| Research Article | ||
Open Vet. J.. 2024; 14(12): 3289-3295 Open Veterinary Journal, (2024), Vol. 14(12): 3289-3295 Research Article Molecular detection of Chlamydophila felis from conjunctiva of cats infected with conjunctivitis and upper respiratory diseaseZahraa Mustafa Al-Jumaa1*, Mohammed Tariq Jaber2, Atheer Abdulrazzaq Al-Doori31Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Mosul, Nineveh, Iraq 2Department of Forensic Biology, Higher Institute of Forensic Sciences, Al-Nahrain University, Baghdad, Iraq 3Department of Microbiology, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq *Corresponding Author: Zahraa Mustafa Al-Jumaa. Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Mosul, Nineveh, Iraq. Email: sandy285 [at] uomosul.edu.iq Submitted: 18/08/2024 Accepted: 15/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
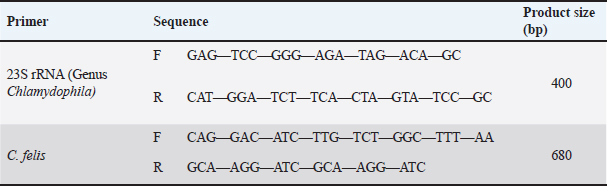
AbstractBackground: Chlamydiae are obligate generally Gram-negative intracellular parasites with bacterial characteristics, including a cell wall, DNA, and RNA, and the main characteristics of infections are ocular conjunctivitis and upper respiratory disease. Aim: The aim of this study was to investigate the prevalence of Chlamydophila infection in a population of shelter cats in Baghdad. Methods: Molecular detection was done using a polymerase chain reaction (PCR) approach. Fifty shelter cats of various ages, sexes, and breeds participated in the study from 1/11/2023 to 1/4/2024. DNA was extracted and amplified using PCR. Results: The study’s findings revealed that the PCR technique showed that 22/44% of positive cats from a total of 50 cats were assured for the 23S rRNA gene and yielded a band at 400 bp, and for 41/27.3%, these findings are regarded as distinctive for the genus Chlamydophila and positive sample 30/20% from a total of 150 samples was assured for Chlamydophila felis, a band at 680 bp. The findings revealed that the prevalence of Chlamydophila in upper respiratory tract infections in female cats older than 1 year was between 14/50 (28%) and 13/50 (26%) conversely, and the infections exhibited greater prevalence and a higher rate of detection in male under 1 year of age. The present investigation highlighted a significant prevalence of Chlamydophila in respiratory swabs obtained from Persian and Himalayan cats, but Scottish and British cats exhibited a comparatively lower rate of positive Chlamydophila. Conclusion: In conclusion of this study, a significant number of cats are infected with C. felis, and PCR provided rapid and sensitive detection of Chlamydophila species in different samples and detected Chlamydophila that did not grow in culture. It was considered the first study for the detection of C. felis from conjunctivitis in shelter cats in Baghdad. Keywords: Co-infection, Feline chlamydophilasis, PCR, Shelter cats, URTI. IntroductionChlamydiae are intracellular parasites that are primarily Gram-negative and obligatory. They possess bacterial features such as a cell wall, DNA, and RNA. In recent years, there has been significant growth in the family Chlamydiaceae (Wu et al., 2013; Kim et al., 2022). The family now consists of 13 species that are classified under the genus Chlamydia. These species are Chlamydophila trachomatis, Chlamydophila pneumoniae, Chlamydophila felis, Chlamydophila muridarum, Chlamydophila psittaci, Chlamydophila gallinacean, Chlamydophila serpentis, and Chlamydophila poikilotheyism (Laroucau et al., 2020). Chlamydophila felis was initially discovered in the United States in 1942 from cats exhibiting upper respiratory tract disease (URTD) (Barimani et al., 2019; AL-Jumaa et al., 2025). Numerous studies have demonstrated that C. felis, feline calicivirus, and feline herpesvirus type-1 are the primary causal agents of URTD in cats (Bommana and Polkinghorne 2019; Barimani et al., 2019). Clinical symptoms of C. felis infection in cats include fever, eye discharge, and sneeze. The main characteristic is the inflammation of the conjunctiva or nictitating membrane. Cats can exhibit a variety of ocular symptoms, including chemosis, hyperemia, blepharospasm, and mucopurulent or serous ocular discharge. Cats may experience a prolonged duration of conjunctivitis lasting for several months (Wu et al., 2013; Kim et al., 2022). Infections caused by C. felis typically develop into long-lasting, gradual diseases that are spread by intimate physical contact or through the air as tiny droplets. Regarding this matter, even though the cats may not show any symptoms of the illness, they carry the organism in their conjunctiva for 2 months or more (Hughes et al., 2024). Without regard to breed or gender, organisms have been recovered from the conjunctiva for up to 7 months following an experimental infection. Previous research has demonstrated that differences in the prevalence of C. felis infection in cats can be attributed to a variety of factors, including the methods used, sample size, geographic region, and kind of cat (feral versus domestic) (Tîrziu et al., 2022). In cats living in households with conjunctivitis, the isolation rate of C. felis can reach 30% (Barimani et al., 2019). Iraq’s cities are home to a large number of stray cats that interact with other animals. This problem raises the risk of illness in both humans and animals (Akçakavak et al., 2024). The survey results from Iraq revealed a significant presence of C. felis in cats. A study conducted in Tehran and Isfahan found that 40 out of 224 instances of cats (17.85%) were infected with C. felis, according to the findings of Walter et al. (2020). The disease was shown to be 20% prevalent among cats in another investigation done in Tehran. In a study, Cantekin et al. (2014) numerous techniques exist for the detection of Chlamydiosis in animals, including culturing cells, analyzing conjunctival swabs, genetic testing, ELISA, polymerase chain reaction (PCR), and direct and indirect fluorescent antibody tests (Sjödahl-Essén et al., 2008; Ravindranath et al., 2022; Sainkaplan et al., 2022). The availability of molecular assays (such as PCR) for the identification of Chlamydia infections in cats is on the rise. The speciation of isolates from cultures or infected tissues can be accomplished with these sensitive and fast procedures. To distinguish between Chlamydiaceae species, one can utilize the PCR technique, which can be followed by restriction digestion or high-resolution melting curve analysis. In addition, neither a living organism nor any other kind of test subject is required for this procedure (Kim et al., 2022). To the best of our knowledge, there is no study addressing Chlamydiosis in this area; therefore, the purpose of the present study was the molecular detection of C. felis in companion cats of Baghdad, Iraq. Materials and MethodsSample population collectionA total of 50 cats and 150 swab samples (nasal, oropharyngeal, and conjunctival) were collected from cats that showed classical signs of pneumonia and conjunctivitis during the period between November 2023 and April 2024 from veterinary clinics in Baghdad. All parameters, including age, gender, breed, and clinical signs, were recorded (Fig. 1). The samples were collected using sterile procedures and then underwent direct DNA extraction techniques. DNA extractionSwabs with each tube were vortexed with GSB buffer, and after centrifuged at 5,000 rpm for 5 minutes, the DNA of the precipitates was extracted according to the instructions of the kit manufacturing company, “gSYNC™ Geneaid extraction Kit Quick Protocol, Taiwan.” The extracted DNAs were kept at −20°C until used. PCRFor the diagnosis of C. felis by the PCR technique, two pairs of PCR primers were used in Table 1 and consisted of a universal primer pair for the genus of Chlamydophila depending on 23S rRNA sequences, and the second pair was specific for it; the efficacy of these primers was confirmed by Barimani et al. (2019). Primers were synthesized by Syntol Co., Russia. PCR was performed in a total volume of 25 μl, including 10 μl of master mix 2.5× (Syntol Co., consisting of 2.5× PCR buffer, 200 μM of the dNTPs, and 0.5 U of Taq DNA polymerase) with 2 μl of primer mix (forward and reverse primer), 1.5 μl MgCl2, 5 μl of extracted DNA (template), and 6.5 μl of PCR grade distilled water. The PCR reactions were performed in Thermocycler (BIONEER®, South Korea). Thermal conditions were an initial denaturation for 5 minutes at 95°C, 35 cycles of 30 seconds at 95°C, 30 seconds at an annealing temperature of 52°C for Genus Chlamydophila, and 59°C for species C. felis; extension made at 72°C for 30 seconds, and final elongation was made at 72°C for 5 minutes. The PCR products were exposed to electrophoresis for 1 hour at 70 volts in a 1.5% agarose gel containing ethidium bromide stain. The bands were visualized and photographed at 245–312 nm through the UV transilluminator.
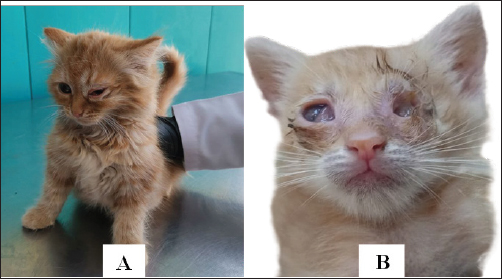
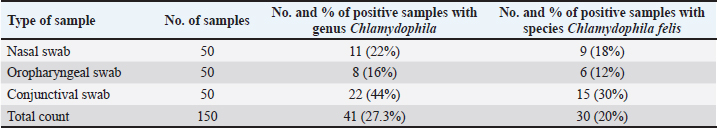
Fig. 1. Diseased cat has typical clinical signs of conjunctivitis with pneumonia. (A) Unilateral conjunctivitis with nasal discharge. (B) Corneal opacity with muco-purulent conjunctivitis (left eye) and unilateral eye-globe rupture with purulent conjunctivitis (right eye). Table 1. Primers used in this study genus- and species-specific primers for the detection of Genus Chlamydophila and C. felis
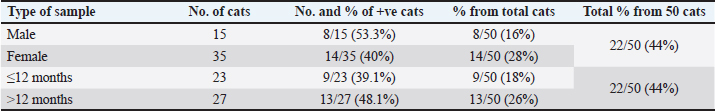
Ethical approvalIn order to carry out this systematic activity at the University of Baghdad’s College of Veterinary Medicine, the commission of scientific morality has granted the institution an endorsement certificate (with the number P.G./2386) and moral approbation. ResultsAffected cats showed signs of conjunctivitis with respiratory abnormalities, the final result every single cat that had clinical suspicions of feline Chlamydophilosis tested positive for C. felis. The cats displayed symptoms of conjunctivitis during the examinations, including redness, swelling, inflammation of the conjunctival tissue, and possibly ocular discharge. Figure 1 shows the accumulation of purulent discharge and a full eye-globe rupture in one cat, whereas keratitis with corneal opacity was seen in another. The eye-globe rupture was noted upon the cat’s rescue, as per the owner’s summary. After exhibiting serious respiratory issues, the felines were admitted to animal clinics. There were also general clinical indications that were noted, such as lameness and inappetence for several days. The results of the PCR with specified primers showed that 100 samples from the respiratory system and 50 samples from the conjunctiva were examined. In terms of “400-bp region of 23S rRNA,” results showed the presence of Chlamydophila spp. when the sample was analyzed for the Chlamydophila genus. Most samples tested positive for C. felis, as shown in Figure 3, even though only a 680-bp area of 23S rRNA was amplified for this species (Fig. 2). The sequencing of the 23S rRNA gene demonstrated that the C. felis primers specifically targeted the corresponding gene and produced a single band at 680 bp, confirming the presence of the 23S RNA gene of C. felis in the isolate. Confirming the presence of Chlamydophila genus was done by detecting the 23S rRNA gene using PCR and agarose gel. The findings exhibited that 19%, 22%, and 44% (from a total of 150 from each nasal, oropharyngeal, and conjunctival swabs, respectively) had the Chlamydophila 23S rRNA gene, which has a 400-bp product size, while results confirmed that 18%, 12%, and 30% of nasal, oropharyngeal, and conjunctival swabs were positive to species C. felis, respectively (Tables 2–4). DiscussionChlamydophila species are present in the typical microflora of the conjunctiva and upper respiratory tract of cats (Ghasemian et al., 2023). Nevertheless, certain Chlamydophilas have been known to induce feline infections, including feline conjunctivitis, lower respiratory tract infections, and polyarthritis. Chlamydophila felis has been identified as a likely reason for feline conjunctivitis and respiratory disorders. Accurate identification of the Chlamydophila species is essential in order to provide rapid and suitable treatment (Momtaz et al., 2014). The conventional method for detecting C. felis in cases of feline pneumonia and conjunctivitis depends on clinical presentation, which is not completely dependable due to the presence of similar symptoms caused by other pathogens (Kang and Park, 2008). Results showed that C. felis was found in every cat that showed clinical signs of feline Chlamydophilosis. During the examinations, the cats exhibited conjunctivitis characterized by inflammation of the conjunctival tissue, resulting in redness and swelling, with or without discharge from the eyes. In this study, one cat had keratitis, which is characterized by corneal opacity. Another cat had a full eye-globe rupture, accompanied by a buildup of purulent discharge, as seen in Figure 1, Based on the owner’s account, the cat’s eye-globe rupture was noticed at the time of its rescue. The felines were admitted to veterinary facilities following the manifestation of acute respiratory ailments. Additional common clinical symptoms, such as difficulty in walking and lack of appetite lasting for many days, were documented. A total of 100 respiratory samples and 50 conjunctival samples were studied using the PCR technique with specific primers, as indicated by the PCR results about “400-bp region of 23S rRNA.” The sample was subjected to amplification for the Chlamydophila genus, and the results indicated a positive presence of Chlamydophila spp. (Fig. 2), while a 680-bp region of 23S rRNA was amplified for the C. felis also most samples positive to it as shown in Figure 3. The sequencing of the 23S rRNA gene demonstrated that the C. felis primers specifically targeted the corresponding gene and produced a single band at 680 bp, confirming the presence of the 23S RNA gene of C. felis in the isolate. Confirming the presence of Chlamydophila genus was done by detecting the 23S rRNA gene using PCR and agarose gel. The findings exhibited that 19%, 22%, and 44% (from a total of 150 from each nasal, oropharyngeal, and conjunctival swabs, respectively) had the Chlamydophila 23S rRNA gene, which has a 400-bp product size (Fig. 3), while results confirmed that 18%, 12%, and 30% of nasal, oropharyngeal, and conjunctival swabs were positive to species C. felis, respectively (Table 2). The combination of clinical signs and PCR is the most recommended approach due to the small genome size of Chlamydophila, and they exhibit more ability than they do to demonstrate various biochemical pathways for species-level identification. (Sibitz et al., 2011; Momtaz et al., 2014; Johnson, 2024). The study found that the 23S rRNA gene successfully identified all the tested feline Chlamydophila. In addition, combining the routine PCR test with traditional identification techniques could be a reliable approach to accurately determine the level of prevalence of Chlamydophila in infected cats. Many researchers have described the use of PCR in the detection of Chlamydophila directly from clinical samples such as nasal swab, tracheal, and conjunctival (Helps et al., 2003; Harley et al., 2007). PCR is an effective method for diagnosing C. felis by amplifying the 23S rRNA gene using particular primers designed for this species. This is possible because the characteristics of different Chlamydophila species are not distinct enough to differentiate them easily (Momtaz et al., 2014; Chan et al., 2023). The increase in C. felis happened might be due to two causes. The subclinical form is commonly observed in most cases of C. felis infection, together with the implemented control methods. The present investigation found a significant prevalence of C. felis infection, which is consistent with previous research (Gruffydd-Jones et al., 2009; Sibitz et al., 2011) that recorded in above 2 months of age. The molecular detection of the 23S rRNA gene specific for Chlamydophila genus (Momtaz et al., 2014) achieved in the existing study was indicated Chlamydophila directly from swabs in the shelter cats suffering from respiratory signs with conjunctivitis (Table 4) (Halánová et al., 2011; Borel et al., 2018). The study found that upper respiratory tract infections were more prevalent in young cats (less than 1 year old) at a rate of 39.1%. These infections were less common in cats older than 1 year, but more frequent in diseased females 40% compared to diseased males 53.3%. The infections were mainly observed in Persian and Himalayan cats. However, the detection rate of Chlamydophila was higher in swabs taken from older female cats. The findings were consistent with (Hartmann et al., 2010), who confirmed a significant association between Chlamydophila infection and young age (≤1 year). However, the results differed from (Kang and Park, 2008) who reported a high rate of Chlamydophila isolation from upper respiratory tract infections in children around 1.5 years old. (Wasissa et al., 2021; Johnson, 2024) also found a higher rate of Chlamydophila isolation in females compared to males and in Toy cats breed compared to other breeds. Generally, cats that are ≤1 year old, immunocompromised, or sensitive to other respiratory tract infections are more likely to experience upper respiratory tract infections. The inconsistencies may have arisen due to variations in diagnostic processes, sample populations, or inclusion criteria (Bressan et al., 2021). A 100% prevalence of Chlamydophila was observed in conjunctival infections in diseased Sphynx cats, whereas other studies reported a lower prevalence of Chlamydophila in such infections (Low et al., 2007; Maazi et al., 2016). This difference may be attributed to the increased breeding of these species for various purposes in recent years. Thus, the current research is the first study in recording C. felis detected from conjunctival and upper respiratory tract infections in shelter cats using the PCR technique by detecting the C. felis species-specific 23SrRNA gene in Iraq, especially in Baghdad. Herein, it provides information on the occurrence of Chlamydophilosis in cats in Baghdad. Serious disease management is suggested to avoid high costs associated with regularly relapsing disease.
Fig. 2. Amplification of genus Chlamydophila 23S rRNA gene on 2% (w/v) agarose gel at 400 bp. M: 1500 bp marker. Lanes 1–10 represent positive samples.
Fig. 3. Amplification of species C. felis gene on 2% (w/v) agarose gel at 680 bp. M: 1500 bp marker. Lanes 1 and 2 represent positive samples. Lanes 3–10 represent negative samples. Table 2. Molecular detection results of genus Chlamydia and species C. felis from swabs by PCR
Table 3. Prevalence of C. felis infection based on sex and age in shelter cats.
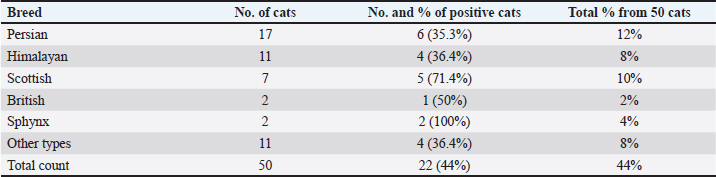
Table 4. Prevalence of isolation rate of C. felis between different breeds of shelter cats.
ConclusionThere are a number of possible clinical signs of feline Chlamydophilosis, but this study’s findings suggest that the molecular assay of C. felis is the gold standard for making a definitive diagnosis. An infected cat could be a vector for the transmission of the disease even though the infection is often not lethal and may only manifest subclinically. AcknowledgmentsThe author would like to express her appreciation to the Forensic DNA Research and Training Centre at AL-Nahrain University, Baghdad (F.DNA R.T.C.), for providing her with the resources she needed to carry out her research. Conflict of interestThe writer confirms that she has no conflicts of interest to declare. FundingThe author has not received any funds for this work. Authors’ contributionsThere is one author for this manuscript. Data availabilityAll data are available in this manuscript. ReferencesAkçakavak, G., Tuzcu, N., Çelik, Z., Tural, A., Dağar, O. and Tuzcu, M. 2024. A molecular and histopathological study on Bronchopneumonia in cats. Man. J. Agr. Vet. L. Sci. 14(1), 30–39. AL-Jumaa, Z.M., Al-doori, A.A. and Jabessaer, M.T. 2025. Laboratory diagnosis of Mycoplasma spp. from the upper respiratory tract and conjunctival infections in shelter cats. Egy. J. Vet. Sci. 56(5), 1105–1112. https://doi.org/10.21608/ejvs.2024.279983.1973 Barimani, M., Mosallanejad, B., Ghorbanpoor, M. and Esmaeilzadeh, S. 2019 Molecular detection of Chlamydia felis in cats in Ahvaz, Iran. Arch. R. Inst. 74(2), 119–126. Bommana, S. and Polkinghorne, A. 2019. Mini review: antimicrobial control of chlamydial infections in animals: current practices and issues. Front. Microbiol. 10, 113. Borel, N., Polkinghorne, A. and Pospischil, A. 2018. A review on chlamydial diseases in animals: still a challenge for pathologists? Vet. Pathol. 55(3), 374–390. Bressan, M., Rampazzo, A., Kuratli, J., Marti, H., Pesch, T. and Borel, N. 2021. Occurrence of Chlamydiaceae and Chlamydia felis pmp9 typing in conjunctival and rectal samples of Swiss stray and pet cats. Path. 10(8), 951. Cantekin, Z., Solmaz, H., Altug, N. and Ozmen, G.O. 2014. Development of pmp Gene-Specific PCR assay with a host specific internal control for Chlamydophila felis. Thi. J. Vet. Med. 44(4), 469–476. Chan, I., Dowsey, A., Lait, P., Tasker, S., Blackwell, E., Helps, C.R. and Barker, E.N. 2023. Prevalence and risk factors for common respiratory pathogens within a cohort of pet cats in the UK. J. S. A. Pract. 64(9), 552–560. Ghasemian, M., Memariani, M., Mahmoodi, S., Kohansal, M., Nikfar, G. and Rajabi-Vardanjani, H., 2023. The emergence potential of Chlamydia psittaci and Chlamydia felis as zoonotic agents causing eye and respiratory infections in humans and animals. Arch. Razi Inst. 79(4), 685–694. Gruffydd-Jones, T., Addie, D., Belák, S., Boucraut-Baralon, C., Egberink, H., Frymus, T., Hartmann, K., Hosie, M.J., Lloret, A., Lutz, H., Marsilio, F., Pennisi, M., Radford, A., Thiry, E., Truyen, U. and Horzinek, M. 2009. Chlamydophila felis infection. ABCD guidelines on prevention and management. J. Feline Med. Surg. 11(7), 575–584. Halánová, M., Sulinová, Z., Cisláková, L., Trbolová, A., Páleník, L., Weissová, T., Halán, M., Kalinová, Z. and Holičková, M. 2011. Chlamydophila felis in cats--are the stray cats dangerous source of infection? Zoo. Public Health 58(7), 519–522. Harley, R., Herring, A., Egan, K., Howard, P., Gruffydd-Jones, T., Azuma, Y., Shirai, M. and Helps, C. 2007. Molecular characterisation of 12 Chlamydophila felis polymorphic membrane protein genes. Vet. Microbiol. 124(3-4), 230–238. Hartmann, A.D., Hawley, J., Werckenthin, C., Lappin, M.R. and Hartmann, K. 2010. Detection of bacterial and viral organisms from the conjunctiva of cats with conjunctivitis and upper respiratory tract disease. J. Feline Med. Surg. 12(10), 775–782. Helps, C., Reeves, N., Egan, K., Howard, P. and Harbour, D. 2003. Detection of Chlamydophila felis and feline herpesvirus by multiplex real-time PCR analysis. J. Clin. Microbiol. 41(6), 2734–2736. Hughes, L., Visser, S., Heddema, E., de Smet, N., Linssen, T., Wijdh, R.J. and Huis, R. 2024. Zoonotic transmission of Chlamydia felis from domestic cats; a case series of chronic follicular conjunctivitis in humans. N. Mic. N. Inf. 59, 101412. Johnson, F.W. 2024. Isolation of Chlamydia psittaci from nasal and conjunctival exudate of a domestic cat. Vet. Rec. 114(14), 342–344. Kang, B.T. and Park, H.M. 2008. Prevalence of feline herpesvirus 1, feline calicivirus and Chlamydophila felis in clinically normal cats at a Korean animal shelter. J. Vet. Sci. 9(2), 207–209. Kim, H.R., Jeon, G.T., Kim, J.M., Baek, J.S., Shin, Y.K., Kwon, O.K., Kang, H.E., Cho, H.S., Cheon, D.S. and Park, C.K., 2022. Prevalence of Bordetella bronchiseptica, Mycoplasma felis, and Chlamydia felis using a newly developed triplex real-time polymerase chain reaction assay in Korean cat population. Kor. J. Vet. Ser. 45(4), 305–316. Laroucau, K., Ortega, N., Vorimore, F., Aaziz, R., Mitura, A., Szymanska-Czerwinska, M. and Caro, M. R. 2020. Detection of a novel Chlamydia species in captive spur-thighed tortoises (Testudo graeca) in southeastern Spain and proposal of Candidatus Chlamydia testudinis. Sys. Appl. Microbiol. 43(2), 126071. Low, H.C., Powell, C.C., Veir, J.K., Hawley, J.R. and Lappin, M.R. 2007. Prevalence of feline herpesvirus 1, Chlamydophila felis, and Mycoplasma spp DNA in conjunctival cells collected from cats with and without conjunctivitis. Am. J. Vet. Res. 68(6), 643–648. Maazi, N., Jamshidi, S., Kayhani, P. and Momtaz, H., 2016. Occurrence of Chlamydophila felis, feline herpesvirus 1 and calcivirus in domestic cats of Iran. Iran. J. Microbiol. 8, 312. Momtaz, H., Alirezaie, M. and Keyhani, P. 2014. Molecular detection of Chlamydophila felis in ocular discharge of domestic cats in Tehran & Isfahan. Biol. J. Mic. 3, 49–57. Ravindranath, B.S., Vishnu Vinayak, S. and Chandra Mohan, V. 2022. RNR inhibitor binding studies of Chlamydia felis: insights from in silico molecular modeling, docking, and simulation studies. J. Biomol. Str. Dyn. 40(19), 9416–9428. Sainkaplan, S., Irdem, D.I. and Ergin, I. 2022. Assessment of ocular lesions in a persian cat concurrently infected with Chlamydia felis, herpesvirus and coronavirus. Kaf. Uni. Vet. Fak. Der. 28(6), 781–784. Sibitz, C., Rudnay, E.C., Wabnegger, L., Spergser, J., Apfalter, P. and Nell, B. 2011. Detection of Chlamydophila pneumoniae in cats with conjunctivitis. Vet. Ophthalmol. 14(Suppl. 1), 67–74. Sjödahl-Essén, T., Tidholm, A., Thorén, P., Persson-Wadman, A., Bölske, G., Aspán, A. and Berndtsson, L. 2008. Evaluation of different sampling methods and results of real-time PCR for detection of feline herpes virus-1, Chlamydophila felis and Mycoplasma felis in cats. Vet. Ophthalmol. 11(6), 375–380. Tîrziu, A., Herman, V., Imre, K., Degi, D.M., Boldea, M., Florin, V., Bochiș, T.A., Adela, M. and Degi, J., 2022. Occurrence of Chlamydia spp. in conjunctival samples of stray cats in Timișoara Municipality, Western Romania. Microbiology 10(11), 2187. Walter, J., Foley, P., Yason, C., Vanderstichel, R. and Muckle, A. 2020. Prevalence of feline herpesvirus-1, feline calicivirus, Chlamydia felis, and Bordetella bronchiseptica in a population of shelter cats on Prince Edward Island. Can. J. Vet. Res. 84(3), 181–188. Wasissa, M., Lestari, F.B., Nururrozi, A., Tjahajati, I., Indarjulianto, S. and Salasia, S.I.O., 2021. Investigation of chlamydophilosis from naturally infected cats. J. of vet. Sci., 22(6), e67. Wu, S.M., Huang, S.Y., Xu, M.J., Zhou, D.H., Song, H.Q. and Zhu, X.Q. 2013. Chlamydia felis exposure in companion dogs and cats in Lanzhou, China: a public health concern. BMC Vet. Res. 9, 104. | ||
| How to Cite this Article |
| Pubmed Style Zahraa Mustafa Al-Jumaa. Molecular detection of Chlamydophila felis from conjunctiva of cats infected with conjunctivitis and upper respiratory disease. Open Vet. J.. 2024; 14(12): 3289-3295. doi:10.5455/OVJ.2024.v14.i12.13 Web Style Zahraa Mustafa Al-Jumaa. Molecular detection of Chlamydophila felis from conjunctiva of cats infected with conjunctivitis and upper respiratory disease. https://www.openveterinaryjournal.com/?mno=216127 [Access: January 12, 2026]. doi:10.5455/OVJ.2024.v14.i12.13 AMA (American Medical Association) Style Zahraa Mustafa Al-Jumaa. Molecular detection of Chlamydophila felis from conjunctiva of cats infected with conjunctivitis and upper respiratory disease. Open Vet. J.. 2024; 14(12): 3289-3295. doi:10.5455/OVJ.2024.v14.i12.13 Vancouver/ICMJE Style Zahraa Mustafa Al-Jumaa. Molecular detection of Chlamydophila felis from conjunctiva of cats infected with conjunctivitis and upper respiratory disease. Open Vet. J.. (2024), [cited January 12, 2026]; 14(12): 3289-3295. doi:10.5455/OVJ.2024.v14.i12.13 Harvard Style Zahraa Mustafa Al-Jumaa (2024) Molecular detection of Chlamydophila felis from conjunctiva of cats infected with conjunctivitis and upper respiratory disease. Open Vet. J., 14 (12), 3289-3295. doi:10.5455/OVJ.2024.v14.i12.13 Turabian Style Zahraa Mustafa Al-Jumaa. 2024. Molecular detection of Chlamydophila felis from conjunctiva of cats infected with conjunctivitis and upper respiratory disease. Open Veterinary Journal, 14 (12), 3289-3295. doi:10.5455/OVJ.2024.v14.i12.13 Chicago Style Zahraa Mustafa Al-Jumaa. "Molecular detection of Chlamydophila felis from conjunctiva of cats infected with conjunctivitis and upper respiratory disease." Open Veterinary Journal 14 (2024), 3289-3295. doi:10.5455/OVJ.2024.v14.i12.13 MLA (The Modern Language Association) Style Zahraa Mustafa Al-Jumaa. "Molecular detection of Chlamydophila felis from conjunctiva of cats infected with conjunctivitis and upper respiratory disease." Open Veterinary Journal 14.12 (2024), 3289-3295. Print. doi:10.5455/OVJ.2024.v14.i12.13 APA (American Psychological Association) Style Zahraa Mustafa Al-Jumaa (2024) Molecular detection of Chlamydophila felis from conjunctiva of cats infected with conjunctivitis and upper respiratory disease. Open Veterinary Journal, 14 (12), 3289-3295. doi:10.5455/OVJ.2024.v14.i12.13 |