| Research Article | ||
Open Vet J. 2024; 14(7): 1596-1606 Open Veterinary Journal, (2024), Vol. 14(7): 1596–1606 Research Article Pathophysiological and pro-inflammatory cytokine surveys on livestock normally infected with Taenia saginata cysticercosisAyed Alshammari1, Abdulbaset Mohammad Kabli2 and Naser Abdelsater3*1Department of Biology, College of Science, University of Hafr Al-Batin, Hafr Al-Batin, 39511, Saudi Arabia 2Department of Laboratory Medicine, Faculty of Applied Medical Sciences, Al-Baha University, Al-Baha, Saudi Arabia 3Zoology Department, Faculty of Science, Al-Azhar University (Assiut Branch), Assiut, 71524, Egypt *Corresponding Author: Naser Abdelsater, Zoology Department, Faculty of Science, Al-Azhar University (Assiut branch), Assiut, Egypt. Email: N.Alazaly [at] azhar.edu.eg Submitted: 14/04/2024 Accepted: 26/06/2024 Published: 31/07/2024 © 2024 Open Veterinary Journal
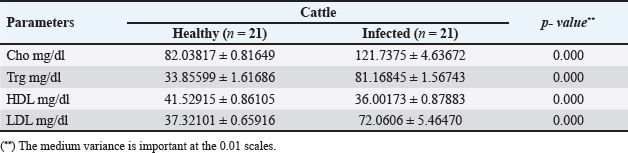
AbstractBackground: Cattle and buffaloes can contract cysticercosis, an infection of the muscles brought on by Taenia saginata larvae. Despite having a global spread, cysticercosis is more prevalent in impoverished nations due to impaired hygiene standards. It has been discovered that Taenia saginata cysticercosis routine visual diagnosis is not very effective, especially in mild infections. Therefore, a more trustworthy in vivo test might be used as an alternative in slaughterhouses and epidemiological studies. Biochemical assays are possibly utilized as an alternative to detect cysticercosis inside a topical environment. Aim: Investigating serum biochemical alterations in cattle with cysticercosis was the goal of the current research. As a further method of diagnosis, it was also determined how Cysticercus bovis affected pro-inflammatory cytokines and histopathology. Methods: Blood samples from 42 slaughtered cattle (21 healthy and 21 sick animals) were taken from Assiut abattoir. Using an ELISA and spectrophotometer, respectively, their serum's pro-inflammatory cytokines and biochemical profile were evaluated. These cattle were chosen between March 2023 and February 2024. Results: A percentage of 4.6% of the 455 cattle examined after being slaughtered had T. saginata cysticerci infections. All values in the serum biochemistry were considerably different (p < 0.01), whereas the majority of biochemical parameters increased significantly (p < 0.01) in infected animals. In contrast, there was a substantial (p < 0.01) decline in HDL-c, SOD, CAT, and GSH. On the other hand, procytokine inflammatory indices for both TNF-α and IL-1β indicated a substantial increase (p < 0.01) in infected cattle. Additionally, the histological results revealed significant alterations in the tissues of infected livestock. Conclusion: This has been inferred cysticercosis possesses negative impacts on cattle's plasma biochemical profiles, indicating the field applicability of biochemical measures in outbreaks of bovine cysticercosis. Pro-inflammatory cytokine indices and histological changes could be included as further indicators of T. saginata cysticercosis in cattle. Keywords: Taenia saginata, Cysticercus bovis, Cattle, Serum enzymes, Pro-inflammatory cytokine. IntroductionCysticercosis is a muscle contagion brought on by the maggot phase of Taenia saginata (Hancock et al., 1989). Taenia saginata is an economically and medically significant cestode parasite (McFadden et al., 2011). Taenia saginata cysticercosis is prevalent throughout the world, although it has the paramount financial influence and communal soundness inferences in the tropics and subtropics (Abdo et al., 2009). After consuming T. saginata eggs (proglottids) from infected humans, cattle get infected as intermediate hosts. After about 10 weeks, cysticerci develop in cow muscle and become infective to individuals (Flisser et al., 2005; McFadden et al., 2011). The disease is found in animals and humans worldwide (Minozzo et al., 2002), with varying prevalence (Doyle et al., 1997). Cysticercosis is more common in underdeveloped nations due to poor sanitation and the ingestion of pickled or insufficiently stewed or sun-dried flesh (Frolova, 1982; Symth, 1994; Minozzo et al., 2002). The illness is also an issue in advanced nations where underdone steak is commonly consumed as a repast. It is competence noting that even great-ranking flesh inspection in developed-country butchery has not resulted in the eradication of this scrounger (Frolova, 1982; Symth, 1994; Eddi et al., 2003). According to (Neva and Brown, 1994; Dural et al., 2015; Symeonidou et al., 2018), intestinal blockage, weakness, weight loss, nausea, vomiting, gastroenteritis, and abdominal discomfort are all signs of cysticercosis in humans. Rarely do movable gravid segments cause a major condition like the appendix or biliary tract infestation. Live cattle with cysticercosis do not exhibit any symptoms, but due to a large infestation of the grubs, there may be inflammatory cardiomyopathy or cardiac defect (Gracey and Collins, 1992; Belay and Afera, 2014). Economic losses in the meat sector are closely related to infection status. If a corpse is determined to have a severe infection or generalized cysticercosis, it must be destroyed. Light cysticercosis results in the confiscation of the diseased-ridden sections; additionally, the cadaver must be held in reserve in a chilly warehouse at a hotness not transcending –70°C for 21 days to incapacitate the spongers (Kandil et al., 2004; Scandrett et al., 2009; Ibrahim and Zerihum, 2012). Cysticercosis cannot be detected in live animals by antemortem testing, hence postmortem analysis of the carcass is the only method used to make the diagnosis at abattoirs. The existing tests (ELISA) need a lot of time, money, and effort to obtain. Therefore, it is beneficial for diagnostics to create some appropriate tests that can recognize diseased animals before slaughter. The tests help to find the disease's origin and may aid in disease management. In the domestic circumference and to meet the requirements of universal exordium for livestock with a sufficient level of reliance, hematological and biochemical trials were possibly performed as an alternative to the verification of Cysticercus bovis (Wrights, 1998; Saeed et al., 2016). Damage to the host (pig) liver tissues by T. saginata asiatica cysticerci results in metabolic disorders of glycogen, fats, and protein, and alterations in ferment anabolism (Linghu et al., 2007). Myocarditis and heart failure in cattle may result from a severe infestation of T. saginata cysticerci (Gracey and Collins, 1992; Belay and Afera, 2014). The literature on the variations in the serum biochemical factors of livestock infected with T. saginata cysticerci is scarce, nevertheless. The objective of the current investigation was to use serum biochemistry to identify Taenia saginata cysticercosis in slaughtered cattle. It was also done to look for changes in pro-inflammatory markers and histopathological changes caused by this infection. Materials and MethodsSamples selection and collection of bloodAt the Assiut abattoir in the Assiut governorate, blood samples were collected from 42 slaughtered cattle. Twenty-one healthy cattle and 21 cysticercosis cattle out of 455 detected by post-mortem analysis were used for biochemical analysis. Each animal's jugular vein was used to collect 5 ml of blood, which was then placed in clean, dry tubes and allowed to clot before the serum was centrifuged out and kept at –20°C to be used for biochemical and pro-inflammatory cytokine analysis. Biochemical determinationsBiochemical analysis was achieved spectrophotometrically as aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and creatine kinase (CK-total) vigors were specified to utilize kits obtained from Human Gesell Schaftfur Biochemical und Diagnostic mbH, Germany. Serum total cholesterol, triglycerides, LDL-cholesterol, and HDL-cholesterol grades were specified via kits purchased from DiaSys Diagnostic System GmbH, Germany. Serum glutathione (GSH), lipid peroxidation (Malondialdehyde) (MDA), and nitric oxide (NO) grades, in addition to that superoxide dismutase (SOD), and catalase (CAT) vigors were valued via kits acquired from Biodiagnostic, Dokki, Giza, Egypt. Determination of pro-inflammatory cytokineUsing ELISA (Dynatech Microplate Reader Model MR 5000, 478 Bay Street, Suite A213 Midland, ON, Canada), serum TNF-α and IL-1β concentricities were mensurated by mouse Elisa kits obtained from SinoGeneClon Biotech Co., Ltd, No.9 BoYuan Road, YuHang District 311112, Hang Zhou, China. Histopathological studyCarcasses were meticulously examined at the time of slaughter, and C. bovis-infected hearts were taken to the pathology lab. Heart samples were then preserved for an overnight period in neutral buffered formalin (10%). The specimens were subjected to dryness in progressive C2H5OH, purified in xylene, and implanted in paraffin, and then have been sectioned tissue into thin sections measured at 5 µm. Then, tissues were deparaffinized, and then stained with H&E, finally it was examined by a light microscope (Sethiya et al., 2014). Statistical analysisFor the datum investigation, the SPSS 25 program was applied. To come across the data, medium ± SD were listed. To statistically compare mean differences in measured variables between healthy and infected animals, a T-test was employed. The current data was tested at a significance level (p ≤ 0.05). Ethical approvalThis study was licensed by the Scientific Committee of the College of Science - Al-Azhar University (Assiut Branch), Egypt. Certificate reference number: AZHAR 12/2023. ResultsThe prevalence of C. bovis was 4.6% in the current investigation employing meat inspection, which was recorded among 455 investigated cattle in Egypt. In Table 1 and Figure 1, the serum cholesterol, triglyceride, LDL, and HDL concentrations of infected and uninfected cattle with cysticercosis are shown. When compared to the control group, T. saginata cysticercosis infection caused a significantly higher level of serum cholesterol, triglycerides, and LDL while also causing a significantly lower amount of HDL. Table 1. Mean ± SD of lipids profile measured in the serum of healthy and C. bovis -infested Cattles (n=21).
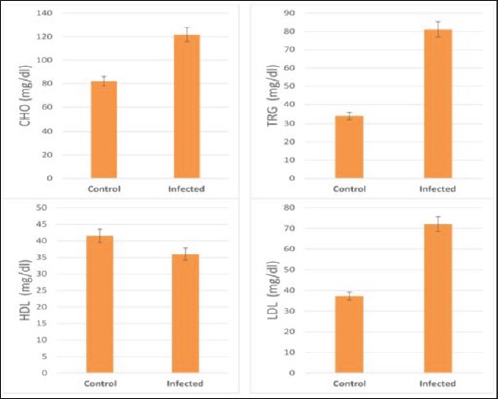
Fig. 1. Serum lipids profile in the control and infected livestock with C. bovis. (Mean ±SD, N=21). Table 2. Mean ±SD of enzymes measured in the serum of healthy and C. bovis-infested cattle (n=21).
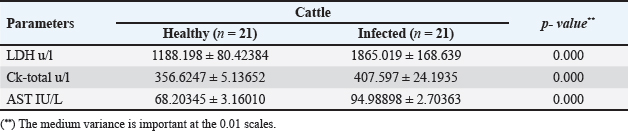
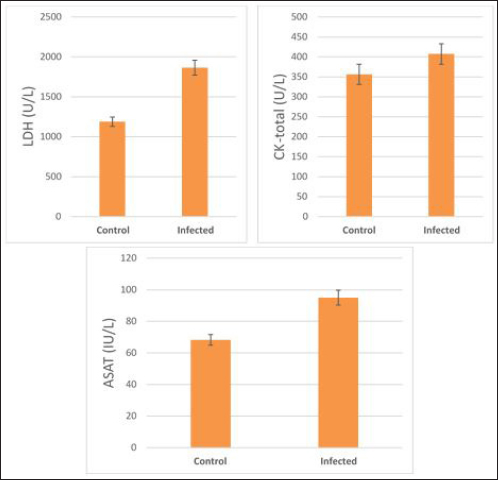
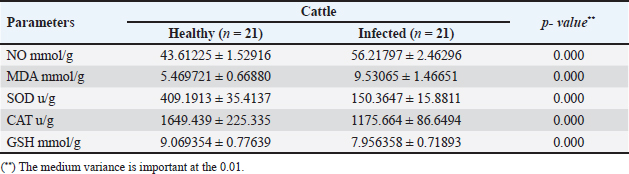
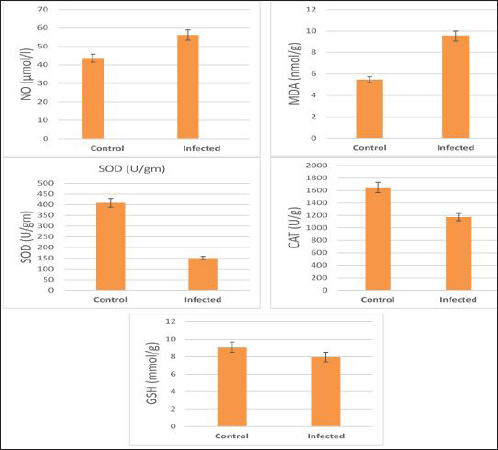
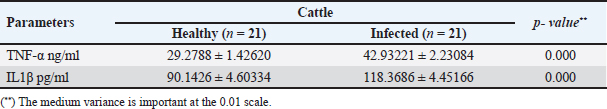
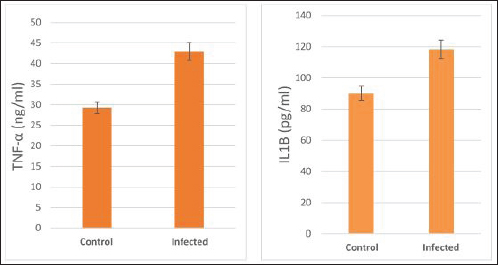
The activity of AST, CK-total, and LDH in the serum of infected cattle showed a substantial rise (p < 0.01, Table 2, and Fig. 2). In cow serum infected with cysticercosis, there is a statistically significant decrease in antioxidant enzymes (SOD, CAT, and GSH). In contrast, there is a statistically significant rise in MDA and NO levels compared to control samples, which is a biomarker of lipid peroxidation (Table 3 and Fig. 3). Our findings demonstrated the influence of T. saginata cysticercosis on particular immunological markers in cattle serum. In rapprochement to the controller set, the grades of TNF-α, and IL1β, were observed to be considerably higher in the infected group (Table 4 and Fig. 4).
Fig. 2. Serum enzymes in the control and infected livestock with C. bovis. (Mean ±SD, N=21). Table 3. Mean ±SD of oxidative stress markers measured in the serum of healthy and C. bovis -infested Cattles (n=21).
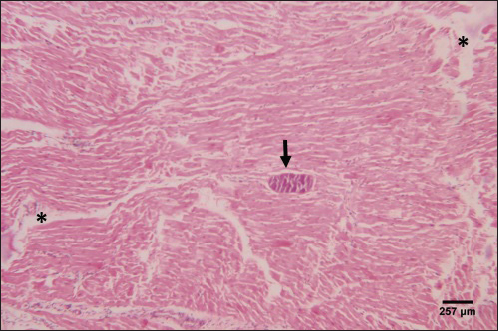
The histological structure of the cardiac myocytes was normal, with central oval nuclei and acidophilic sarcoplasm. In the intercellular gaps, a few tiny blood capillaries could be seen (Figs. 5 and 6). A cross-section of a proglottid of T. saginata revealing thick outer tegument, degenerating myocardiocytes surrounding it, and altered histological architecture was visible in the cardiac muscle tissue of infected calves. Mononuclear infiltration, cardiac oedema, and hemorrhage were all signs of a mild inflammatory reaction (Figs. 7 and 8). DiscussionIn this study, 4.6% of cattle were infected with C. bovis. Our outcome is deemed upper than formerly logged by Haridy et al. (1999) (0. 23%), Rodriguez-Hidalgo et al. (2003), (0. 37%), Abdo et al. (2009) (1. 65%), Kandil et al. (2012a,b) (4%) and (4.4%), respectively, Yassien et al. (2013), (0.58%), El-Alfy et al. (2017) (0.51%), and Abdel Aziz et al. (2022) (0.47%). Contrarily, our study found fewer instances of C. bovis than did Oryan et al. (1995) (7. 7%), Abdel-Hafeez et al. (2015) (20 %), and Dyab et al. (2017) (7.5 %). The variance in infection rates may be brought on by epidemiological elements such as climate, various sanitation practices, the number of samples taken, and the use of control methods and programmes (Usip et al., 2011).
Fig. 3. Serum oxidative stress in the controller and infected livestock with C. bovis. (Mean ±SD, N=21. Table 4. Mean ±SD of pro-inflammatory cytokines measured in the serum of healthy and C. bovis-infested Cattles (n=21).
Increased values of cholesterol, triglycerides, and LDL of infected cattle with decreased HDL were seen in this study. These findings conflict with those of (Kandil et al., 2012b; Saeed et al., 2016), who discovered that total cholesterol was present in the sera of infected cattle in low concentrations. Decreases in cholesterol may result from the function that cholesterol moves in aetiology by allowing the grubs to survive in steward tissues, as suggested by Kandil et al. (2012b), or they may result from commotions in hepatic enzymes and changes in hormonal exudation triggered by parasite existence. Furthermore, it was shown that adding cholesterol to the RPMI-1640 culture medium increased the survival of ascariasis larval growth, and it may be that certain laborers or ferments enable the parasite to break down and ingest fats/cholesterol (Urban et al., 1984; Wiedermann et al., 1991). In contrast, Scare et al., (2019) found that the addition of cholesterol did not improve worm longevity or viability. The activity of enzymes in the serum of infected cattle showed values higher than uninfected ones. Hepatic impairment may result from an abundance of C. bovis (Scandrett et al., 2009). Our discoveries are regular with those of (Pathak and Gaur 1981; Bamorovat et al., 2014), who found that goats empirically disease-ridden with Cysticercus tenuicollis had elevated AST, ALT, and ALP levels. They claimed that the significant loss of liver parenchymal cells brought on by grubs transmigration may be the reason for this increase. ALT, AST, and ALP levels rose in C. tenuicollis- infective pigs, according to Pathak et al. (1984), who also linked these alterations to the metacestode's migration. Infected swine with C. cellulosae had significantly higher levels of ALT, AST, ALP, and LD, according to Pathak and Gaur (1985), who speculated that these alterations might be the result of muscle tissue being harmed by this metacestode. In addition to that ALT, AST, ALP, and GGT levels rose in adult goats infected with liver flukes, according to Aslam et al. (2020), who also linked these alterations to the larval migration. Also, Singh et al. (2004) reported an increase of AST levels 2 weeks post-infection synchronizing with the migratory phase of Fasciola hepatica in the liver parenchyma. They reported that liver damage is the most important cause of the increase in serum ALT activity in the infected sheep. While Radfar et al. (2014) noted a notable rise in ALT, AST, and ALP in goats with C. tenuicollis infection. Calves that had been empirically infected with 85 thousand and 200 thousand T. saginata ova showed elevated CK activity, according to (Blazek et al., 1985; Kursa et al., 1986). In addition to that, Anwar et al. (2024) showed a significant increase in LDH activity in infected animals. On the other hand, the current findings conflict with (Kandil et al. 2012b; Saeed et al., 2016). According to (Pinzani and Rombonts, 2004; Otto et al., 2010), some large digits of cysts and consequent continuing deterioration of the liver parenchyma may be to blame for the drop in AST activity in infected cattle.
Fig. 4. Serum pro-inflammatory cytokines in the control and infected livestock with C. bovis. (Mean ±SE, N=21).
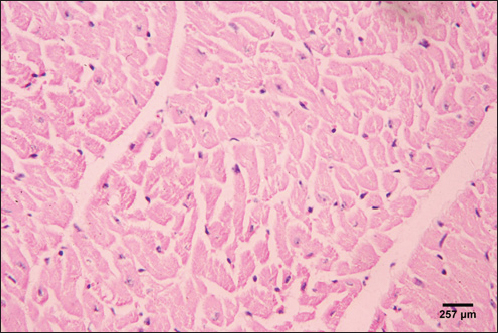
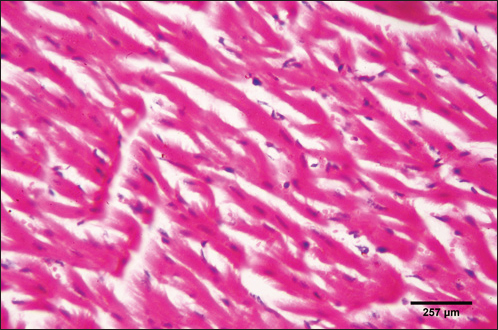
Fig. 5. Photomicrograph of the ventricle of control cattle demonstration of a regular arrangement of cardiac muscle fibers, acidophilic sarcoplasm and central oval vesicular nuclei of the cardiac myocytes (H&E, low power).
Fig. 6. Photomicrograph of the ventricle of control cattle demonstration of normal cardiac myocytes with acidophilic sarcoplasm and central oval vesicular nuclei and normal histological appearance (H&E, high power).
Fig. 7. Photomicrograph of the ventricle of infected cattle demonstration irregular and disturbed arrangement of cardiac muscle fibers with mild cardiac edema (*). cross-section of a proglottid of Taenea sp. showing thick outer tegument (short arrow) (H&E, low power). In our results, cattle serum infected with cysticercosis shows a decrease in antioxidant enzymes, while oxidative stress levels rise. These findings concur with (Atteya et al., 2015). When parasites are present, host cells typically suffer an oxidative assault. According to Kataria et al. (2010), alterations in antioxidant capacity (oxidative stress) contribute to parasite persistence in host tissues and are followed by pathological changes and damage that result in the development of various clinical disorders.
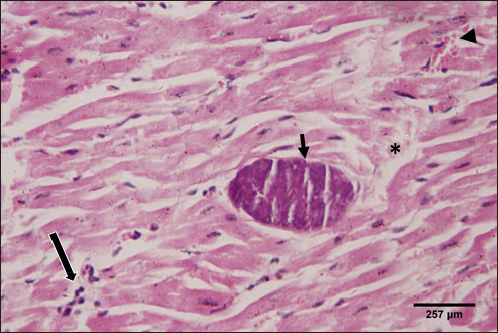
Fig. 8. Photomicrograph of the ventricle of infected cattle demonstration irregular and disturbed arrangement of cardiac muscle fibers. Few myocytes represent degenerated nuclei and minimal vacuolization of the cytoplasm with a disarrangement pattern (*) around the proglottid of Taenea sp. showing thick outer integument (short arrow). Mild haemorrhage was noticed (arrow-head) and infiltration of inflammatory cells (long arrow) (H&E, high power). When compared to healthy animals, cows and buffalo with fascioliasis and cysticercosis had lower antioxidant values. According to El-Moghazy (2011) and Atteya et al. (2015), while MDA levels are rising, CAT, SOD, GSH, and GST activity is declining. Infected sheep with distomatosis had lower levels of non-enzymatic antioxidants and antioxidant ferment actions in their livers than the non-infected group (Kolodziejczyk et al., 2005; Değer et al., 2008). Total antioxidant status decreased, indicating a reduction in the antioxidant capability of the Fasciola hepatica-infected rat liver tissue. Increased oxidative alterations of lipids and proteins were the outcome of diminished antioxidant capacities (Siemieniuk et al., 2008). Additionally, there is a statistically considerable rise in the action of the antioxidant ferment SOD in the T. saginata-disease-ridden skeletal muscles compared to the non-infected cattle's control value (Luszczak et al., 2011). Reactive oxygen species (ROS) are released as a result of altered liver and heart parasites' antioxidant capacities, causing damage to cell membranes and other components that ultimately result in cell death. Once the pathogen has been eradicated, the cell is equipped with several antioxidant defense enzymes that protect the cell from oxidative damage (Belkaid, 2007). To defend their tissues from inflaming damage, hosts have an administrative web built on cytokines that control the resolve of inflaming (Belkaid, 2007). Our findings demonstrated that TNF-α and IL1β were higher in the infected group. The current returns are compatible with Palma et al. (2019), who examined the variations in the oncosphere growth of Taenia solium and T. saginata. Analyze the manifestation of cytokines and MMPs in human peripheral blood mononuclear cells (PBMCs) after these activated oncospheres (AO) and postoncospheral (PO) antigens stimulated them. Rats received intracranial inoculations of the AO and PO styles of either T. solium or T. saginata. Neurocysticercosis (NCC) was manifested in rats infected with the AO and PO shapes of T. solium but not in those infected with T. saginata. Antigens of the AO and PO shapes of both species were used to stimulate human PMBCs, and the amount of cytokines and MMPs produced was assessed. In comparison to T. saginata AO, the T. solium AO antigen triggered a greater generation of IL-4, IL-5, IL-13, IFN-γ, and IL-2 cytokines. When contrasted to T. solium, the PO antigen from T. saginata boosted the construction of the cytokines IL-4, IL-5, IL-13, IFN-γ, IL-1β, IL-6, IL-10, TNF-α, and IL-12. Due to the early inflammatory response in cardiac muscle and the increased visibility of macroscopic lesions, the cardiac was validated as the ideal location for the diagnosis of cysticercosis (Scandrett et al., 2009). Heart inflammation brought on by the emergence of a pseudoepithelial boundary and a granulation tissue zone was the heart's reaction to the presence of C. bovis. Later, when the Cysticercus began to experience necrotic alterations, a new type of inflammatory response began to emerge (Sterba et al., 1979; Kumar et al., 2013). In the current investigation, routine H&E staining and histological analysis of the control group's heart muscles revealed a regular arrangement of cardiac muscle fibers with what appeared to be normal striations and branching. The histological results revealed significant alterations in the tissues of infected livestock. These findings are consistent with those of (Atteya et al., 2015), who demonstrated that microscopic lesions in the heart included cellular infiltration of lymphocytes, eosinophils, plasma cells and macrophages, necrosis of the tissue, proliferation of fibroblasts and development of granulation tissue along with the disintegration of cysts. Hashemnia et al. (2016) examined the ovine cysticercosis pathological lesions in sheep that had been killed. Severe degenerative and necrotic changes in the muscle fibers and also the sections of cysticerci in the affected organs were surrounded by a zone of degenerative, necrotic changes and polymorphonuclear leucocytes, lymphocytes, and fibroblasts were the predominant histopathological abnormalities. Abdel Aziz et al. (2022) showed that, severe fibrosis among cardiac muscles, fibrous tissue infiltrated with mononuclear inflammatory cells, and necrosis and atrophy in cardiac myocytes. ConclusionIn the epilogue, cysticercosis has detrimental influences on the serum biochemical profile in livestock, and trialing these factors can be used as a backup experiment to diagnose T. saginata cysticercosis in lifelike livestock and assess the precision of the flesh-checking transaction for detecting cysticercosis in livestock cadavers. AcknowledgmentThe authors would like to thank Dr. Mostafa Abozeid, the veterinarian at Assiut abattoir, for his assistance in collecting the infected organs and blood samples from the infected animals. Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Authors’ contributionAA provided the study’s concept, while the methodology, validation, and formal analysis were created by NA. The investigations, preparation of the original draft, revision of the manuscript, and review and editing of the paper were carried out by AA and AMK. The version that was presented and written by all authors has been confirmed. FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Data availabilityEvery dataset is included in the publication. ReferencesAbdel Aziz, A., AbdAlrahman, N., El-Seify, M. and Sultan, K. 2022. Cysticercus bovis at Sohag Province: prevalence, morphological and molecular characterizations. Sohag J. Jun. Sci. Res. 2, 23–32. Abdel-Hafeez, E.H., Kamal, A.M., Abdelgelil, N.H. and Abdel-Fatah, M. 2015. Parasites transmitted to humen by ingestion of different types of meat, El-Minia City, El-Minia governorate. Egypt. J. Egypt. Soc. Parasitol. 45, 671–680. Abdo, B.R.N., Sayed, A.S.M., Hussein, A.A.A. and Arafa, M.I. 2009. Occurrence of cisticercosis in cattle and buffaloes and Taenia saginata in man in Assuit governance of Egypt. Vet. World. 2, 173–176. Anwar, F.A.S., Negm, E.A., Abdelhaseib, M., Abdel-Maksoud, F.M., Mohammed, A.A., Mohamed, S.A., Gareh, A., Elbarbary, N.K., El-Khadragy, M.F., Hassan, E.A. and Elmahallawy, E.K. 2024. High prevalence of bovine cardiac cysticercosis in upper Egypt: an epidemiological and histopathological study. Animals (Basel). 14, 158–174. Aslam, A., Akbar Khan, S., Tunio, M.T. and Shehzad, M. 2020. Hematobiochemical alterations and gross pathology of liver fluke infestation in goat (Capra hircus) in Poonch Azad Kashmir. Pure Appl. Biol. 9, 595–608. Atteya, M.A., Wahba, A.A., Ghobashy, M.A. and Dessouky, A.A. 2015. Oxidative stress and histopathological changes in cattle affected with fascioliasis and cysticercosis. Egypt. J. Med. Sci. 36, 191–204. Bamorovat, M., Radfar, M.H., Derakhshanfar, A., Molazadeh, M. and Zarandi, M.B. 2013. A comparative evaluation of hematological, biochemical and pathological changes among infected sheep with Cysticercus tenuicollis and non-infected control group. J. Parasit. Dis. 38, 399–403. Belay, S. and Afera, B. 2014. Prevalence of Cysticercus bovis in cattle at municipal abbatoir of Shire. J. Vet. Sci. Technol. 5, 1–3. Belkaid, Y. 2007. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7, 875–888. Blazek, K., Kursa, J., Schramlova, J. and Prokopic, J. 1985. Contribution to the symptomatology of experimental bovine cysticercosis. Folia Parasitol. (Praha). 32, 323–332. Değer, Y., Ertekin, A., Değer, S. and Mert H. 2008. Lipid Peroxidation and antioxidant potential of sheep liver infected naturally with distomatosis, Turkiye Parazitol. Derg. 32, 23–26. Doyle, M., Beuchat, L. and Montaville, T. 1997. Food microbiology. Fundamentals and Frontiers. Center for Food Safety and Quality Enhancement. Washington, DC. Department of Food Science and Technology, University of Georgia. Dural, A.C., Celik, M.F., Temizgonul, B., Unsal, M.G., Akarsu, C., Gonenc, M., Kalayci, M.U. and Alis, H. 2015. Unusual clinical case: extraluminal manifestation of a tapeworm from the eviscerated midline incision in a post-surgery patient. J. Infect. Dev. Ctries. 9, 428–430. Dyab, A.K., Marghany, M.A., Othman, R.A., Ahmed, M.A. and Abd- Ella, O.H. 2017. Taenia Saginata in man and cysticercosis in cattle and buffaloes in Aswan Governorate, Egypt. J. Egypt. Soc. Parasitol. 47, 389–394. Eddi C., Nari, A. and Amanfu, W. 2003. Taenia solium cysticercosis/ taeniosis: Potential linkage with FAO activities; FAO support possibilities. Acta Trop. 87, 145–148. El-Alfy, E.N., Al-Kappany, Y.M. and Abu-Elwafa, S.A. 2017. Parasitological and pathological studies on Tissue parasites among slaughtered animals in Dakahlia province, Egypt. Egypt. Vet. Med. Soc. Parasitol. J. 13, 78–98. El-Moghazy, F.M. 2011. Effect of parasitic infestation on oxidant-antioxidant status in buffaloes. Middle East J. Sci. Res. 7, 585–593. Flisser, A., Correa, D., Avilla, G. and Marvilla, P. 2005. Biology of Taenia solium, Taenia saginata and Taenia saginata asiática. In: WHO/FAO/OIE guidelines for the surveillance, prevention and control of taeniosis/cisticercosis. Ed., Murrel. K.D., Paris, France: OIE, p: 1–9. Frolova, A. 1982. Epidemiology of Taeniasis. In: Zoonoses Control. Collection of Teaching Aids for International Training Course. Ed., Lysenko, A, Moscow, Russia: Center of International Projects GKNT, vol. 2, pp: 202–207 Gracey, J.F. and Collins, D.S. 1992. Meat Hygiene. Philadelphia, PA: W.B. Saunders Company Ltd., pp: 669–678. Hancock, D., Wikse, S., Lichtenwalner, A., Wescott, R. and Gay, C. 1989. Distribution of bovine cysticercosis in Washington. Am. J. Vet. Res.50, 564–570. Haridy, F.M., Ibrahim, B.B., Morsy, T.A. and Ramadan, N.I. 1999. Human taenaisis and cisticercosis in slaughtered cattle, buffaloes and pigs in Egypt. J. Egypt. Soc. Parasitol. 29, 375–394. Hashemnia, M., Shahbazi Y. and Frajani Kish, G. 2016. Prevalence and pathological lesions of ovine cysticercosis in slaughtered sheep in western Iran. J. Parasit. Dis. 40, 1575–1578. Ibrahim, N. and Zerihum, F. 2012. Prevalence of Taenia saginata cysticercosis in cattle slaughtered I Addis Ababa municipal abattoir, Ethiopia. Glob. Vet. 8, 467–471. Kandil, O.M., Mahdy, O.A., Abou-El-Doubal, S.K. and Mousa, W.M. 2004. Purification and characterization of the three larval taeniid antigens by gel filtration. Vet. Med. J. 52, 449–456. Kandil, O.M., Mahmoud, M.S., Shalaby, H.A., El Namaky, A.H., Hendawy, S.H.M. and Arafa M.I. 2012a. Value of Taenia saginata crude antigen in diagnosis of bovine cysticercosis with reference to its characterization. Glob. Vet. 9, 474–478. Kandil, O.M., Nasr S.M., Mahmoud, M.S., Nassar, S.A., El-Metanawey, T., Abd El-Aziz, M.A. and Abu El Ezz, N.M.A. 2012b. Serological and biochemical studies on cattle naturally infested with Taenia saginata cysticercosis. Glob. Vet. 9, 571–579. Kataria, N., Kataria, A.K., Maan, R. and Gahlot, A.K. 2010. Evaluation of oxidative stress in brucella infected cows. J. Stress Physiol. Biochem. 6, 19–25. Kolodziejczyk, L., Siemieniuk, E. and Skrzydlewska, E. 2005. Antioxidant potential of rat liver in experimental infection with Fasciola hepatica. Parasitol. Res. 96, 367–372 Kumar, S.N., Prasad, T.S., Narayan, P.A. and Muruganandhan, J. 2013. Granuloma with langhans giant cells: An overview. J. Oral Maxillofac. Pathol. 17, 420–423. Kursa, J., Blazek, K. and Schramlova, J. 1986. Clinical picture and haematological findings in experimental Cysticercus bovis. Sbornik Vyoke Skoly Zemedelske V Praze Fakulta Agronomika B Zivocisna Vyroba. 1, 89–108. Linghu, Y., Zhu, W., Bao, H. and Chen, Y. 2007. Observations on pathological and histochemical changes in piglet livers infected with Taenia saginata asiatica. Front. Med. China 1, 258–263. Luszczak, J., Ziaja-Soltys, M. and Rzymowska, J. 2011. Anti-oxidant activity of superoxide dismutase and glutathione peroxidase enzymes in skeletal muscles from slaughter cattle infected with Taenia saginata. Exp. Parasitol. 128, 163–165. McFadden, A.M.J., Heath, D.D., Morley, C.M. and Dorny, P. 2011. Investigation of an outbreak of Taenia saginata cysts (Cysticercus bovis) in dairy cattle from two farms. Vet. Parasitol. 176, 177–184. Minozzo, J.C., Gusso, R.L.F., de Castro, E.A., Lago, O. and Soccol, V.T. 2002. Experimental bovine infection with Taenia saginata eggs: recovery rates and cysticerci location. Brazilian Arch. Biol. Tech. 45, 45–1455. Neva, A.F. and Brown, W.H. 1994. Basic clinical parasitology. 6th Ed. Hoboken, NJ: Prentice-Hall International Inc. pp: 181–200. Oryan, A., Moghaddar, N. and Gaur, S.N.S. 1995. Taenia saginata cysticercosis in cattle with special reference to its prevalence, pathogenesis and economic implications in Fars Province of Iran. Vet. Parasitol. 57, 319–327. Otto, M.A., Da Silva, A., Wolkmer, S.P., Traesel, C.K., Schmidt, C., Tonin, A.A.R.A., Zanette, R.A., Lopes, S.T.A. and Monteiro, S.G. 2010. Levels of liver enzymes and urea in rats naturally infected with larval forms of Taenia taeniformis. Comp. Clin. Pathol. 19, 527–529. Palma, S., Chile, N., Carmen-Orozco, R.P., Trompeter, G., Fishbeck, K., Cooper, V., Rapoport, L., Bernal-Teran, E.G., Condori, B.J., Gilman, R.H. and Verastegui, M.R. 2019. In vitro model of postoncosphere development, and in vivo infection abilities of Taenia solium and Taenia saginata. PLoS Negl. Trop. Dis. 13, e0007261. Pathak, K.M.L. and Gaur, S.N.S. 1981. Serum proteins in goat experimentally infected with Cysticercus tenuicollis of Taenia hydatigena. Haryana Vet. 20, 109–113. Pathak, K.M.L. and Gaur, S.N.S. 1985. Changes in serum enzyme activities in pigs naturally infected with the metacestodes of Taenia soliurn. Vet. Res. Commun. 9, 143–146. Pathak, K.M.L., Kumar, M. and Gaur, S.N.S. 1984. Changes in blood cellular components, serum proteins and serum enzyme activities in pigs naturally infected with Serum Cysticercus tenuicollis. Res. Vet. Sci. 36, 263–265. Pinzani, M. and Rombonts, K. 2004. Liver fibrosis: from the bench to clinical targets. Dig. Liver Dis. 36, 231–242. Radfar, M.H., Zarandi, M.B., Bamorovat, M., Kheirandish, R. and Sharifi, I. 2014. Hematological, biochemical and pathological findings in goats naturally infection with Cysticercus tenuicollis. J. Parasit. Dis. 38, 68–72. Rodríguez-Hidalgo, R., Benítez-Ortiz, W., Dorny, P., Geerts, S., Geysen, D., Ron-Román, J., Proaño-Pérez, F., Chávez-Larrea, M.A., Barrionuevo-Samaniego, M., Celi-Erazo, M., Vizcaíno-Ordóñez, L. and Brandt, J. 2003. Taeniosis- cisticercosis in man and animals in the Sierra of Northern Ecuador. Vet. Parasitol. 118, 51–60. Saeed, M., Durrani, A.Z., Khan, M.A., Maqbool, A., Avais, M., Younus, M., Aqib, A. I., Ahmad, I. and Ijaz, M. 2016. Cysticercus bovis induced hemato-biochemical changes in cattle and buffaloes. J. Anim. Plant Sci. 26, 1187–1190. Scandrett, B., Parker, S., Forbes L., Gajadhar, A., Dekumyoy, P., Waikagul, J. and Haines, D. 2009. Distribution of Taenia Saginata Cysticerci in tissues of experimentally infected Cattle. Vet. Parasitol. 164, 223–231. Scare, J.A., Steuer, A.E., Shaffer, C.L., Slusarewicz, P., Mousley, A. and Nielsen, M.K. 2019. Long live the worms: methods for maintaining and assessing the viability of intestinal stages of Parascaris spp. in vitro. Parasitology. 146, 685–693. Sethiya, N.K., Trivedi, A. and Mishra, S. 2014. The total antioxidant content and radical scavenging investigation on 17 phytochemical from dietary plant sources used globally as functional food. Biomed. Prev. Nutr. 4, 439–444. Siemieniuk, E., Kolodzicjczyk, L. and Skrzydlewska, E. 2008. Oxidative modification of rat liver cell components during Fasciola hepatica infection. Toxicol. Mech. Methods. 18, 519–524. Singh, J., Bal, M., Aradhana, S. and Gumber, S. 2004. Efficacy of different flukicides against fascioliosis in sheep and goats. J. Res. 41, 287–289. Sterba, J., Dykova I. and Machnicka, B. 1979. Tissue reaction in the heart of cattle with a spontaneous and artificial Cysticercus bovis infection. Folia Parasitol. (Praha) 26, 27–33. Symeonidou, I., Arsenopoulos, K., Tzilves, D., Soba, B., Gabriël, S. and Papadopoulos, E. 2018. Human taeniasis/cysticercosis: a potentially emerging parasitic disease in Europe. Ann Gastroenterol. 31, 406–412. Symth, J.D. 1994. Introduction to Animal Parasitology. 3rd ed. New York, NY: Cambridge University Press. Urban, J.F., Douvres, F.W. and Xu, S. 1984. Culture requirement of Ascaris suum larvae using a stationary multi-well system: increased survival, development and growth with cholesterol. Vet. Parasitol. 14, 33–42. Usip, L., Isaac, L., Amadi, E., Utah, E. and Akpaudo, U. 2011. The occurrence of cysticercosis in cattle and taeniasis in man in Uyo, capital city of Akwa Ibom state, Nigeria. Niger. J. Agric. Food Environ. 7, 47–51. Wiedermann, U., Stemberger, H., Unfried, E., Widhalm, K., Kundli, M., Altenriederer, M., Savedra, M. and Wiedermann, G. 1991. Intestinal worm burden and serum cholesterol or lipid concentration in a Shipibo population (Peru). Zentrabl Bakteriol. 275, 279–286. Wrights, P.F. 1998. International Standards for Test Methods and Reference Sera for Diagnostic Tests for Antibody Detection. Rev. Sci. Tech. 17, 527–549. Yassien, M.A., Ahmed, A.M., Soliman., S.A. and Youssef, A. I. 2013. Prevalence of parasitic infection of slaughtered cattle and buffalo in Municipal Abattoir at Ismailia, Egypt. In: Parasitic Zoonoses in Asian-Pacific Region Eds., Tokoro, M., Uga, S, Sankeisha Publishing Agent, 19–24. | ||
| How to Cite this Article |
| Pubmed Style Alshammari AE, Kabli AM, Youssef NA. Pathophysiological and pro-inflammatory cytokine surveys on livestock normally infected with Taenia saginata cysticercosis. Open Vet J. 2024; 14(7): 1596-1606. doi:10.5455/OVJ.2024.v14.i7.9 Web Style Alshammari AE, Kabli AM, Youssef NA. Pathophysiological and pro-inflammatory cytokine surveys on livestock normally infected with Taenia saginata cysticercosis. https://www.openveterinaryjournal.com/?mno=196569 [Access: June 29, 2025]. doi:10.5455/OVJ.2024.v14.i7.9 AMA (American Medical Association) Style Alshammari AE, Kabli AM, Youssef NA. Pathophysiological and pro-inflammatory cytokine surveys on livestock normally infected with Taenia saginata cysticercosis. Open Vet J. 2024; 14(7): 1596-1606. doi:10.5455/OVJ.2024.v14.i7.9 Vancouver/ICMJE Style Alshammari AE, Kabli AM, Youssef NA. Pathophysiological and pro-inflammatory cytokine surveys on livestock normally infected with Taenia saginata cysticercosis. Open Vet J. (2024), [cited June 29, 2025]; 14(7): 1596-1606. doi:10.5455/OVJ.2024.v14.i7.9 Harvard Style Alshammari, A. E., Kabli, . A. M. & Youssef, . N. A. (2024) Pathophysiological and pro-inflammatory cytokine surveys on livestock normally infected with Taenia saginata cysticercosis. Open Vet J, 14 (7), 1596-1606. doi:10.5455/OVJ.2024.v14.i7.9 Turabian Style Alshammari, Ayed Eid, Abdulbaset Mohammad Kabli, and Naser Abdelsater Youssef. 2024. Pathophysiological and pro-inflammatory cytokine surveys on livestock normally infected with Taenia saginata cysticercosis. Open Veterinary Journal, 14 (7), 1596-1606. doi:10.5455/OVJ.2024.v14.i7.9 Chicago Style Alshammari, Ayed Eid, Abdulbaset Mohammad Kabli, and Naser Abdelsater Youssef. "Pathophysiological and pro-inflammatory cytokine surveys on livestock normally infected with Taenia saginata cysticercosis." Open Veterinary Journal 14 (2024), 1596-1606. doi:10.5455/OVJ.2024.v14.i7.9 MLA (The Modern Language Association) Style Alshammari, Ayed Eid, Abdulbaset Mohammad Kabli, and Naser Abdelsater Youssef. "Pathophysiological and pro-inflammatory cytokine surveys on livestock normally infected with Taenia saginata cysticercosis." Open Veterinary Journal 14.7 (2024), 1596-1606. Print. doi:10.5455/OVJ.2024.v14.i7.9 APA (American Psychological Association) Style Alshammari, A. E., Kabli, . A. M. & Youssef, . N. A. (2024) Pathophysiological and pro-inflammatory cytokine surveys on livestock normally infected with Taenia saginata cysticercosis. Open Veterinary Journal, 14 (7), 1596-1606. doi:10.5455/OVJ.2024.v14.i7.9 |