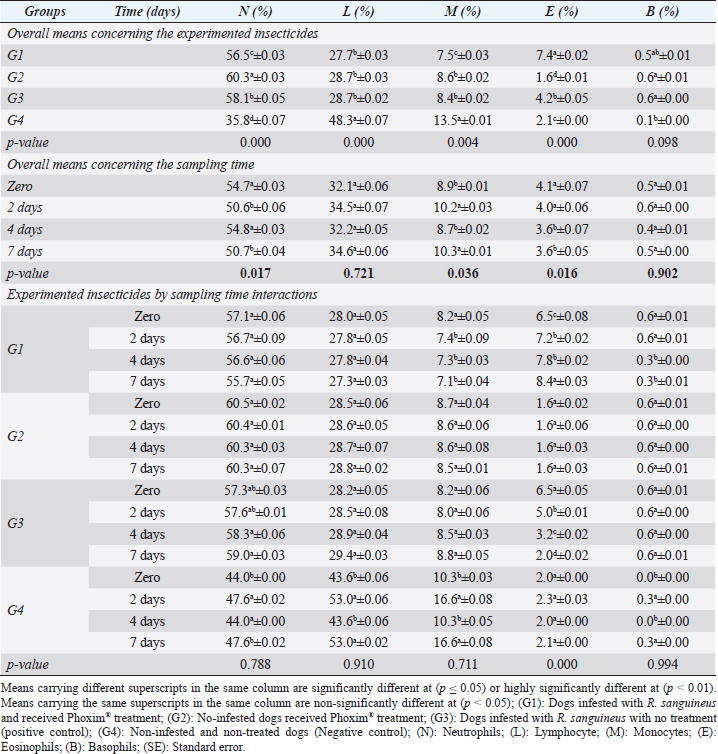
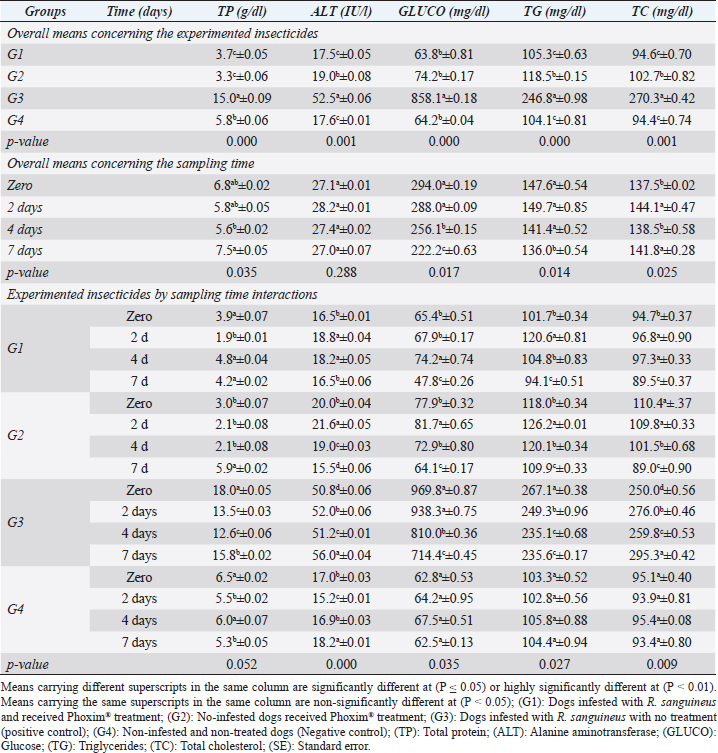
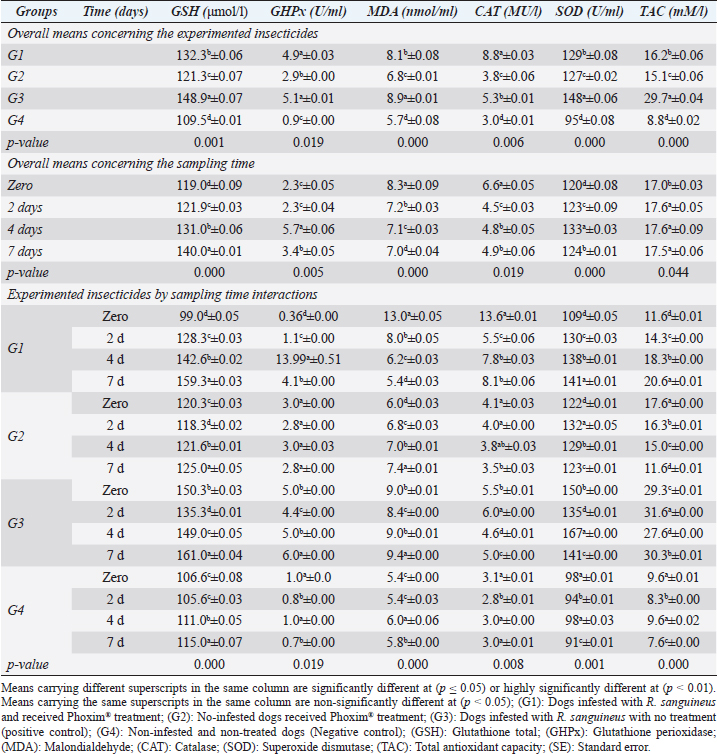
Fig. 2. Experimental design showing the grouping of the experimental dogs into dogs infested with R. sanguineus and received Phoxim® treatment (G1), Non-infested dogs who received Phoxim® treatment (G2), Dogs infested with R. sanguineus with no treatment (Positive control, G3), and Non-infested and non-treated dogs (Negative control, G4). Hematological profileWhole blood samples (n=80) were examined for erythrocytic count (RBCs, ×106/µl), hematohiston (Hb, g/dl), hematocrit (PCV, %), mean corpuscular volume (MCV, fl), hemoglobin centering (MCH, pg), hemoglobin consolidation (MCHC, g%), platelet counts (PLT, ×103/µl), leukocytic count (WBCs, ×103/µl), neutrophils (N, %), lymphocytes (L, %), monocytes (M, %), eosinophil (E, %), and basophils (B, %) using Sysmex XP-300 Automated Hematology Analyzer. Biochemical, antioxidant, and hormonal profilesSera samples (n=80) were examined for total protein (TP, g/dl), alanine aminotransferase (ALT, IU/L), glucose (GLUCO, mg/dl), total cholesterol (TC, mg/dl), triglycerides (TG, mg/dl), glutathione total (GSH, μmol/l), glutathione peroxidase (GHPx, U/ml), malondialdehyde (MDA, nmol/ml), catalase (CAT, MU/L), superoxide dismutase (SOD, U/ml), and total antioxidant capacity (TAC, U/ml) calorimetrically using H144211 ROCHE COBAS Integra 800 chemical analyzer. Cortisol hormone (CORT, mcg/dl) was measured by using ROCHE Elecsys 1010 Immunoassay Analyzer (Wu et al., 2017). Statistical analysisThe statistical analysis was run using a statistical package for social sciences version 20 IBM Corp (SPSS, 2016 - IBM SPSS Statistics 20). The data were analyzed with a Two-tailed multifactorial Analysis of Variance (Two-tailed analysis of variance) to estimate the influence of Phoxim® as a treating and prophylactic agent on infested and non-infested dogs concerning different sampling times. The statistical model empathized as follows: Yijk=µ + αi + βj + (αβ)ij + Ɛijk Where Yijk was the measurement of dependent variables; µ was the overall mean; αi was the fixed effect of the Phoxim® treatment, βj was the fixed effect of sampling time, (αβ)ij was the interactions of Phoxim® treatment by sampling time, and Ɛijk was the random error. The results were expressed as highly significant at (p ≤ 0.01), significant at (p ≤ 0.05), and non-significant at (p > 0.05). Ethical approvalThe experimental design, study protocol, guidance on authorship, transparency, and ensuring accuracy away from potential complications were approved by the Scientific Research Ethics Committee at the Faculty of Veterinary Medicine, Suez Canal University, Egypt with approval number (2022008). The experimental German Shepherd dogs were subjected to infestation with all the precautions required and provided the animals with human management and approach. The samples were collected with minimum pain during collection using all the necessary precautions. ResultsParasitological examinationThe parasitological examination revealed R. sanguineus ticks (The brown dog tick, Rhipicephalus sanguineus Latreille, 1806) (Nava et al., 2015) are characterized where by a distinct anal groove (Fig. 3). Male has adenal and accessory adenal shields and caudal process. The basis capitulum is hexagonal and laterally produced. Palpi are short. Mouthparts as long as basis capitulum. The second segment of the palpi is as long and wide and is not produced laterally and the festoons are present. The spiracular plate is comma-shaped and consists of a stigma, peritreme, and tail. Hematological assessmentThe erythrocyte count, hematohiston, and hematocrit disclosed (Table 1) highly significant (p < 0.01) increases in treated non-infested dogs (G2) and highly significant (p < 0.01) declines in both G1 and G3 dogs. Mean corpuscular volume revealed a highly significant (p < 0.01) improvement in the G1 with no significant differences between the positive control (G3) and negative control (G4). Hemoglobin centering and hemoglobin consolidation recorded (Table 1) highly significant (p < 0.01) declines in G1, G2, and G3 compared to the G4 concerning the G2; the treated non-infested group showed highly significant (p < 0.01) increases compared to other groups. Platelet count illustrated (Table 2) highly significant (p < 0.01) increases in the G2 dogs compared to the other groups. Meanwhile, leukocytes (Table 2) recorded highly significant (p < 0.01) improvement in G2 dogs compared to the other groups. Neutrophils and basophils (Table 3) disclosed highly significant (p < 0.01) increases in the G2 dogs compared to other groups. Agranulocytes revealed highly significant (p < 0.01) declines in all groups. The sampling time revealed no significant differences (Table 1) in erythrocytes, hematohiston, hematocrit, mean corpuscular volume, hemoglobin centering, and hemoglobin consolidation between all the treated and non-treated groups. On the other hand, platelets (Table 2) recorded highly significant (p < 0.01) increases on the 4th-day post-treatment and highly significant (p < 0.01) declines in leukocytes (Table 2) and differential counts (Table 3) post-treatment. Biochemical assessmentPhoxim® treatment in both infested and non-infested animals (Table 4) illustrated highly significant (p < 0.01) improvement of all assessed biochemical parameters concerning the higher improvement of alanine aminotransferase, glucose, triglycerides, and total cholesterol in G2 (treated non-infested) dogs compared to other animals. The time scale revealed highly significant (p < 0.01, Table 4) declines in alanine aminotransferase, glucose, and triglycerides on the 7th-day post-treatment and total protein and total cholesterol on the 4th-day post-treatment. Antioxidant profile and hormonal stress markerThe glutathione total, glutathione peroxidase, malondialdehyde, catalase, superoxide dismutase, and total antioxidant capacity (Table 5) recorded highly significant (p < 0.01) declines in the G2 dogs compared to G1 and G3 animals.
Fig. 3. Rhipicephalus sanguineus, (A) ventral view; (B) dorsal view. (Sp): Spicular plate, (fe): festoon, (ca): capituli, (ad): adanal shield, (h): hypostome. Table 1. Corpuscular indices (Mean ±SE) of Phoxim® treated and untreated dog groups.
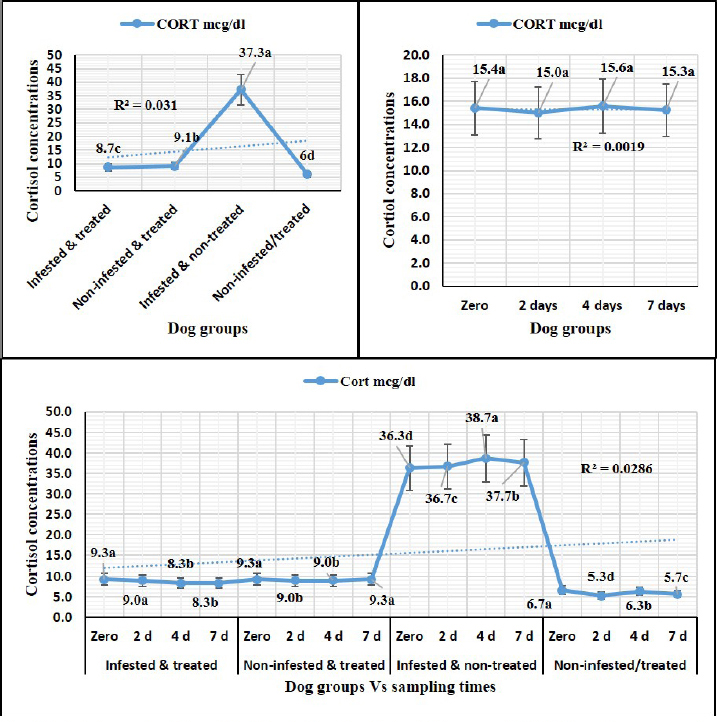
Sampling times (Table 5) illustrated highly significant (p < 0.01) declines of glutathione total and total antioxidant capacity at the zero-time; glutathione peroxidase, catalase, and superoxide dismutase on the 2nd day; and malondialdehyde at the 7th-day post-treatment. Cortisol stress markers (Fig. 4) illustrated highly significant (p < 0.01) declines in both G1 and G3 groups concerning the G1 compared to other groups. Sampling times showed no significant differences in all treated and non-treated groups. DiscussionEctoparasitic infestations in dogs are usually influenced and associated with numerous associations and determinants such as animal associates including breed, sex, age, body configurations, and coat color as revealed by Földvári et al. (2016); Kumsa et al. (2019); Aboelela et al. (2022a), macro and microclimatic associates such as geographical locations, seasonal variations, housing system, living pattern, and type of food as revealed by Kruse and Schuz (2016); Hasib et al. (2020); Minabaji et al. (2020), and agent associates including the type and characteristics of infesting parasites as revealed by Moravvej et al. (2015). Thus, control, elimination, and eradication strategies and plans for external parasites have been included in all biosecurity programs on pet animal houses, kennels, and shelters (Alho et al., 2017, 2018). Table 2. White blood cells and platelet counts (Mean ±SE) of Phoxim® treated and untreated dog groups.
Pesticides are conventional in the control of agricultural pests and insects contributing to extensive ecosystem damage and public health concerns with toxicity that occurs with direct exposure to insecticides or the indirect consumption of contaminated feed and water (Hussein et al., 2012). Organophosphorus pesticides like Phoxim® recorded by Kalender et al. (2006) to suppress the activity of acetylcholinesterase and pseudocholinesterase with partial influences on immunity (Handy et al., 2002), liver (Kalender et al., 2005), and blood indices (Kalender et al., 2006). Chemical insecticides were and unfortunately still are the predominating tools for the eradication of insects and external parasites (Singh, 2010). The use of chemical insecticides has been subjected to evaluation for their numerous disadvantages such as non-specific targets, resistance, and residual actions (Saeed et al., 2016). Table 3. Differential leukocytic counts (mean ±SE) of Phoxim® treated and untreated dog groups.
Organophosphorus pesticide toxicity is usually associated with their chemical composition (oxygen, sulfur, or phosphorus atoms) and the replacement of the sulfur with oxygen during the detoxication process causing further activation of the compound. The toxic influence usually arises from their binding affinity to some amino acids concerning serine and prevents their catalytic activity as recorded by Mahananda and Mohanty (2012). The continuous and repetitive use of insecticides is contraindicated for the development of multifactorial resistance build-up against these compounds as recorded by Aguilar et al. (2018); Cossío-Bayúgar et al. (2018); and Kumar (2019). Table 4. Biochemical profile (Mean ±SE) of Phoxim® treated and untreated dog groups.
Our results illustrated highly significant increases in erythrocytes, hematohiston, hematocrit, platelets, neutrophils, and basophils in treated non-infested animals (G3) compared to both infested treated and infested non-treated dogs. Mean corpuscular volume revealed highly significant improvements in the infested treated dogs (G1). Hemoglobin centering, hemoglobin consolidation, and agranulocytes recorded highly significant declines in all groups concerning the non-infested treated group (G3) which showed highly significant increases. Leukocytes illustrated highly significant improvements in treated non-infested dogs (G3). The results were in contrast to those obtained by Mossa and Abbassy (2012) and Holy et al. (2015) who recorded significant declines in erythrocytes, hematohiston, and hematocrit of male rats treated with phoxim at a rate of 2,007.13 mg/kg body weight. Meanwhile, Aboelela et al. (2022b) in agreement with our study recorded significant improvements in hematological profile and erythrocyte sedimentation rates. Table 5. Antioxidant profile (Mean ±SE) of Phoxim® treated and untreated dog groups.
The significant increase in the total leukocytic count might be attributed to the regeneration and relocations of leukocytes from marginal granulocytes affected by the high background of adrenaline. The recorded significant increase in the total leukocytic count was consistent with those reported by Al-Sahhaf (2006) and Yassa and Girgis (2018) after using the organophosphorus compound as an insecticidal agent in rats. Meanwhile, Mossa and Abbassy (2012) recorded a significant decline in total leukocytic count in chlorpyrifos-treated rats. The recorded significant increase in the inflammatory agranulocytes (lymphocytes and monocytes) can be considered a typical picture that developed usually in any animal under stress, and in this case, the treatment itself might be considered a stress factor on the animals that might shift the blood picture to a little extent.
Fig. 4. Cortisol stress marker (Mean ±SE) of Phoxim® treated and untreated dog groups. (A) CORT overall means concerning experimental groups. (B) CORT overall means concerning sampling times. (C) CORT overall means concerning experimental groups by time interactions. Phoxim® treatment in both infested and non-infested animals showed highly significant improvement of all measured biochemical parameters concerning higher improvements in the treated noninfested dogs (G3) of alanine aminotransferase, glucose, triglycerides, and total cholesterol compared to the infested treated animals (G1). Yassa and Girgis (2018) were consistent with our results and recorded nonsignificant modifications in total protein and albumin in Phoxim®-treated rats. Meanwhile, they recorded a significant increase in liver enzymes contrary to the current results. On the contrary, Abo El-Soud et al. (2015) recorded a significant decrease in total protein and significant increases in liver enzyme concentrations of rats treated with profenofos 65 days post-treatment. Al-Sahhaf (2006) recorded a significant decrease in total protein concentrations of rats treated with oral doses of sumithiom. The significant improvement of the biochemical profiles concerning proteinogram and liver enzymes might be attributed to the adaptation abilities of the animal to the treatment stress which contributed significant decline of these biochemical indicator levels in the treated and non-infested animals compared to the infested non-treated animals and infested treated animals. Al-Sahhaf (2006) and Abo El-Soud et al. (2015) reported in contrast to the current results significant increase in serum creatinine and they contributed this elevation to the lower glomerular filtration capacity. Yassa and Girgis (2018) also reported coagulative necrosis of the epithelial lining of the glomeruli in Phoxim®-treated rates with marked hypercellularity and this occurred using an acute toxic dose of Phoxim. Antioxidant enzymes are excited in certain circumstances such as shortage or cessation of the oxidative conditions of some nutrients such as lipids and proteins. Oxidative stress is a condition during which the reactive oxygen overwhelms the antioxidative defense mechanism of the body causing shifting in the macromolecules, cellular injuries, and apoptosis (Trevisan et al., 2001). The current results revealed that glutathione total, glutathione peroxidase, malondialdehyde, catalase, superoxide dismutase, total antioxidant capacity, and cortisol stress marker showed highly significant declines in the treated non-infested dogs compared to other animal groups. The results were consistent with those reported by Aboelela et al. (2022b) who recorded significant improvements in the biochemical profile and cortisol hormone of the two-week and four-week post-treatment samples. Also, El-Naggar et al. (2017) reported neutral influences of their tested insecticides on the tissue architecture and operational conditions of the brain, liver, and kidney in male Albino mice. Herbal and eco-friendly insecticides that are based on a plant extract like peppermint, citrolina, garlic, onions, pomegranate, camphor, and clove might vanquish all the negative impacts contributed by the usage of some synthesized insecticides after Abd-Ella (2014, 2016); Gil et al. (2015); Nettles et al. (2016); and Aboelela et al. (2022b). They act by disrupting the insect’s central nervous system by calcium channel blockage or direct enteric nervous system effect causing hyperexcitation of contaminated insects’ nerves and muscles, a repellent that masks the scent that is attractive to insects and it would be difficult for an insect to locate their target, and interrupt the action potential which is essential for insects’ activity and synaptic transmission resulting in suffocation and distressing of the cuticular waxes. ConclusionPhoxim® as an organophosphorus insecticidal agent contributed to significant increases and improvements in erythrocytes, hematohiston, hematocrit, hemoglobin consolidation, total leukocytic count, granulocytes, alanine aminotransferase, glucose, triglycerides, and total cholesterol, as well as significant declines of glutathione total, glutathione peroxidase, malondialdehyde, catalase, superoxide dismutase, total antioxidant capacity, and cortisol stress marker in non-infested treated dogs rather than infested treated, infested non-treated, and negative control. Phoxim® was able to prove a significant prophylactic rather than treating influences to be included in the prophylactic schedule of the biosecurity programs for eradication of external parasites concerning R. sanguineus ticks in dogs. Garlic, onions, pomegranate, camphor, and clove herbal insecticides are much more recommended in the prophylaxis and treatment of external parasitism because of their lower toxicity, lower ecological impact, higher efficacy, and specific actions. Authors’ ContributionsMME, AEM, and DAA participated in the clinical investigation of the animals (infested and non-infested), supervised the Phoxim® treatment, performed the animals sampling at the scheduled intervals, conducted the antioxidant assay, and participated in manuscript writing. NHS performed the parasitological examination and participated in manuscript writing. AAA. Performed the Phoxim® treatment, conducted the hematological assessment, and participated in manuscript writing. ESS planned and illustrated the experimental design, carried out the biochemical and hormonal analysis, participated in manuscript writing, and reviewed the final edit of the manuscript. AcknowledgmentThanks should be provided to Prof. WF Khalil for his guidance and help in the work. FundingThe authors received no financial support for the research, authorship, and/or publication of this article Conflict of interestThe authors declare that they have no financial or personal conflicts which may have inappropriately influenced them in writing this manuscript. ReferencesAbd-Ella, A.A. 2014. Toxicity and persistence of selected neonicotinoide insecticides on cowpea aphid, Aphis craccivora Koch (Homoptera: Aphididae). Arch. Phytopathol. Pflanzanschutz. 47(3), 366–376. Abd-Ella, A.A. 2016. Evaluation of certain neonicotinoid insecticide seed treatments against cereal aphids on some wheat cultivars. J. Phytopathol. Pest Manag. 3(1), 21–33. Abo El-Soud, S.M., El-Hadad, S.F., El-Said, F.G. and Sanousi, S.A. 2015. Cytogenetic, fertility and pathological studies organophosphorus insecticide of (Profenofose) on male rats. Egypt. J. Chem. Environ. Health 1(1), 303–314. Aboelela, E.M., Sobieh, M.A., Abouelhassan, E.M., Farid, D.S. and Soliman, E.S. 2022a. Retrospective seasonal parasitological survey on prevalence and epidemiological determinants of ectoparasitic infestations in dogs and cats of Damietta, Egypt. Adv. Anim. Vet. Sci. 10(8), 1854–1867. Aboelela, E.M., Sobieh, M.A., Abouelhassan, E.M., Farid, D.S. and Soliman, E.S. 2022b. In-vivo and in-vitro effectiveness of three insecticides types for eradication of the tick Rhipicephalus sanguineus in dogs. Open Vet. J. 12(2), 290–302. Adenubia, O.T., Fasinab, F.O., McGawa, L.J., Eloffa, J.N. and Naidoo, V. 2016. Plant extracts to control ticks of veterinary and medical importance. S. Afr. J. Bot. 105(2016), 178–193. Aguilar, G., Olvera, A.M., Carvajal, B.I. and Mosqueda, J. 2018. SNPs and other polymorhisms associated with acaricide resistance in Rhipicephalus microplus. Front. Biosci. – Landmark 23(1), 65–82. Alho, A.M., Lima, C., Colella, V., de Carvalho, L.M., Otranto, D. and Cardoso, L. 2018. Awareness of zoonotic diseases and parasite control practices: a survey of dog and cat owners in Qatar. Parasit. Vectors 11, 133. Alho, A.M., Lima, C., Latrofa, M.S., Colella, V., Ravagnan, S., Capelli, G., de Carvalho, L.M., Cardoso, L. and Otranto, D. 2017. Molecular detection of vector-borne pathogens in dogs and cats from Qatar. Parasit. Vectors 10, 298. Al-Sahhaf, Z.Y. 2006. Toxicity of Sumithion in albino rats: hematological and biochemical studies. J. Appl. Sc. 6(14), 2959–2962. Apanaskevich, D.A., Chaloemthanetphong, A., Vongphayloth, K., Ahantarig, A., Apanaskevich, M.A., Brey, P.T., Hertz, J.C., Lakeomany, K., Sutherland, I.W. and Trinachartvanit, W. 2020. Description of a new species of Dermacentor Koch, 1844 (Acari: Ixodidae) from Laos and Thailand. Syst. Parasitol. 96(2), 1–10. Cochi, S., de Quadros, C., Dowdle, W., Goodman, R., Ndumbe, P. and Salisbury, D. 1998. Post-Conference small group report. In Global disease elimination and eradication as public health strategies. Eds., Goodman, R.A., Foster, K.L., Trowbridge, F.L, and Figueroa, J.P. Bulletin of the World Health Organization 76(Suppl. 2), 113. Available via https://apps.who.int/iris/bitstream/handle/10665/42172/bulletin_1998_76(supp2).pdf Cossío-Bayúgar, R., Martínez-Ibañez, F., Aguilar-Díaz, H. and Miranda-Miranda, E. 2018. Pyrethroid acaricide resistance is proportional to P-450 cytochrome oxidase expression in the cattle tick Rhipicephalus microplus. BioMed Res. Int. Article ID: 8292465; doi:10.1155/2018/8292465 De Leeuw, W. 2003. The Council of Europe. In: Proceedings of an ILAR international workshop; Washington, DC. November 15–17, 2003. Dendoncker, P.A., De Rooster, H., Abma, E., Wydooghe, E. and Dewulf, J. 2018. Biosecurity measures for dog merchants and canine breeding kennels. In Biosecurity in animal production and veterinary medicine: from principles to practice. Eds., J. Dewulf and F. Van Immerseel,. Leuven, Belgium; The Hague, The Netherlands: ACCO, pp: 433–452. El-Naggar, S.A., Eltantawi, H., Ibrahim, M.A. and Alm-Eldeen, A. 2017. Assessment of the toxicity of sub-chronic low and high doses of the bio-insecticide spinosad on the liver, kidney and the cerebellum in male albino mice. Braz. Arch. Biol. Technol. 60; doi:10.1590/1678-4324-2017160179 Farid, D.S., Abouelhassan, E.M., El- Sebae, A.A., Enany, M.E. and Youssef, A.I. 2021a. Ectoparasite fauna of commensal rodents collected from the North Sinai Governorate, Egypt and its public health significance. Adv. Anim. Vet. Sci. 9(4), 563–570. Farid, D.S., Sallam, N.H., Salah Eldein, A.M. and Soliman, E.S. 2021b. Cross-sectional seasonal prevalence and relative risk of ectoparasitic infestations of rodents in North Sinai, Egypt. Vet. World 14(11), 2996–3006. Földvári, G., Široký, P., Szekeres, S., Majoros, G. and Sprong, H. 2016. Dermacentor reticulatus: a vector on the rise. Parasit. Vectors 9(1), e314. Gil, F.N., Moreira-Santos, M., Chelinho, S., Pereira, C., Feliciano, J.R., Leitão, J.H., Sousa, J.P., Ribeiro, R. and Viegas, C.A. 2015. Suitability of a Saccharomyces cerevisiae-based assay to assess the toxicity of pyrimethanil sprayed soils via surface runoff: Comparison with standard aquatic and soil toxicity assays. Sci. Total Environ. 505, 161–171. Handy, R.D., Abd-El Samei, H.A., Bayomy, M.F.F., Mahran, A.M., Abdeen, A.M. and El-Elaimy, E.A. 2002. Chronic diazinon exposure: pathologies of spleen, thymus, blood cells, and lymph nodes are modulated by dietary protein or lipid in the mouse. Toxicol. 172, 13–34. Hasib, F., Kabir, H., Barua, S., Akter, S. and Chowdhury, S. 2020. Frequency and prevalence of clinical conditions and therapeutic drugs used in dog and cat at Teaching Veterinary Hospital, Chattogram Veterinary and Animal Sciences University. J. Adv. Vet. Anim. Res. 7(1), 156–163. Holy, B., kenanagha, B. and Onwuli, D.O. 2015. Haemato-pathological effect of dichlorvos on blood picture and liver of albino rats. J. Toxicol. Environ. Health Sci. 7(2), 18–23. Hussein, H.K., Elnaggar, M.H. and AlDailamy, J.M. 2012. Protective role of Vitamin C against hepatorenal toxicity of fenvalerate in male rats. Glob. Adv. Res. J. Environ. Sci. Toxicol. 1(4), 60–65. Kalender, S., Ogutcu, A., Uzunhisarcikli, M., Acikgoz, F., Durak, D., Ulusoy, Y. andKalender, Y. 2005. Diazinon-induced hepatotoxicity and protective effect of vitamin E on some biochemical indices and ultrastructural changes. Toxicol. 211(3), 197–206. Kalender, Y., Uzunhisarcikli, M., Ogutcu, A., Acikgoz, F. and Kalender, S. 2006. Effects of diazinon on pseudocholinesterase activity and haematological indices in rats: the protective role of vitamin E. Environ. Toxicol. Pharmacol. 22(1), 46–51. Kruse, M. and Schulz, S.C. 2016. Chapter 1: Overview of schizophrenia and treatment approaches. In Schizophrenia and psychotic spectrum disorders. Eds., Charles Schulz, S., Green, M.F. and Nelson, K.J. New York: Oxford University Press, p: 7. ISBN 978-0-19-937806-7. Kumar, R. 2019. Molecular markers and their application in the monitoring of acaricide resistance in Rhipicephalus microplus. Exp. Appl. Acarol. 78, 149–172. Kumsa, B., Abiy, Y. and Abunna F. 2019. Ectoparasites infesting dogs and cats in Bishoftu, central Oromia, Ethiopia. Vet. Parasitol.: Reg. Stud. Rep. 15, 100263. Kwak, M.L., Chavatte, J.M., Chew, K.L. and Lee, B.P.Y.H. 2021. Emergence of the zoonotic tick Dermacentor (Indocentor) auratus Supino, 1897. (Acari: Ixodidae) in Singapore. Ticks Tick Borne Dis. 12(1), 101574. Larsen, K.S., Sciuto, M. and Dahl, J. 2018. The use of phoxim and bendiocarb for control of fleas in farmed mink (Mustela vison). Acta Vet. Scand. 60, 58. Mahananda, M.R. and Mohanty, B.P. 2012. Toxicity on biochemical and hematological parameters in Bufo melanostictus (Schneider) (Common Indian Toad) exposed to Malathion. Pesticides—advances in chemical and botanical pesticides. INTECH. ISBN: 978-953-51-0680-7. http://dx.doi.org/10.5772/2609. Mansour, R., Grissa-Lebdi, K., Suma, P., Mazzeo, G. and Russo, A. 2017. Key scale insects (Hemiptera: Coccoidea) of high economic importance in a Mediterranean area: host plants, bio-ecological characteristics, natural enemies and pest management strategies—a review. Plant Protection Science. Czech Acad. Agricul. Sci. 53(1), 1–14. Metcalf, R.L. 2002. Insect Control. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH. Doi:10.1002/14356007.a14_263. ISBN 978-3527306732. Meyer-Kühling, B., Pfister, K., MüllerLindloff, J. and Heine, J. 2007. Field efficacy of phoxim 50% (ByeMite®) against the poultry red mite Dermanyssus gallinae in battery cages stocked with laying hens. Vet. Parasitol. 147 (3-4), 289–296. Minabaji, A., Moshaverinia, A. and Khoshnegah J. 2020. Frequency of Ectoparasite Infestation in Dogs in Mashhad, Northeast Iran. J. Vet. Res. 75(3), 280-287. Moore, G. 2003. Assessment of animal housing needs in the research setting using a peer-reviewed literature approach: Dogs and Cats. The Development of science-based guidelines for laboratory animal care. In: Proceedings of the November 2003 International Workshop. Moravvej, G., Hamidi, K., Nourani, L. and Bannazade, H. 2015. Occurrence of ectoparasitic arthropods (Siphonaptera, Acarina, and Anoplura) on rodents of Khorasan Razavi Province, northeast of Iran. Asian Pac. J. Trop. Dis. 5(9), 716–720. Mossa, A.H. and Abbassy, M.A. 2012. Adverse Haematological and Biochemical Effects of Certain Formulated Insecticides in Male Rats. Res. J. Environ. Toxicol. 6(4), 160–168. Nava, S., Estrada-Peña, A., Petney, T., Beati, L., Labruna, M.B., Szabó, M.P., Venzal, J.M., Mastropaolo, M., Mangold, A.J. and Guglielmone, A.A. 2015. The taxonomic status of Rhipicephalus sanguineus (Latreille, 1806). Vet Parasitol. 208(1-2), 2-8. Nettles, R., Watkins, J., Ricks, K., Boyer, M., Licht, M., Atwood, L.W., Peoples, M., Smith, R.G., Mortensen, D.A. and Koide, R.T. 2016. Influence of pesticide seed treatments on rhizosphere fungal and bacterial communities and leaf fungal endophyte communities in maize and soybean. Appl. Soil Ecol. 102(2016), 61–69. Rajput, Z.I., Song-hua, H., Chen, W., Abdullah, G.A. and Xiao, C. 2006. Importance of ticks and their chemical and immunological control in livestock, 2006. J. Zhejiang Univ Sci B. 7(11), 912–921. Saeed, R., Razaq, M. and Hardy, I.C.W. 2016. Impact of neonicotinoid seed treatment of cotton on the cotton leafhopper, Amrasca devastans (Hemiptera: Cicadellidae), and its natural enemies. Pest Manag. Sci. 72(6), 1260–1267. Singh, S.S. 2010. Comparative efficacy of certain bio-rational insecticide and Bacillus thuringinesis based bio-insecticides against Leucinodes orbonalis Guenee. In brinjal, Indian J. Hortic. 67(3), 353–356. Soliman, E.S. and Abdallah, M.S. 2020. Assessment of biosecurity measures in broiler’s farms in the Suez Canal area – Egypt using a seasonal prevalence of Salmonellosis. Vet. World 13(4), 622–632. Soliman, E.S. and Hassan, R.A. 2019. Impact of lighting color and duration on productive performance and Newcastle disease vaccination efficiency in broiler chickens. Vet. World 12(7), 1052–1059. Soliman, E.S., Ali, A.A. and Gafaar, R.E.M. 2021. Impact of Heating Systems on Air and Litter Quality in Broiler Houses, Performance, Behavior, and Immunity in Broiler Chickens. Adv. Anim. Vet. Sci. 9(2), 301–314. Soliman, E.S., Hamad, R.T. and Ahmed, A. 2017. Prophylactic and immune modulatory influences of Nigella sativa Linn. in broilers exposed to biological challenge. Vet. World 10(12), 1447–1455. Soliman, E.S., Sallam, N.H. and Abouelhassan, E.M. 2018. Effectiveness of poultry litter amendments on bacterial survival and Eimeria oocyst sporulation. Vet. World 11(8), 1064–1073. SPSS, 2016. Statistical Packages of Social Sciences. Version 21 for windows. SPSS. Inc. USA. Taktak, N.E.M., Badawy, M.E.I., Awad, O.M., Abou El-Ela, N.E. and Abdallah, S.M. 2021. Enhanced mosquitocidal efficacy of pyrethroid insecticides by nanometric emulsion preparation towards Culex pipiens larvae with biochemical and molecular docking studies. J. Egypt. Public Health Assoc. 96, 21. Tong, L., Nieh, J.C. and Tosi, S. 2019. Combined nutritional stress and a new systemic pesticide (flupyradifurone, Sivanto®) reduce bee survival, food consumption, flight success, and thermoregulation. Chemosphere 237, 124408. Trevisan, M., Browne, R., Ram, M., Muti, P., Freudenheim, J., Carosella, A.M. and Armstrong, D. 2001. Correlates of markers of oxidative status in the general population. Am. J. Epidemiol. 154(4), 348–356. Villarroel, A., Dargatz, D.A., Lane, M.V., McCluskey, B.J. and Salman, M.D. 2007. Suggested outline of potential critical control points for biosecurity and biocontainment on large dairy farms. J. Am. Vet. Med. Assoc. 230(6), 808. Walker, A.R., Bouattour, A., Camicas, J.L., Estrada-Peña, A., Horak, I.G., Latif, A.A., Pegram, R.G. and Preston, P.M. 2003. Ticks of domestic animals in africa: a guide to identification of species. Biosci. Rep., pp: 228. Wang, K.Y., Zhang, Y., Wang, H., Xia, X.M. and Liu, T.X. 2010. Influence of three diets on susceptibility of selected insecticides and activities of detoxification esterases of Helicoverpa assulta (Lepidoptera: Noctuidae)". Pestic. Biochem. Phys. 96(1), 51–55. WHO, 2000. Toxicological evaluation of certain veterinary drug residues in food. WHO food additives series: 43. IPCS—International Programme on Chemical Safety, WHO. Wu, Y.N., Yan, F.F., Hu, J.Y., Chen, H., Tucker, C.M., Green, A.R. and Cheng, H.W 2017. The effect of chronic ammonia exposure on acute-phase proteins, immunoglobulin, and cytokines in laying hens. Poult. Sci. 96(6), 1524–1530. Yassa, V.F. and Girgis, S.M. 2018. Influence of organophosphorus insecticide, Phoxim, on hematological, biochemical, histopathological, genotoxicity and fertility in male rats. Anim. Health Res. J. 6(1), 129–140. Zendehfili, H., Zahirnia, A.H., Maghsood, A.H., Khanjani, M. and Fattah, M. 2015. Ectoparasites of rodents captured in Hamedan, Western Iran. Short Communication. J. Arthropod-Borne Dis. 9(2), 267–273. | ||
| How to Cite this Article |
| Pubmed Style El-maghraby MM, Mahmoud AE, Abdelkhalek DA, Sallam NH, Aly AN, Abd-elaziz AA, Soliman ES. Insecticidal Efficacy and Safety of Phoxim and Influence on Hematological, Biochemical, and Antioxidant Profiles in German Shepherd Dogs. Open Vet J. 2022; 12(6): 888-902. doi:10.5455/OVJ.2022.v12.i6.15 Web Style El-maghraby MM, Mahmoud AE, Abdelkhalek DA, Sallam NH, Aly AN, Abd-elaziz AA, Soliman ES. Insecticidal Efficacy and Safety of Phoxim and Influence on Hematological, Biochemical, and Antioxidant Profiles in German Shepherd Dogs. https://www.openveterinaryjournal.com/?mno=90861 [Access: July 01, 2025]. doi:10.5455/OVJ.2022.v12.i6.15 AMA (American Medical Association) Style El-maghraby MM, Mahmoud AE, Abdelkhalek DA, Sallam NH, Aly AN, Abd-elaziz AA, Soliman ES. Insecticidal Efficacy and Safety of Phoxim and Influence on Hematological, Biochemical, and Antioxidant Profiles in German Shepherd Dogs. Open Vet J. 2022; 12(6): 888-902. doi:10.5455/OVJ.2022.v12.i6.15 Vancouver/ICMJE Style El-maghraby MM, Mahmoud AE, Abdelkhalek DA, Sallam NH, Aly AN, Abd-elaziz AA, Soliman ES. Insecticidal Efficacy and Safety of Phoxim and Influence on Hematological, Biochemical, and Antioxidant Profiles in German Shepherd Dogs. Open Vet J. (2022), [cited July 01, 2025]; 12(6): 888-902. doi:10.5455/OVJ.2022.v12.i6.15 Harvard Style El-maghraby, M. M., Mahmoud, . A. E., Abdelkhalek, . D. A., Sallam, . N. H., Aly, . A. N., Abd-elaziz, . A. A. & Soliman, . E. S. (2022) Insecticidal Efficacy and Safety of Phoxim and Influence on Hematological, Biochemical, and Antioxidant Profiles in German Shepherd Dogs. Open Vet J, 12 (6), 888-902. doi:10.5455/OVJ.2022.v12.i6.15 Turabian Style El-maghraby, Mamdouh M., Ahmed E. Mahmoud, Dina A. Abdelkhalek, Nahla H. Sallam, Abdelfatah N. Aly, Aliaa A. Abd-elaziz, and Essam S. Soliman. 2022. Insecticidal Efficacy and Safety of Phoxim and Influence on Hematological, Biochemical, and Antioxidant Profiles in German Shepherd Dogs. Open Veterinary Journal, 12 (6), 888-902. doi:10.5455/OVJ.2022.v12.i6.15 Chicago Style El-maghraby, Mamdouh M., Ahmed E. Mahmoud, Dina A. Abdelkhalek, Nahla H. Sallam, Abdelfatah N. Aly, Aliaa A. Abd-elaziz, and Essam S. Soliman. "Insecticidal Efficacy and Safety of Phoxim and Influence on Hematological, Biochemical, and Antioxidant Profiles in German Shepherd Dogs." Open Veterinary Journal 12 (2022), 888-902. doi:10.5455/OVJ.2022.v12.i6.15 MLA (The Modern Language Association) Style El-maghraby, Mamdouh M., Ahmed E. Mahmoud, Dina A. Abdelkhalek, Nahla H. Sallam, Abdelfatah N. Aly, Aliaa A. Abd-elaziz, and Essam S. Soliman. "Insecticidal Efficacy and Safety of Phoxim and Influence on Hematological, Biochemical, and Antioxidant Profiles in German Shepherd Dogs." Open Veterinary Journal 12.6 (2022), 888-902. Print. doi:10.5455/OVJ.2022.v12.i6.15 APA (American Psychological Association) Style El-maghraby, M. M., Mahmoud, . A. E., Abdelkhalek, . D. A., Sallam, . N. H., Aly, . A. N., Abd-elaziz, . A. A. & Soliman, . E. S. (2022) Insecticidal Efficacy and Safety of Phoxim and Influence on Hematological, Biochemical, and Antioxidant Profiles in German Shepherd Dogs. Open Veterinary Journal, 12 (6), 888-902. doi:10.5455/OVJ.2022.v12.i6.15 |