| Research Article | ||
Open Vet. J.. 2024; 14(8): 1843-1849 Open Veterinary Journal, (2024), Vol. 14(8): 1843–1849 Research Article Immunohistochemical study of scrapie in naturally affected sheep in the east of LibyaFawzia Mohamed1*, Ayiman Aboulqassim1, Monier Sharif1, Salh Belgasem2, Abraheem Omar2 and Nagi Saeed21Faculty of Veterinary Medicine, Omar Al-Mukhtar University, Al-Bayda, Libya 2Agricultural Research Center, Al-Bayda, Libya *Corresponding Author: Fawzia Mohamed. Faculty of Veterinary Medicine, Omar Al-Mukhtar University, Al-Bayda, Libya. Email: fawzia.fathi [at] omu.edu.ly Submitted: 11/04/2024 Accepted: 05/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: The most common natural prion disease that primarily affects sheep and goats is scrapie. It belongs to a group of disorders known as transmissible spongiform encephalopathies, which impact both humans and animals. Aim: The research is aimed to examine and confirm the presence of scrapie in Libya using immunohistochemistry (IHC) techniques. Methods: Brain samples were collected from thirty-three sheep older than two years of age showing clinical signs resembling to scrapie during the period between 2018 and 2023, regardless of race or gender. Three animals, six months old, healthy, and without any symptoms, were used as negative controls. Different parts of the brain, including the obex and cerebellum, were taken from each case. The IHC technique used in this study involved staining with monoclonal antibody L42 and DAB (3,3′-diaminobenzidine) as a chromogenic substrate. Results: The IHC examination showed the expression of prion proteins in brain tissue in twenty-three samples. The staining intensity was markedly observed in the neuronal cell bodies and around blood vessels. Conclusion: The findings of this study provide evidence that scrapie exists in Libya. Keywords: Immunohistochemistry, Libya, Prion, Scrapie, Sheep. IntroductionTransmissible spongiform encephalopathies (TSEs), also known as prion diseases, involve a variety of progressive neurodegenerative disorders that impact the central nervous system (CNS) of diverse mammalian species such as sheep and goats (referred to as scrapie), cattle (known as Bovine spongiform encephalopathy, or BSE), as well as humans (Creutzfeldt-Jakob disease). The etiological agent responsible for these diseases is a prion, an infectious protein (Prusiner, 1998; Corbière et al., 2013). Scrapie was first recognized as a TSE more than 250 years ago, and it continues to serve as the prototype for this group of disorders. The manifestation of this disorder mainly affected sheep and goats (Adeola et al., 2023). This disease has a slow progression without any indication of inflammation or immune reaction; therefore, affected animals show a reduced chance of early detection. The importance of this disease has experienced a recent increase, attributed to discoveries that establish a link between the scrapie prion and the bovine prion (BSE), as well as a variant that affects humans, thus strengthening its classification as a zoonotic condition. The importance of this particular species was elucidated upon the discovery that an infected goat had the ability to transmit scrapie to members of its species as well as sheep (Farias et al., 2017). The present status of scrapie in Libya remains unknown and overlooked. Within laboratories in Libya, there is a lack of utilization of confirmatory methods for the identification and validation of scrapie-infected instances. The diagnosis of scrapie disease in sheep and goats is based on the recognition of clinically observable neurological manifestations and the observation of a combination of distinctive histopathological lesions. When the typical neuroanatomic distribution of the characteristic vacuolar alterations in the brain is observed, the existence of other histologic features, such as florid plaques, astrogliosis, and neuronal loss, can aid in making a positive diagnosis but is not diagnostic in the absence of TSE neuronal vacuolations. In cases where the neuronal vacuolations are unclear and the changes are mild or inconclusive, it is crucial to verify the diagnosis by utilizing more sensitive techniques, such as immunohistochemical or western blot analyses (Gavier-Widén et al., 2005). The principal goal of this investigation was to confirm the existence of scrapie in Libya using immunohistochemistry (IHC) techniques for cases previously diagnosed by routine histopathology examination (Abdalla and Sharif, 2022). Materials and MethodsSamples collectionThirty-three cases were used in the study, including three cases serving as a negative control at six months of age. The remaining thirty cases were collected between 2018 and 2023, regardless of race and gender, and exhibited various nervous clinical signs. The cases were collected from various sources, including the Veterinary Teaching Hospital, during field investigations, and from slaughterhouses in eight different regions in east Libya. Brain tissue samples were selected based on the areas where scrapie lesions are typically found, with a specific focus on the medulla oblongata sectioned at the level of the obex. Histopathology and IHCThe samples were placed in a 10% formalin solution for 72 hours and then dehydrated through a series of ethyl alcohols of increasing concentration. The samples were then embedded in paraffin wax. A microtome was used to cut the samples into 2-micron sections. The sections were placed on charged slides. The sections for histopathology undergo a staining process with Harris’s haematoxylin and eosin. The sections for IHC undergo a series of washes with xylene, isopropanol, ethanol, and water. Various solutions and reagents were prepared, including tris buffered saline (TBS), Mayer’s Hematoxylin, and a 10x PK buffer. The samples were treated with formic acid 98%–100% (CHEM KING) for 5 minutes and washed with tap water, then treated with H2O2 3% (CITY PHARMA) for 30 minutes and washed with TBS. The samples were left in PK buffer for 5 minutes, treated with PK (BIO Basic Canada Inc.) for 10 minutes, and incubated at 38°C. The primary antibody (MAB L42) (FLI Germany) was prepared at 40:500 in 10% goat serum in TBS and applied to the samples, which were then incubated for 2 hours at room temperature and washed. The secondary antibody (anti-house) (VECTOR Laboratories) was prepared at 5:500 in 10% goat serum in TBS and applied to the samples, which were then incubated for 2 hours at room temperature and washed. An ABC complex (VECTOR laboratory) was applied to the samples, which were then incubated for 30 minutes and washed. A DAB-Kit (VECTOR laboratory) was applied to the samples, which were then incubated for 10 minutes and washed. The samples were placed in distilled water and subsequently counterstained with Mayer’s hematoxylin for 30 seconds, then rinsed with tap water. The samples undergo a series of washes with ethanol, isopropanol, and xylene, and are then covered with a slip. All steps were performed inside a closed container at room temperature. Ethical approvalThis research received approval from the Libyan National Committee for Biosafety and Biosecurity. The collected samples were properly and safely disposed of following the international guidelines.
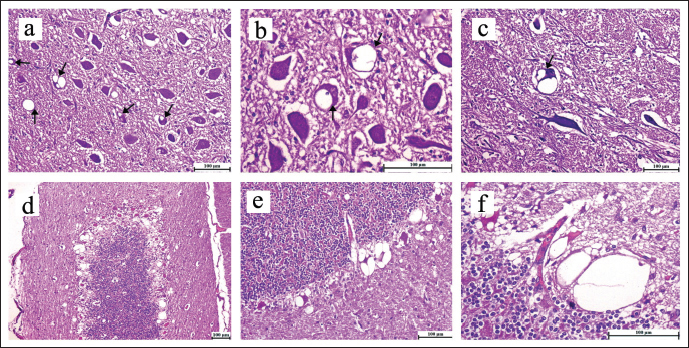
Fig. 1. Brain, sheep (a–f). Spongiform change (spongiosis) of neuropil. Intraneuronal vacuolation (arrows) is presence in the neuropil of the pons, particularly in the neurons found within the DMVN (a–c). Spongiform change (spongiosis) of neuropil and severe cell damage was detected within the Purkinje cells layer located in the cerebellum, characterized by the presence of intraneuronal and perineuronal vacuolations (d–f). H–E staning, (scale bar, 100 µm). ResultsIn twenty-one cases, the histopathological examination of the brain revealed spongiform alteration (spongiosis), gliosis, and neuron loss in various brain regions. The most affected sites were the brainstem at the obex level and the dorsal motor nucleus of the vagus nerve (DMVN) (Fig. 1a–c). The cerebellum’s Purkinje cell layer showed the presence of intraneuronal and perineuronal vacuoles, which were severe in one case and mild in another two cases. Purkinje cells were often shrunken or lost (Fig. 1d–f). Two cases were clinically suspected to be scrapie with a degenerative neuronal disease, but lacked the characteristic neuronal vacuolization lesions and, hence were not considered positive. The remaining seven cases were diagnosed as negative for scrapie. Immunohistochemical analysis detected prion proteins in the brain tissue of twenty-three samples. The presence of PrPSc deposition was consistently observed in brain regions showing spongiform degeneration of neurons, particularly within the DMVN in the brainstem (Fig. 2g–i), with clear intraneural deposition (Fig. 2j and k). In addition, PrPSc deposits were also found in the granular layer and molecular layer of the cerebellum (Fig. 2l). The deposition patterns of PrPSc involved perivascular areas in the obex (Fig. 2m and n). In the negative control, neither vacuoles nor immunostaining expression of PrPSc were observed (Fig. 2o). DiscussionScrapie remains a challenging and enigmatic neurodegenerative disorder. With its elusive nature, the pathogenesis, transmission mechanisms, and molecular intricacies of scrapie continue to puzzle researchers worldwide. Comprehending the fundamental mechanisms driving this devastating ailment is essential for creating practical diagnostic tools, devising therapeutic interventions, and formulating preventive strategies.
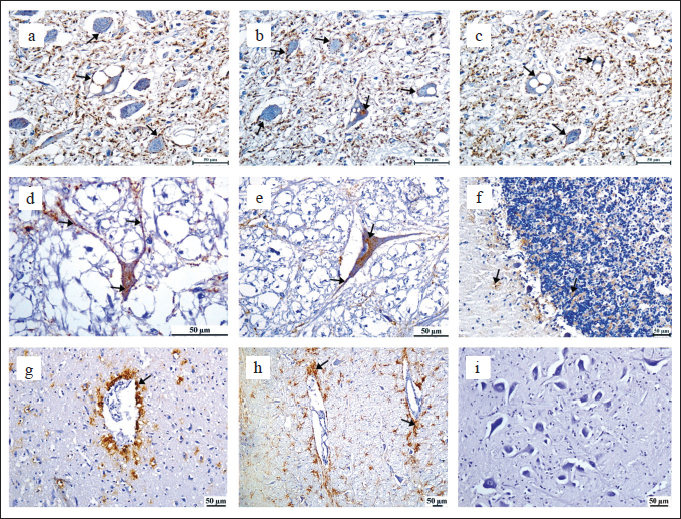
Fig. 2. Brain, sheep (a–i). Main PrPSc deposition patterns observed in the brain intraneuronal and extraneuronal in the neuropil (a–h), intraneural and not vacuolated, also present in the neuropil and axons (d and e), small PrPSc deposits in the molecular and granular layer of the cerebellum (c), PrPSc perivascular deposition (g and h), negative control, no vacuoles were observed, nor was the presence of immunostaining expression of PrPSc were observed (i). IHC with mAb L42 and Mayer’s hematoxylin counterstain (Scale bar, 50 µm). The Libyan National Center for Animal Health reported for the first time the occurrence of scrapie in 2014, as reported on the World Organisation for Animal Health (WOAH) website. The disease in Libya remains unrecognized and neglected, and no confirmatory techniques have been used to detect and confirm scrapie-infected cases in Libyan laboratories. This study focused on confirming the existence of scrapie cases and contributing to applying one of the confirmatory methods (IHC) in Libya. In our study, we examined twenty-nine cases exhibiting clinical signs of resampling of scrapie. The cases were older than 2 years of age and collected from 2018 to 2023, regardless of the race and gender of the animals. The depiction of the disease situation in Libya was solely based on these cases. Consequently, it may not necessarily reflect the true epidemiological characteristics of the illness in the eastern region of Libya. Observation of typical clinical neurological signs and detection of a combination of distinct histological abnormalities are essential for confirming the diagnosis of scrapie in sheep and goats. In confirming scrapie through examination of brain tissue and its respective most histopathological changes (spongiform degeneration), which include vacuolization and loss of neurons accompanied by gliosis. However, they are not enough for diagnosis when TSE vacuolations are absent or the histological lesions are mild or equivocal. According to WOAH guidelines (2018), the diagnosis needs more sensitive methods, such as immunohistochemical diagnosis, to be confirmed, which is more sensitive and confirmatory in the detection of PrPsc in the brain tissues. Despite that, the histopathological diagnosis remains a critical diagnostic instrument, particularly in detecting the distribution and severity of vacuolar lesions in the CNS. Additionally, it is useful in the differential diagnosis in cases where there is a negative result for scrapie (Miller et al., 1993; Gavier-Widén et al., 2005; Monleon et al., 2005; Tang, 2014; Farias et al., 2017; Amara et al., 2023). Scrapie diagnosis in deceased animals is both accurate and more employed. In this diagnostic approach, histopathological examination is conducted on brain stem samples taken at the obex level. The observed alterations in the CNS were indicative of a spongiform encephalopathy characterized mainly by the presence of vacuoles in neuronal cell bodies, concomitant with gliosis. Pons was affected, and changes were present in thirty cases where a diagnosis could be made, except in two cases where the changes were not clear and they were suspected of being infected with scrapie. The other seven cases were negative for scrapie. The largest number of vacuolated neuronal perikarya were found in the nucleus of the pons (dorsal motor nucleus of the vagus). Vacuolated neurons were usually accompanied by neuropil vacuolations in the surrounding areas, which were variable in severity. In vacuolated areas, there was a clear increase in the glial cell population in all affected cases. The lesions in the cerebellum were variable and were not seen in most cases, possibly due to the focal nature of the lesions, which may not be found in all parts of the cerebellum. This may require examining several slides from different parts of the cerebellum instead of examining a single slide. The presence of intraneuronal and perineuronal vacuoles, which were severe in one case and mild in two other cases. Neuropil vacuolations sometimes extend into the granular layer and show an astrocytic reaction, which are linked to scrapie and are consistent with the findings discussed in the aforementioned results (Gonzalez et al., 2002; Dustan et al., 2008; Orge et al., 2021; Amara et al., 2023). The characteristic vacuolations were not clearly apparent in two cases, which were confirmed by using IHC. IHC stands out as the gold standard. It boasts an impressive 100% sensitivity in detecting this prion disease (Tang, 2014). The IHC technique has been recognized by the World Organization for Animal Health (WOAH) since 2000 as the most reliable, secure, and conclusive assay for detecting scrapie to other immunodiagnostic methods such as rapid tests (Farias et al., 2017). The definitive diagnosis relies on a combination of factors, including clinical signs, histopathological findings, and identification of the prion using IHC. The IHC analysis performed during this study employed a commercially available kit containing a monoclonal antibody that specifically targets MAB L42 to detect PrPSc. The chromogenic substrate DAB was used to demonstrate the reaction. Obtained results indicated that twenty-three cases of sheep developed brown granular immune expression, and there have been eight confirmed disease outbreaks in these regions. In addition to the observation of vacuoles, which confirmed the existence of the prion associated with scrapie. The results were in agreement with those described by the study (Sofianidis et al., 2006; Amara et al., 2023). Significantly, none of the three negative controls displayed any immune expression. Furthermore, neither vacuoles nor spongiosis of nervous tissue were observed. This confirms that twenty-three of thirty cases of the analyzed samples were positive for scrapie. A TSE naturally affects small ruminants, it has existed for centuries and is not considered a significant public health risk under experimental conditions. However, it must be noted that small ruminants are susceptible to BSE, the pathogenesis as well as clinical signs associated with this condition are often indistinguishable from those of scrapie. In addition, the exclusionary practice of removing meat and bone meal from ruminant feedstuffs after the exposure of small ruminants to BSE-contaminated food has resulted in the possibility of BSE affecting sheep and goats on commercial farms, which may be misidentified as scrapie. Although BSE is primarily a disease affecting cattle, Humans have also been infected (causing variant Creutzfeldt-Jacob disease (vCJD)). Furthermore, it has the potential to be orally transmitted to sheep. Concerns have been raised about the natural transmission of BSE to the sheep population. BSE in cattle and vCJD in humans are the most highly publicized prion diseases. In 2005, the identification of BSE in a farmed goat in France resulted in the recognition of scrapie as a possible hazard to human health, as well as to goats and sheep as a source of prion reservoir (Spiropoulos et al., 2011). Moreover, the implementation of strict control measures, including the ban on the use of ruminant meat and bone meal in animal feed, has successfully reduced the incidence of BSE in cattle. However, more robust control measures are required to prevent the spread of TSEs in small ruminants and other animal species. These measures include implementing effective surveillance programs, developing sensitive and specific diagnostic tests, and implementing strict biosafety measures to prevent the spread of the disease. Early and reliable identification is an essential aspect of diagnosis that holds significant value, especially in animals that are intended for human consumption. It is necessary to eliminate the occurrence of BSE in sheep in Libya to prevent any potential association with unknown neurological conditions that are not diagnosed or reported in humans due to cultural practices. Therefore, it is highly recommended to apply other methods that would be very informative, especially for the exclusion of BSE in sheep, which can harm public health. Scrapie is a disease that has a wide distribution in many countries, often being transmitted through the process of importation (Gravenor et al., 2004). There is a possibility of transmission from the dam to the offspring during the timeframe between parturition and weaning, as well as the potential for it to take place in utero (Adams, 2016). Infected animals are a source of risk. Animals are the disease’s incubators, and even in situations where they are not showing any symptoms, they remain able to infect others. The infectious material has the ability to persist in pastures and buildings that were previously inhabited by infected animals. The fetal membranes have been identified as a possible source of infection, and it has been observed that the transmission of the disease can occur via the ingestion of milk obtained from animals that exhibit clinical symptoms of the disease (Gallardo and Delgado, 2021). It is not possible to detect PrPSc in the brain until a few months have elapsed post-challenge, thus a negative test outcome might not indicate the animal’s lack of infection (Cassmann and Greenlee, 2020). Scrapie disease in Libya has both been neglected and misdiagnosed. Although limited data have been gathered from animal owners, there is a belief that the disease has persisted in various regions of Libya for a long time. The actual prevalence of the disease may not be apparent due to the restricted commercial lifespan of sheep or the potential for exposure to occur too late in life to produce clinical symptoms. WOHA has identified scrapie as the eighth most prevalent factor contributing to losses in sheep and goats on a global scale. A reduction in productivity, loss of exports, and an increase in the cost of disposing of animal carcasses. Due to the unique characteristics of the disease agent, including prolonged incubation periods (ranging from months to decades), resistance to high temperatures, common disinfectants, ultraviolet, and ionizing radiation, as well as the absence of genetic material, efforts to eradicate and control this disease remain challenging and elusive. For those countries or areas where a disease is currently endemic, the eradication efforts have taken decades. In most cases, these elimination efforts have not been successful. The traits of the causative agent have been the primary cause of this lack of success. Several countries including the EU and USA have implemented breeding policies pertaining to sheep in order to control and eliminate scrapie. To accomplish this, the frequency of the ARR allele is heightened in affected flocks and the general sheep population. Several countries are currently contemplating the implementation of prion protein gene (PRNP) genotype selection programs to manage and eliminate scrapie due to the successful outcomes witnessed in countries such as Australia and New Zealand, which are now scrapie-free (Esteves et al., 2021). It is challenging to get rid of scrapie once it has entered a country; complete removal is only possible with the help of special breeding programs. When evaluating the appropriateness of these programs, it is important to conduct a thorough analysis:
Up to now, there is no information on the PRNP genotyping in Libyan sheep, which is a clear connection between scrapie susceptibility/resistance and the use of sheep in resistance breeding programs. This could have interfered with the analysis of the samples or, in the future massive sampling since there could be some genetic resistance in the Libyan sheep breeds. This poses a vital need to promote and exploit those who resist the disease. Increasing veterinary awareness and education about scrapie can improve disease management and control. To conclude, substantial progress has been made in improving histological diagnosis, mainly by introducing immunohistochemical techniques. According to the EU legislation (Andreoletti et al., 2007), all confirmed cases of TSE in small ruminants must undergo thorough examination through discriminatory testing. This is necessary to unveil possible BSE infections in both sheep and goats through the utilization of defined immunoblot protocols. Therefore, it is highly recommended to apply immunoblot methods that are very informative for excluding BSE in sheep and more information about the protein. Also, we need more studies to determine the genotype of the PRNP of Libyan sheep breeds. ConclusionScrapie is a widely indicated disease in Libya, with almost all cases being diagnosed based on clinical signs, and histopathological lesions, and Mab L42, a monoclonal antibody, was used for immune detection of prion proteins in the CNS through IHC. Twenty-three of thirty cases were confirmed with clear signs of a TSE infection. The results of this study have the potential to enhance disease diagnosis among veterinary laboratories and help identify and confirm the distribution of the disease in Libya, facilitating and improving future national control measures. AcknowledgmentThe authors would like to thank the Digital Pathology Laboratory at Cairo University’s Faculty of Veterinary Medicine for photographing microscope lesions. FundingNone. Conflict of interestThe authors declare no conflict of interest. Authors contributionsFawzia Mohamed is the main author, conducted the entire experiment, performed the laboratory investigation, and wrote the manuscript. Monier Sharif is the main supervisor of the research and assisted in diagnosis, confirming the results, and reviewing the final version of the manuscript. Salh Belgasim and Ayiman Aboulqassim contributed to the sample preparation and provided the necessary tools for reviewing the manuscript. Abraheem Omar and Nagi Saeed contributed to collecting samples. All authors provided critical feedback and helped shape the research and manuscript. Data availabilityData supporting the findings of this study are available within the manuscript. ReferencesAbdalla, F.F. and Sharif, M.A. 2022. Scrapie in Eastern Libya: case report in sheep. Al-Mukhtar J. Sci. 37(1), 41–47. Adams, D. 2016. Prenatal transmission of scrapie in sheep and goats: a case study for veterinary public health. Open Vet. J. 6(3), 194–214. Adeola, A.C., Bello, S.F., Abdussamad, A.M., Mark, A.I., Sanke, O.J., Onoja, A.B. and Rogo, L.D. 2023. Scrapie-associated polymorphisms of the prion protein gene (PRNP) in Nigerian native goats. Gene, 855, 147121. Amara, A., Elmehatli, K., Di Bari, M.A., Pirisinu, L., Andolsi, R., Gachout, S. and Khorchani, R. 2023. Characterization of the first case of classical scrapie in a sheep in Tunisia. Transbound. Emerg. Dis. 2023, 253316. Andreoletti, O., Budka, H., Buncic, S., Colin, P., Collins, J. D., De Koeijer, A., Griffin, J., Havelaar, A., Hope, J., Klein, G., Kruse, H., Magnino, S., López, A.M., McLauchlin, J., Nguyen-The, C., Noeckler, K., Noerrung, B., Maradona, M.P., Roberts, T., Vågsholm, I. and Vanopdenbosch, E. 2007. Opinion of the Scientific Panel on Biological Hazards on certain aspects related to the risk of Transmissible Spongiform Encephalopathies (TSEs) in ovine and caprine animals. EFSA JOURNAL, 5(3), 466. Cassmann, E.D. and Greenlee, J.J. 2020. Pathogenesis, detection, and control of scrapie in sheep. Am. J. Vet. Res. 81(7), 600–614. Corbière, F., Chauvineau-Perrin, C., Lacroux, C., Lugan, S., Costes, P., Thomas, M. and Schelcher, F. 2013. The limits of test-based scrapie eradication programs in goats. PLoS One, 8(1), e54911. Dustan, B., Spencer, Y., Casalone, C., Brownlie, J. and Simmons, M. 2008. A histopathologic and immunohistochemical review of archived UK caprine scrapie cases. Vet. Pathol. 45(4), 443–454. Esteves, A., Vieira-Pinto, M., Quintas, H., Orge, L., Gama, A., Alves, A. and Mendonça, A.P. 2021. Scrapie at Abattoir: Monitoring, control, and differential diagnosis of wasting conditions during meat inspection. Animals 11(11), 3028. Farias, G., Frohlich, E., Sanhueza, P., Jara, P. and Lecocq, C. 2017. Differential diagnosis of scrapie in post mortem goats by immuno histo chemistry. J. Dairy Vet. Anim. Res. 5(4), 121. Gallardo, M.J. and Delgado, F.O. 2021. Animal prion diseases: a review of intraspecies transmission. Open Vet. J. 11(4), 707–723. Gavier-Widén, D., Stack, M.J., Baron, T., Balachandran, A. and Simmons, M. 2005. Diagnosis of transmissible spongiform encephalopathies in animals: a review. J. Vet. Diagn. Invest. 17(6), 509–527. Gonzalez, L., Martin, S., Begara-McGorum, I., Hunter, N., Houston, F., Simmons, M. and Jeffrey, M. 2002. Effects of agent strain and host genotype on PrP accumulation in the brain of sheep naturally and experimentally affected with scrapie. J. Comp. Pathol. 126(1), 17–29. Gravenor, M., Papasozomenos, P., McLean, A. and Neophytou, G. 2004. A scrapie epidemic in Cyprus. Epidemiol. Infect. 132(4), 751–760. Miller, J.M., Jenny, A.L., Taylor, W.D., Marsh, R.F., Rubenstein, R. and Race, R.E. 1993. Immunohistochemical detection of prion protein in sheep with scrapie. J. Vet. Diagn. Invest. 5(3), 309–316. Monleon, E., Monzon, M., Hortells, P., Bolea, R., Acin, C., Vargas, F. and Badiola, J. 2005. Approaches to Scrapie diagnosis by applying immunohistochemistry and rapid tests on central nervous and lymphoreticular systems. J. Virol. Methods 125(2), 165–171. Orge, L., Lima, C., Machado, C., Tavares, P., Mendonça, P., Carvalho, P. and Pereira, J.C. 2021. Neuropathology of animal prion diseases. Biomol. 11(3), 466. Prusiner, S.B. 1998. Prions. Proceedings Nat. Academ. Sci. 95(23), 13363–13383. Sofianidis, G., Psychas, V., Billinis, C., Spyrou, V., Argyroudis, S., Papaioannou, N. and Vlemmas, I. 2006. Histopathological and immunohistochemical features of natural goat scrapie. J. Comp. Pathol. 135(2–3), 116–129. Spiropoulos, J., Lockey, R., Sallis, R.E., Terry, L.A., Thorne, L., Holder, T.M. and Simmons, M.M. 2011. Isolation of prion with BSE properties from farmed goat. Emerg. Infect. Dis. 17(12), 2253. Tang, J. 2014. Prevalence estimation and geographic distribution of scrapie in the Canadian sheep population via abattoir surveillance. Guelph, Canada: University of Guelph. | ||
| How to Cite this Article |
| Pubmed Style Mohamed F, Aboulqassim A, Sharif M, Belgasem S, Omar A, Saeed N. Immunohistochemical study of scrapie in naturally affected sheep in the east of Libya. Open Vet. J.. 2024; 14(8): 1843-1849. doi:10.5455/OVJ.2024.v14.i8.12 Web Style Mohamed F, Aboulqassim A, Sharif M, Belgasem S, Omar A, Saeed N. Immunohistochemical study of scrapie in naturally affected sheep in the east of Libya. https://www.openveterinaryjournal.com/?mno=197391 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.12 AMA (American Medical Association) Style Mohamed F, Aboulqassim A, Sharif M, Belgasem S, Omar A, Saeed N. Immunohistochemical study of scrapie in naturally affected sheep in the east of Libya. Open Vet. J.. 2024; 14(8): 1843-1849. doi:10.5455/OVJ.2024.v14.i8.12 Vancouver/ICMJE Style Mohamed F, Aboulqassim A, Sharif M, Belgasem S, Omar A, Saeed N. Immunohistochemical study of scrapie in naturally affected sheep in the east of Libya. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1843-1849. doi:10.5455/OVJ.2024.v14.i8.12 Harvard Style Mohamed, F., Aboulqassim, . A., Sharif, . M., Belgasem, . S., Omar, . A. & Saeed, . N. (2024) Immunohistochemical study of scrapie in naturally affected sheep in the east of Libya. Open Vet. J., 14 (8), 1843-1849. doi:10.5455/OVJ.2024.v14.i8.12 Turabian Style Mohamed, Fawzia, Ayiman Aboulqassim, Monier Sharif, Salh Belgasem, Abraheem Omar, and Nagi Saeed. 2024. Immunohistochemical study of scrapie in naturally affected sheep in the east of Libya. Open Veterinary Journal, 14 (8), 1843-1849. doi:10.5455/OVJ.2024.v14.i8.12 Chicago Style Mohamed, Fawzia, Ayiman Aboulqassim, Monier Sharif, Salh Belgasem, Abraheem Omar, and Nagi Saeed. "Immunohistochemical study of scrapie in naturally affected sheep in the east of Libya." Open Veterinary Journal 14 (2024), 1843-1849. doi:10.5455/OVJ.2024.v14.i8.12 MLA (The Modern Language Association) Style Mohamed, Fawzia, Ayiman Aboulqassim, Monier Sharif, Salh Belgasem, Abraheem Omar, and Nagi Saeed. "Immunohistochemical study of scrapie in naturally affected sheep in the east of Libya." Open Veterinary Journal 14.8 (2024), 1843-1849. Print. doi:10.5455/OVJ.2024.v14.i8.12 APA (American Psychological Association) Style Mohamed, F., Aboulqassim, . A., Sharif, . M., Belgasem, . S., Omar, . A. & Saeed, . N. (2024) Immunohistochemical study of scrapie in naturally affected sheep in the east of Libya. Open Veterinary Journal, 14 (8), 1843-1849. doi:10.5455/OVJ.2024.v14.i8.12 |