| Research Article | ||
Open Vet. J.. 2024; 14(10): 2599-2608 Open Veterinary Journal, (2024), Vol. 14(10): 2599–2608 Research Article Zinc-based fixative as a novel approach for histological preservation: A comparative study with formalin-based fixativesOmar Younis Altaey*, Ali Ahmed Hasan and Adnan Ali HassoDepartment of Anatomy, College of Veterinary Medicine, University of Mosul, Mosul, Iraq *Corresponding Author: Omar Younis Altaey. Department of Anatomy, College of Veterinary Medicine, University of Mosul, Mosul, Iraq. Email: Omar.younes [at] uomosul.edu.iq Submitted: 01/07/2024 Accepted: 08/09/2024 Published: 31/10/2024 © 2024 Open Veterinary Journal
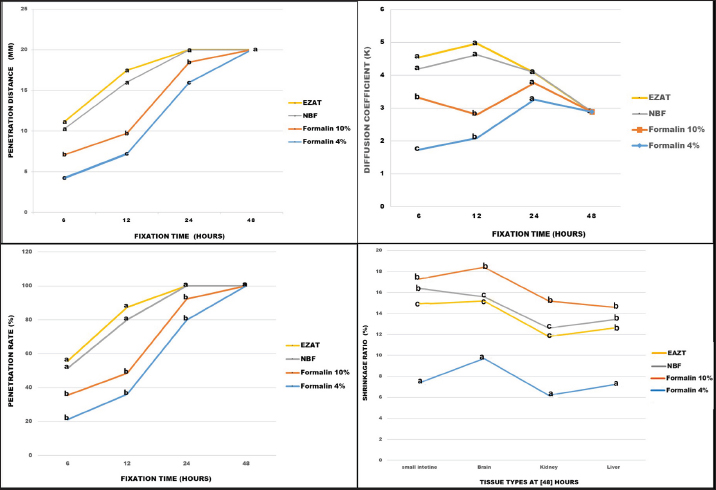
AbstractBackground: Formalin is a widely used histological fixative despite its carcinogenic properties, inadequate nucleic acid preservation, and prolonged fixation time. Aim: The study intended to prepare a novel zinc-based fixative that is, formalin-free, and cost-effective, provides optimal preservation, with rapid penetration rates, and enhanced Hematoxylin/Eosin-stained sections. Methods: Liver, kidney, brain, and small intestine specimens were collected from 10 adult laboratory rats (Rattus norvegicus) and preserved in formalin 4%, formalin 10%, neutral buffered formalin (NBF), and the experimental EZAT solution. The penetration depth (discolored edges) was measured at 6, 12, 24, and 48 hours. The shrinkage ratio was calculated by measuring the width and length of the samples before and after 48 hours of fixation. The histological evaluation, included staining affinity, cellular outline, cytoplasmic and nuclear attributes, and overall tissue structure, was conducted by a panel of academic experts and rated using a scale of poor (1), fair (2), good (3), very good (4), and excellent (5) grades. The data were later statistically analyzed to determine the significant differences among the tested fixative types. Results: A higher penetration rate was observed with the EZAT solution at 6 and 12 hours’ time and the samples reached optimal fixation after 24 hours; with an accelerated diffusion coefficient, and a minimal shrinking effect compared to formalin 10% and NBF. The microscopic evaluation of hematoxylin/eosin-stained sections revealed better staining affinity, refined histological details, and enhanced cytoplasmic and nuclear properties. The overall structural evaluation revealed an excellent microscopic appearance with the EZAT solution compared to formalin-based fixatives. Conclusion: The EZAT fixative should be considered as an everyday preservative in histology and histopathology laboratories. Future studies should be focused on the potential of EZAT in cellular histochemistry and immunohistochemistry practices. Keywords: Formalin, Histology, Laboratory rats, Zinc-based fixative. IntroductionIn the field of histology, fixation refers to the process of preserving cellular and tissue morphology in a “life-like” state, this process is crucial for microscopic examination of normal and diseased tissue (Sampedro-Carrillo, 2022). Formaldehyde has been widely used as a fixative of choice, due to its ability to preserve tissue architecture and prevent tissue degradation (Buesa, 2008). However, prolonged exposure to formaldehyde-based fixatives is considered hazardous to health, and classified as carcinogenic by the World Health Organization; while short-time exposure may result in nasopharyngeal, skin, and ocular irritation (Hasan and Abdelazizalzoubaa, 2019; Protano et al., 2022). Furthermore, formalin is an inadequate preservative of nucleic acids and exhibits slow penetration, requiring 48–72 hours (Buesa, 2008). These facts have motivated researchers to explore alternative fixation chemicals. Some scholars employed heavy metals containing fixatives, recommending their potential to enhance histological details and their effectiveness in special techniques like immunohistochemistry (IHC) (L’Hoste and Ann Tourres, 1995; Mori et al., 2015). Zinc was initially introduced with formalin and found to improve histochemical demonstration, enhance immunostaining of lymphoid cell markers, and reduce fixation and de-calcification time compared to formalin alone (L’Hoste and Ann Tourres, 1995; Bonds et al., 2005). However, a persistent risk is still associated with formalin in these fixatives. Recent studies have shown a growing interest in the use of formalin-free, zinc-containing fixatives to address health-related concerns and improve the quality of histological presentations. Grigorev and Korzhevskii, 2018, found that zinc chloride and zinc sulfate-containing solutions exhibited better cellular, extracellular, and antigenic preservation compared with formalin. Jensen et al., 2010, reported the capability of zinc-buffered fixatives in preserving enzyme and nucleic acid protein and their preference in applications of flow cytometry, fluorescent microscopic analysis, and in the preservation of intracellular and surface epitope profiles. Korzhevskiĭ et al. (2013) suggested using zinc in combination with ethanol or alcoholic fixatives as it presents promising immunocytochemical practice and improves confocal and laser microscopic presentation. However, formalin was still part of the previous experimental fixatives. The objective of this study is to develop a novel fixative using zinc-based compounds with a new chemical composition, volumes, and concentrations that differ from commercial zinc-containing chemicals. The advantages of the experimental solution are: readily accessible, easy to prepare, formalin-free, cost-effective, provides optimal preservation of cellular and tissue morphology, exhibit rapid penetration rates, and enhances the histological details of hematoxylin/eosin-stained sections. Material and MethodsAnimalsTen (n=10) adult white laboratory rats (Rattus norvegicus) were used in this study, obtained from the Animal House—College of Veterinary Medicine, University of Mosul—Iraq. Euthanasia was performed by intraperitoneal injection of 150 mg/kg of Pentobarbital sodium (Penbital® Bioveta-Czeck) in accordance with the guidelines of the American Veterinary Medical Association (Underwood and Anthony, 2020). The experiment was conducted from December 2023 to March 2024 in the Anatomy Department Laboratories, College of Veterinary Medicine. Fixatives preparationSpecimens of liver, kidney, brain (cerebral cortex), and small intestine were collected from the dissected animals, and preserved in labeled containers containing 80 ml of (formalin 10%, formalin 4%, and neutral buffered formalin (NBF) 10% prepared according to (Carson and Cappellano, 2015). Additional samples were kept in an EZAT solution consisting of absolute ethanol 40 %, Glacial Acetic acid 12 % (Scharlab®, Spain), zinc chloride 2 %, zinc tartrate 1%, thymol 0.02% (ThermoFisher®, USA), and distilled water 45%. All samples were kept for 48 hours at room temperature, around 25C○. Macroscopic and physical characteristicsThe specimens were examined for gross appearance, color, and consistency. The penetration depth was determined by creating a longitudinal cut along the middle plane of 2 cm3 liver samples. The fresh surface was exposed and the distance of penetration (discolored edges) was measured using a digital vernier caliper (LOUISWARE, China) for the assessment of fixative permeability within liver tissue. The measurement was performed at certain times (6, 12, 24, and 48 hours) and repeated 10 times in multiple samples, and data were recorded accordingly. The penetration coefficient was calculated using Medawar’s equation X=K √t, in this equation, (X) represents the distance of penetration, (t) represents the time of fixation, and (K) represents the penetration coefficient (Medawar, 1941; Steicke et al., 2018). To determine the shrinkage ratio, a number of pictures were taken using a (KODAK Pixpro, USA) 16MP digital camera. These pictures were captured alongside a metric ruler before and after 48 hours of fixation. The length and width of the samples from the liver, kidney, brain, and small intestine were measured using the ImageJ 1.54 (NIH, USA) software. The shrinkage ratio is represented by measuring the extent of dimensional decreases observed in the samples before and after the fixation process (Kerns et al., 2008). Histological processingAfter the fixation process, the samples were passed to the dehydration step using a series of ethyl alcohol at different concentrations, including 70%, 80%, and 95% (2 changes) for 30 minutes each. Finally, samples were immersed in 100% alcohol (2 changes) for 45 minutes each. Next, the samples were cleared using xylene for 45 minutes, and embedded in paraffin wax (2 changes) for 1 hour each. Later, the samples were cast into a paraffin block and sectioned using a rotary microtome (BIOBASE BK-2218, China) into 4 µm thick sections. The sections were stained with Harris Hematoxylin and Eosin Y stains. Then, the sections were mounted with DPX and coverslipped. Finally, the stained slides were examined under a light microscope (Olympus-CX21, Japan) (Suvarna et al., 2019). Microscopic evaluationA blind microscopic evaluation was conducted by a panel of a total of 7 professors and assistant professors from the Anatomy and Pathology and Poultry Diseases Departments in the College of Veterinary Medicine, University of Mosul, to assess the adequacy of fixation for formalin-based fixatives and EZAT experimental solution. The evaluation focused on the staining affinity, cellular outline, cytoplasmic attributes, nuclear attributes, and overall tissue structure parameters in the liver, kidney, brain, and small intestine sections. The observations of the panel members were rated on grades of poor (1), fair (2), good (3), very good (4), and excellent (5). The questionnaire responses (appendix 1: can be provided by the corresponding author upon request) were recorded and organized for subsequent statistical analysis to determine the effectiveness of the fixation methods. Statistical analysisThe data from the microscopic evaluation grades were expressed as the mean and standard error of the mean (M ± SEM). The statistical differences among fixative groups were analyzed using one-way analysis of variance and Duncan’s post hoc test, while, the penetration and shrinkage ratios were analyzed using chi-Squar and Bonferroni adjustment test to determine the differences among each distinct set of data. All statistical analysis were conducted using (IBM Spss.v27, Chicago-USA) software, at a significant level of p ≤ 0.05 (Petrie and Watson., 2013). Ethical approvalThe study was approved by the Animal Care and Use Committee of the college, with (Ref number: UM.VET.2023.099). ResultsThe gross examination of the samples during the fixation process revealed various physical changes. Initially, there was a hardening of the soft texture in the liver, kidney, and brain, accompanied by alterations in shape, size, and color. Samples kept in the EZAT solution turned light grey and brown, whereas those in formalin-based fixatives appeared totally grey or white. EZAT solution exhibited rapid penetration efficiency, the penetration distance was significantly deeper within liver samples during the periods of 6,12, and 24 hours compared with formalin 4%, 10%, and NBF. The diffusion coefficient (K) of the EZAT solution increased significantly (p < 0.05) after 12 hours of preservation to reach 4.965, and the fixation rate reached to 87.5% compared with formalin 4%, 10%, and NBF whose diffusion coefficient was 2.084, 2.805 and 4.018 respectively. The complete penetration was noticed after 24 hours of preservation in EZAT solution; While the samples in formalin 4 % and 10% required 48 hours for complete penetration. No significant differences were observed in the penetration distances and ratios with NBF at 6, 12, and 24 hours of fixation (Figs. 1 and 3). During the fixation process, the samples showed dimensional shrinkage. Formalin 4% demonstrated less shrinkage compared with other types of fixatives reached 7.2% in liver, 6.2% in brain, 9.7% in kidney, and 7.4% in small intestine. EZAT solution ranked second in terms of minimal shrinking effect with values of (12.6%,11.8%,15.2%, and 14%) in the studied organs, respectively. Moreover, the EZAT preservative showed significantly (p < 0.05) low contraction in the kidney, brain, and small intestine samples compared with the formalin 10% (14.6%,15.2%,18.4%, and 17.3%) and NBF (13.4%,12.6%,15.6%, and 16.4). The brain tissue experienced more shrinkage during 48 hours of fixation compared with liver and kidney samples, and the shrinkage was less with the EZAT solution compared to formalin 10% and NBF fixatives (Figs. 2 and 3).
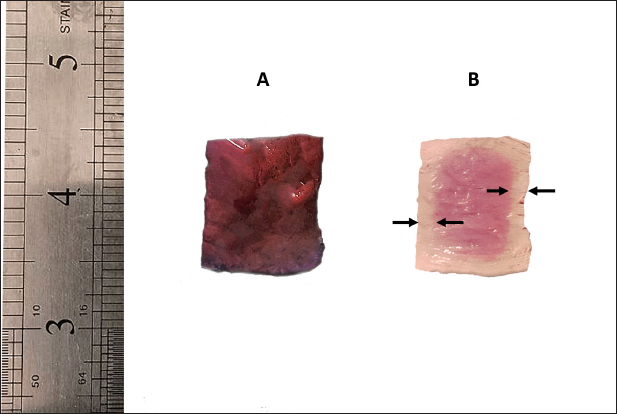
Fig. 1. Penetration distance through liver sample edges (discolored edges), the figure illustrating the sample before fixation (A), and after 6 hours of fixation with EZAT solution (B).
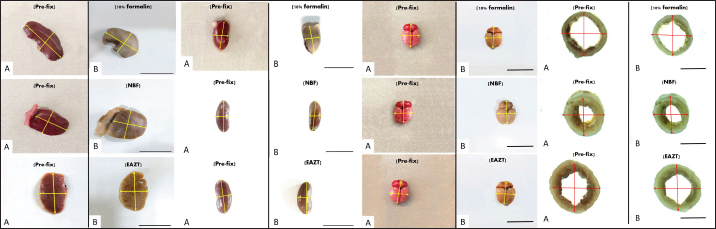
Fig. 2. Dimensional shrinkage (length and width) in the liver, kidney, brain and small intestine samples, pre-fixed samples set (A), and samples fixed with formalin 10%, NBF and EZAT solution (B). Scale bar=2 cm, Scale bar (small intestine)=1 mm.
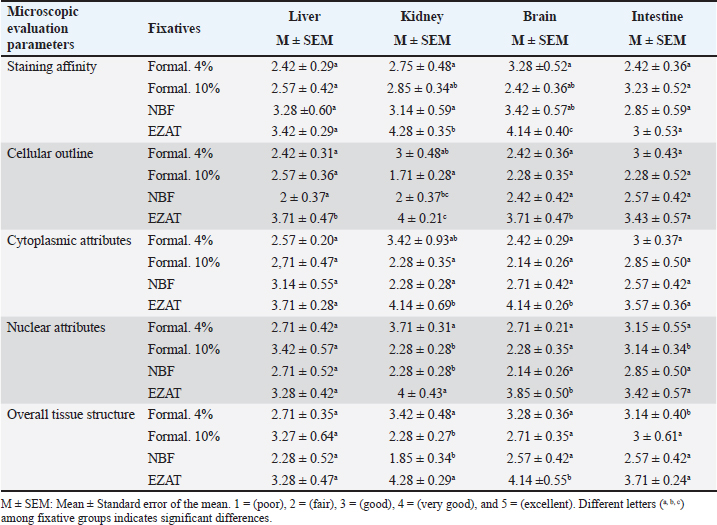
Fig. 3. Penetration distance, penetration ratio, and diffusion coefficient in formalin 4%, 10%, NBF, and EZAT fixatives over 6, 12, 24, and 48 hours of fixation. The figure also shows the shrinkage ratio in the liver, kidney, brain, and small intestine, after 48 hours of fixation. Different letters (a, b, c) indicate significant differences among fixative types, at a significant level of p ≤ 0.05. Microscopic evaluationThe microscopic evaluation of the liver, kidney, brain, and small intestine sections was analyzed to assess the adequacy of fixatives, the parameters of staining affinity, cellular outline, cytoplasmic attributes, nuclear attributes, and overall structural evaluation were targeted. EZAT solution showed a significantly higher staining affinity with hematoxylin and eosin particularly in the kidney and brain slides. The grade was (very good) compared with (fair) to (good) grades in the formalin-based solutions. There were no significant differences noticed in the slides of the liver and small intestine, and staining grades were closely equivalent. EZAT solution also was the best for cellular outline demonstration, cellular architecture and tissue details were clearer in the liver, kidney, brain (cerebral cortex), and small intestine sections compared with formalin-based slides. Grades ranged from very good (4 ± 0.21) in kidney to good (3.71 ± 0.47), (3.71 ± 0.47), and (3.43 ± 0.57) in liver, brain, and small intestine with EZAT slides, and was the worst with formalin 10% which ranged from fair (2.57 ± 0.36), (2.28 ± 0.52), and (2.28 ± 0.35) in liver, brain, and small intestine to poor (1.71 ± 0.28) in kidney. According to evaluators, the cytoplasmic attributes were also enhanced in the EZAT solution, and the grade ranged from very good (4.14 ± 0.69), (4.14 ± 0.26) in kidney and brain sections to good (3.71 ± 0.47), (3.57 ± 0.36) in liver, and small intestine slides. Statistical differences were noted between EZAT and formalin-based fixatives in kidney and brain slides, while there were no differences observed in liver and small intestine slides. Furthermore, the nuclear attributes were superior with EZAT solution compared with formalin-based fixatives, and grades were significantly different in the kidney, brain, and small intestine, varying from very good (4 ± 0.43) in the kidney to good (3.85 ± 0.50), (3.42 ± 0.57), and (3.28 ± 0.42) in the brain, small intestine, and liver. The overall structural evaluation revealed excellent microscopic appearance with EZAT slides across all types of tissues, characterized by less tissue disintegration, fine cellular details, sufficient nuclear staining, and improved morphological presentation, grades for this parameter were between 4.28 and 4.14 in the kidney and brain slides, which is significantly better than formalin 10 % and NBF (Figs. 4–7; Table 1). These results suggest that EZAT provides better tissue preservation and staining quality compared with traditional common fixatives. DiscussionFormalin is well-known for its carcinogenic properties, inadequate nucleic acid preservation, and prolonged fixation time. The current study aimed to develop a novel fixative that is formalin-free provides optimal cellular and tissue preservation, exhibits rapid fixation, and enhances the histological details with routine stains.
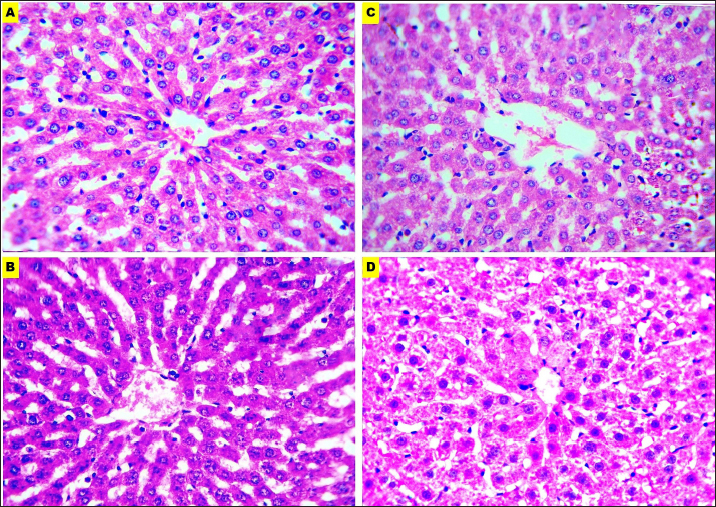
Fig. 4. Microphotographs of Hematoxylin and eosin-stained liver sections preserved in different fixatives included, formalin 10% (A), NBF (B), formalin 4% (C), and EZAT solution (D) at 400x magnification.
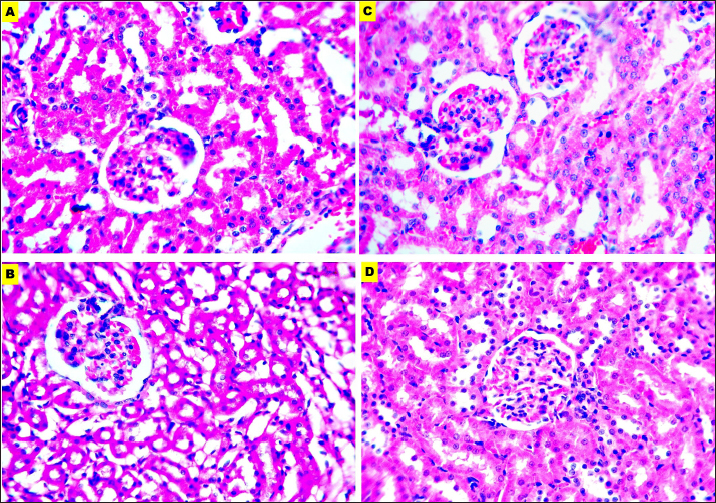
Fig. 5. Microphotographs of Hematoxylin and eosin-stained kidney sections preserved in different fixatives included, formalin 10% (A), NBF (B), formalin 4% (C), and EZAT solution (D) at 400x magnification. The EZAT solution showed a higher penetration rate, deeper penetration within a specific time frame, and accelerated diffusion coefficient, compared to the formalin fixatives. It also had a minimal shrinking effect compared with formalin 10% and NBF. These powerful properties of the EZAT solution are attributed to its chemical components such as ethanol, acetic acid, and zinc chloride. Each component was separately studied in different biological tissues and demonstrated their superiority over formalin-based fixatives. A study by (Howat and Wilson, 2014) mentioned that acetic acid had a higher penetration rate than formalin and noted that acetic acid reverses the shrinking effect of formalin fixatives. (Howat and Wilson, 2014), also reported that ethanol-containing fixatives achieve complete fixation within 24 hours, at a penetration distance of 1–1.5 mm/hour. Furthermore, Steicke et al. (2018) found that alcoholic fixative penetrates tissue faster than formalin and that fixatives containing ethanol, methanol, and acetic acid have higher diffusion coefficients. Lykidis et al. (2007) mentioned that zinc-based fixatives such as Z7 and Z4 formulas have a higher level of hydrophobicity, enabling faster and more effective tissue penetration compared with formalin. In contrast, Hewlett (2002) found that the penetration distance of formalin 10% reached 18 mm in 25 hours of fixation at an average of 0.9 mm/hour, which is lower than EZAT penetration activity in our study. Furthermore, Hewlett (2002) explained that formalin loses its ability to form methylene glycol after 12-hour lead to an interrupted fixation process and inadequate cross-linking which in turn reduces its diffusion through the tissue. The EZAT solution as mentioned earlier comprises 45% water with 12% glacial acetic acid. These chemical components reverse the zinc chloride shrinking effect and decrease the tissue contraction, a similar observation reported by Hewlett (2002), Howat and Wilson (2014), and Steicke et al. (2018). The microscopic evaluation of (H &E) stained sections revealed better staining affinity with the EZAT solution compared to the formalin fixatives. Wester et al. (2003) observations in skin biopsies showed intense staining quality with zinc-based fixatives compared with NBF. González et al. (2001) also demonstrated advantageous immunohistochemical staining with zinc-containing fixative. Moreover, Haque et al. (2020) referred to the superior staining affinity of ethanol-containing fixative in (HandE) stained sections.
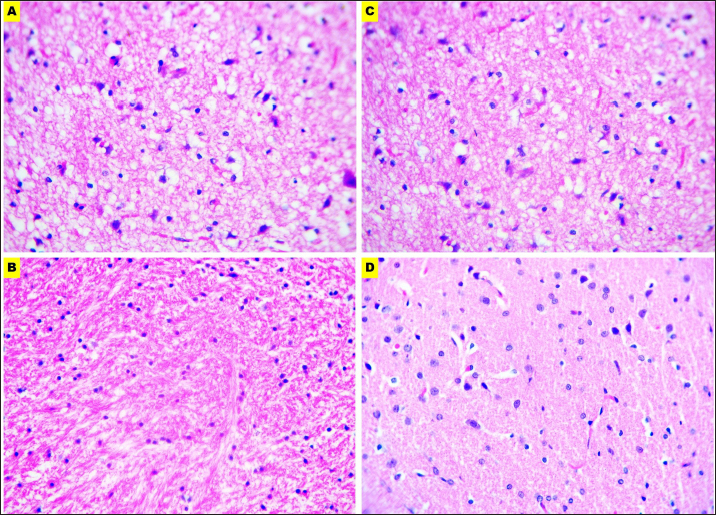
Fig. 6. Microphotographs of Hematoxylin and eosin-stained brain sections preserved in different fixatives included, formalin 10% (A), NBF (B), formalin 4% (C), and EZAT solution (D) at 400x magnification.
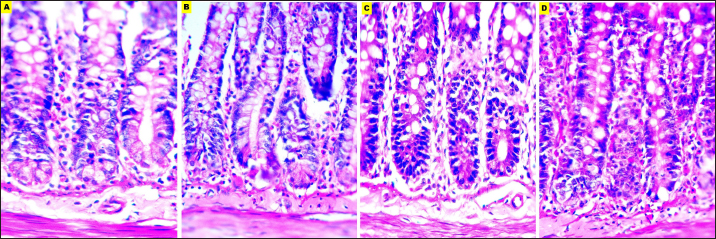
Fig. 7. Microphotographs of Hematoxylin and eosin-stained small intestine sections preserved in different fixatives included, formalin 10% (A), NBF (B), formalin 4% (C), and EZAT solution (D) at 400x magnification. EZAT fixative enhanced morphological and histological details compared with formalin-based fixatives in this study, these findings are consistent with the findings of Wester et al. (2003), which attributed that improvement to the efficient preservation of the cellular protein and nucleic acid. Beckstead (1994) also recommended using zinc-containing fixatives to provide superior morphological preservation for routine and immuno-stained tissue sections. Table 1. Microscopic evaluation grades for EZAT solution and formalin-based fixatives.
The chemical composition of the EZAT solution enhanced cytoplasmic and nuclear properties, which properties included: nuclear membrane integrity, nuclear homogeneity, and better DNA, and RNA presentation. While cytoplasmic properties included: structural integrity, permeability, enzymatic activity, and ionic balance (Howat and Wilson, 2014; Steicke et al., 2018). (Hewlett, 2002; Wester et al., 2003; Lykidis et al., 2007; Howat and Wilson, 2014; Steicke et al., 2018), suggested that zinc fixative can be an alternative for DNA and nuclear protein expression and has superior staining quality over NBF and formalin 10% fixatives. Jensen et al. (2010) also recommended zinc salt fixatives for cellular integrity, and intercellular and cellular protein preservation, the study suggested using the mentioned fixatives in immunostaining and surface epitopes expression. According to Webb (2020), zinc-containing fixative corrected many formalin—related histological artifacts like autolysis, fixation pigments, decalcification, over and under-fixation, difficult sectioning, streaming, and fade staining artifacts. The overall structural evaluation revealed excellent microscopic appearance with EZAT slides, these observations corresponded with Wester et al. (2003), Bonds et al, (2005), Lykidis et al. (2007), Jensen et al. (2010), and Mori et al. (2015) findings in different zinc-containing formulas compared with NBF, formalin 4% and 10 % fixatives. ConclusionThe zinc-based fixative is recommended as a safer and less hazardous alternative to formalin, which is commonly known for its carcinogenic properties and associated health risks. The current study introduces a novel, easily prepared chemical formula that addresses challenges like prolonged fixation time and higher shrinkage rate, and enhancing routine histological staining. EZAT fixative provides superior histological preservation, low shrinkage ratio, better staining affinity, and microscopic details with enhanced nuclear and cytoplasmic attributes. EZAT fixative should be considered as an everyday preservative in histology and histopathology laboratories. Future studies should pay attention to the evaluation of EZAT to assess its preservative quality for carbohydrates, minerals, and lipids through histochemical stains and IHC practices. AcknowledgmentsThe authors would like to thank the staff of the Veterinary Anatomy Department, College of Veterinary Medicine, University of Mosul, and all professors who kindly participated in the microscopic evaluation for their support and encouragement. Conflict of interestThe authors declare there is no conflict of interest. FundingThis study had no financial support from any sponsor as it is a self-funded study. Authors’ contributionsOmar Younis conceptualized the study, wrote the initial draft, and conducted statistical analysis. Ali Ahmed performed penetration and shrinkage ratio tests and conducted histological sectioning. Adnan Ali conducted the microscopic evaluation questionnaire, and collected and analyzed data, All authors contributed equally to the analysis, review, and presentation of the final work. Data availabilityThe data of this study are available within the manuscript. ReferencesBeckstead, J.H. 1994. A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J. Histochem. Cytochem. 42(8), 1127–1134. Bonds, L.A., Barnes, P., Foucar, K. and Sever, C.E. 2005. Acetic acid–zinc-formalin: a safe alternative to B-5 fixative. American. J. Clin. Pathol. 124(2), 205–211. Buesa, R.J. 2008. Histology without formalin? Ann. Diagn. Pathol. 12(6), 387–396. Carson, F.L. and Cappellano, C.H. 2015. Histotechnology. a self-instructional text. In Fixation, Eds. Carson, F.L. and Cappellano, American-Society, ASCP press. Chicago, USA, pp: 12–15. González, L., Anderson, I., Deane, D.C.S.D.B., Summers, C. and Buxton, D. 2001. Detection of immune system cells in paraffin wax-embedded ovine tissues. J. Comp. Pathol. 125(1), 41–47. Grigorev, I.P. and Korzhevskii, D.E. 2018. Current technologies for fixation of biological material for immunohistochemical analysis. Mod Technol Med, 10(2), 156–164. Haque, Z., Rahman, A., Khan, Z.I., Hussan, M.T. and Alam, M. 2020. Fijadores a base de alcohol pueden preservar mejor la morfología tisular que la formalina. Int. J. Morphol. 38(5), 1371–1375. Hasan, T. and Abdelazizalzoubaa, M. 2019. Formalin hazards and health insurance coverage needs among exposed personnel. Res. Adv. Biomed. Sci. Technol. 1, 18–21. Hewlett, B.R. 2002. Penetration rates of formaldehyde. Microscopy Today, 10(6), 30–31. Howat, W.J., and Wilson, B.A. 2014. Tissue fixation and the effect of molecular fixatives on downstream staining procedures. Methods, 70(1), 12–-19. Jensen, U.B., Owens, D.M., Pedersen, S. and Christensen, R. 2010. Zinc fixation preserves flow cytometry scatter and fluorescence parameters and allows simultaneous analysis of DNA content and synthesis, and intracellular and surface epitopes. Cytometry Part A, 77(8), 798–-804. Kerns, M.J.J., Darst, M.A., Olsen, T.G., Fenster, M., Hall, P. and Grevey, S. 2008. Shrinkage of cutaneous specimens: formalin or other factors involved? J. Cutan. Pathol. 35(12), 1093–1096. Korzhevskiĭ, D.E., Sukhorukova, E.G., Gilerovich, E.G., Petrova, E.S., Kirik, O.V. and Grigor’ev, I.P. 2013. Advantages and disadvantages of zinc-ethanol-formaldehyde as a fixative for immunocytochemistry and confocal laser microscopy. Morfologiia (Saint Petersburg, Russia), 143(2), 81–85. L’Hoste Jr, R.J. and Ann Tourres, M. 1995. Using zinc formalin as a routine fixative in the histology laboratory. Lab. Med. 26(3), 210–214 Lykidis, D., Van Noorden, S., Armstrong, A., Spencer-Dene, B., Li, J., Zhuang, Z. and Stamp, G.W. 2007. Novel zinc-based fixative for high quality DNA, RNA and protein analysis. Nucleic acids research, 35(12), e85. Medawar, P.B. 1941. The rate of penetration of fixatives. J. R. Microsc. Soc, 61(1–2), 46–57. Mori, H., Soonsawad, P., Schuetter, L., Chen, Q., Hubbard, N.E., Cardiff, R.D. and Borowsky, A.D. 2015. Introduction of zinc-salt fixation for effective detection of immune cell–related markers by immunohistochemistry. Toxicol. Pathol. 43(6), 883–889. Petrie, A. and Watson, P. 2013. Hypothesis tests, the chi-squared test: comparing proportions. In Statistics for veterinary and animal science 3E. Eds., Petrie, A. and Watson, P., (3rd ed). Hoboken, NJ, USA: Wiley Blackwell, pp: 112–126. Protano, C., Buomprisco, G., Cammalleri, V., Pocino, R.N., Marotta, D., Simonazzi, S., Cardoni, F., Petyx, M., Iavicoli, S. and Vitali, M. 2022. The carcinogenic effects of formaldehyde occupational exposure: a systematic review. Cancers, 14(1), 165. Sampedro-Carrillo, E.A. 2022. Sample preparation and fixation for histology and pathology. Methods Mol. Biol. 2422, 33–45. Steicke, M., Yang, G., Dinh, T.N., Dunster-Jones, M., Sargisson, O., Ahmady, F., Wang, Y. 2018. The penetration of methanol into bovine cardiac and hepatic tissues is faster than ethanol and formalin. European J. Histochem. 62(1), 2880. Suvarna, S.K., Layton, C. and Bancroft, J.D. 2019. Tissue processing. Bancroft’s theory and practice of histological techniques. Eds., Suvarna, S.K., Layton, C. and Bancroft, J.D. Amsterdam, The Netherlands: Elsevier, pp: 83–92. Underwood, W. and Anthony, R. 2020. AVMA guidelines for the euthanasia of animals: 2020 edition. 2013(30), 2020–1. Webb, S. 2020. Fixative artefacts in histology: mitigation and interpretation. Nelson, New Zealand: Cawthron Institute, pp: 3–12. Wester, K., Asplund, A., Bäckvall, H., Micke, P., Derveniece, A., Hartmane, I., Malmström, P.U. and Pontén, F. 2003. Zinc-based fixative improves preservation of genomic DNA and proteins in histoprocessing of human tissues. Lab. Invest. 83(6), 889–899. | ||
| How to Cite this Article |
| Pubmed Style Altaey OY, Hasan AA, Hasso AA. Zinc-based fixative as a novel approach for histological preservation: A comparative study with formalin-based fixatives. Open Vet. J.. 2024; 14(10): 2599-2608. doi:10.5455/OVJ.2024.v14.i10.9 Web Style Altaey OY, Hasan AA, Hasso AA. Zinc-based fixative as a novel approach for histological preservation: A comparative study with formalin-based fixatives. https://www.openveterinaryjournal.com/?mno=207832 [Access: January 11, 2026]. doi:10.5455/OVJ.2024.v14.i10.9 AMA (American Medical Association) Style Altaey OY, Hasan AA, Hasso AA. Zinc-based fixative as a novel approach for histological preservation: A comparative study with formalin-based fixatives. Open Vet. J.. 2024; 14(10): 2599-2608. doi:10.5455/OVJ.2024.v14.i10.9 Vancouver/ICMJE Style Altaey OY, Hasan AA, Hasso AA. Zinc-based fixative as a novel approach for histological preservation: A comparative study with formalin-based fixatives. Open Vet. J.. (2024), [cited January 11, 2026]; 14(10): 2599-2608. doi:10.5455/OVJ.2024.v14.i10.9 Harvard Style Altaey, O. Y., Hasan, . A. A. & Hasso, . A. A. (2024) Zinc-based fixative as a novel approach for histological preservation: A comparative study with formalin-based fixatives. Open Vet. J., 14 (10), 2599-2608. doi:10.5455/OVJ.2024.v14.i10.9 Turabian Style Altaey, Omar Younis, Ali Ahmed Hasan, and Adnan Ali Hasso. 2024. Zinc-based fixative as a novel approach for histological preservation: A comparative study with formalin-based fixatives. Open Veterinary Journal, 14 (10), 2599-2608. doi:10.5455/OVJ.2024.v14.i10.9 Chicago Style Altaey, Omar Younis, Ali Ahmed Hasan, and Adnan Ali Hasso. "Zinc-based fixative as a novel approach for histological preservation: A comparative study with formalin-based fixatives." Open Veterinary Journal 14 (2024), 2599-2608. doi:10.5455/OVJ.2024.v14.i10.9 MLA (The Modern Language Association) Style Altaey, Omar Younis, Ali Ahmed Hasan, and Adnan Ali Hasso. "Zinc-based fixative as a novel approach for histological preservation: A comparative study with formalin-based fixatives." Open Veterinary Journal 14.10 (2024), 2599-2608. Print. doi:10.5455/OVJ.2024.v14.i10.9 APA (American Psychological Association) Style Altaey, O. Y., Hasan, . A. A. & Hasso, . A. A. (2024) Zinc-based fixative as a novel approach for histological preservation: A comparative study with formalin-based fixatives. Open Veterinary Journal, 14 (10), 2599-2608. doi:10.5455/OVJ.2024.v14.i10.9 |