| Review Article | ||
Open Vet. J.. 2024; 14(12): 3181-3188 Open Veterinary Journal, (2024), Vol. 14(12): 3181-3188 Review Article A narrative review of diffusion kurtosis MRI parameters in diagnosing degenerative spine diseases in animal modelsNeil Abraham Barnes1, Winniecia Dkhar1*, Rajagopal Kadavigere2, Suresh Sukumar1, K. Vaishali3 and Abhimanyu Pradhan11Department of Medical Imaging Technology, Manipal College of Health Professions, Manipal Academy of Higher Education, Manipal, India 2Department of Radiodiagnosis and Imaging, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, India 3Department of Physiotherapy, Manipal College of Health Professions, Manipal Academy of Higher Education, Manipal, India *Corresponding Author: Winniecia Dkhar. Department of Medical Imaging Technology, Manipal College of Health Professions, Manipal Academy of Higher Education, Manipal, India. Email: winniecia.dkhar [at] manipal.edu Submitted: 17/09/2024 Accepted: 06/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
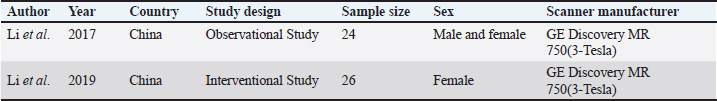
AbstractDegenerative spine disease is a significant health concern in animals due to its impact on daily activities such as movement, balance, and thermoregulation. Early diagnosis is essential for effective management and treatment, with MRI playing a crucial role in identifying degenerative changes. Advanced MRI sequences, particularly diffusion-weighted imaging and diffusion kurtosis imaging (DKI) have determined an important tool for detecting early microstructural changes. This review evaluates the efficacy of DKI parameters for the early diagnosis of degenerative spine disease in animal models. Following the scale for the assessment of narrative review articles guidelines, a systematic literature review was conducted to assess the association between specific DKI metrics and early stage spinal degeneration. Two relevant studies were identified that compared DKI parameters with conventional T2-weighted fast spin echo sequences for disc grading, analyzed gender differences, and performed ROC analysis. The relationship between DKI parameters and post-puncture procedures was also explored. The findings suggest that DKI is more sensitive than conventional MRI and diffusion tensor imaging in detecting subtle microstructural changes in the intervertebral disc space and spinal cord. This highlights DKI’s potential to improve diagnostic accuracy and inform targeted treatments, reducing mortality and life expectancy in affected animals. Further research is warranted to explore the broader applications of DKI in diagnosing degenerative spine conditions. Keywords: Diffusion kurtosis imaging, Degenerative spine, Animal model, Intervertebral disc space, Spinal cord. IntroductionThe spine in mammals is crucial for flexibility, balance, and mobility, while protecting the spinal cord and central nervous system. It also supports physiological functions and regulates body temperature, contributing to overall well-being (Harrison et al., 2013; MacKenzie et al., 2015). Over time, the spine undergoes degeneration, resulting in reduced mobility and posture, as well as impaired thermoregulation, which can lead to severe health issues and life-threatening conditions (Wen et al., 2015). Degenerative disc disease (DDD) involves decreased water content, lower proteoglycan levels, nucleus pulposus cell loss, and annulus fibrosus rupture, affecting disc biomechanics (Rider et al., 2019). Conventionally, spinal degeneration is diagnosed after the manifestation of noticeable symptoms, such as movement impairment, often necessitating surgical interventions that pose significant risks, including infection, nerve damage, and anesthesia complications. These surgical procedures may not restore mobility or function, and recovery can be prolonged and challenging, particularly for elderly animals or those with severe degeneration (Kim et al., 2011; Issy et al., 2013; Che et al., 2019; Barcellona et al., 2022; Santos et al., 2022). Diagnosing and intervening in degenerative disc diseases at an early stage is crucial for improving outcomes and reducing the need for invasive surgical procedures. X-ray and T2-weighted magnetic resonance imaging (MRI) have been instrumental in detecting changes in degenerative disc animal models. X-rays are mainly used to evaluate disc height while T2-weighted MRI provides essential information regarding both disc height and T2 signal intensity (Abdalkader et al., 2020). However, there is a growing need for a new non-invasive in vivo method, which has significantly enhanced the early diagnosis of degenerative spinal diseases (Ruiz Santiago et al., 2022). The advent of advanced clinical MRI parameters and sequences such as diffusion-weighted imaging (DWI) and diffusion kurtosis imaging (DKI), has significantly enhanced the diagnostic accuracy of degenerative spine conditions (Bammer 2003; Fukunaga et al., 2013). DWI sequence is used in assessing the Gaussian movement of the water molecules as compared to DKI sequence which helps in assessing the non-Gaussian movement of the water molecules, which makes the technique a vital tool in early diagnosis of degenerative changes in the spine, which was undetected by DWI, this was made possible by the various parameters of the DKI including, mean Kurtosis (MK), axial Kurtosis (AK), radial Kurtosis (RK), fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). These parameters play a vital role in the early diagnosis of degenerative changes in the spine, which helps in timely treatment and improves the overall life expectancy of the animal (Budjan et al., 2018; Hansen 2019). The review aims to evaluate the effectiveness of the DKI parameters to evaluate the microstructural changes in the degenerative spine. MethodologyQualitative designA qualitative design was followed by the guidelines of the scale for the assessment of narrative review articles (SANRAs), a quick critical appraisal tool for non-systematic studies (Baethge et al., 2019). The initial list of DKI parameters was developed based on a literature review by the project team, comprised of radiologists and medical imaging technologists. Identifying the research questionsThe primary objective in conducting the narrative review was to evaluate how the DKI parameters help to evaluate the subtle microstructural changes in degenerative spines in rats? The research question was developed through an iterative process involving multiple stages: Initial formulationsThe project team initiated the discussion based on the potential research question based on a preliminary search. This aimed to explore the broader implications of DKI parameters in evaluating degenerative spine conditions. Literature reviewA comprehensive literature review was conducted, revealing key studies that highlighted gaps in understanding how DKI parameters correlate with degenerative spine. These insights led to the refinement of our primary research question to ensure it encompassed relevant literature while maintaining a focused scope. Feedback mechanismsThroughout the process, team members provided feedback during regular meetings, which facilitated the adjustment of the question. The iterative dialogue allowed for a consensus on the final formulation, ensuring that it reflected the teams collective expertise and addressed identified gaps in the literature. This question was developed through an iterative process involving discussion among the research team and preliminary literature searches. The question was designed to be broad enough to incorporate a wide range of relevant literature while maintaining a clear focus on the relationship between the DKI parameter and its ability to detect degenerative spine. Identifying relevant studiesA comprehensive search strategy was developed. The following electronic databases were searched: Scopus, Embase, PubMed, and Web of Science. The search was limited to studies published between January 2010 to August 2024, reflecting the period when DKI was started to use from diagnosis degenerative spine. The search strategy included a combination of controlled vocabulary (MeSH terms) and free-text terms. Key search terms included: “MRI” OR “MRI” OR “DKI ” OR “DKI” AND Degenerative Spine” OR “Spinal Degenerative Disease” AND “Lumbar Spine” OR “L-Spine” AND “Spinal Cord” OR “Intervertebral Disc Space.” Study selectionStudies were assessed for eligibility based on predefined selection criteria to ensure the relevance of the included publications. A total of three criteria were used to filter all search results: (1) the studies published in English only since 2010 to ensure the use of modern MRI systems, (2) the studies involving only animal participants and therefore excluding human studies. Studies were selected for full-text screening if their research aim aligned with examining DKI parameters related to the degenerative spine. While reviews were excluded, publications cited within these reviews were considered for inclusion. In addition to excluding full-text publications that did not provide all DKI parameters, or used different methods of DWI application that hampered diagnostic capabilities. Additionally, studies that reported on MRI scans with a field strength of less than 1.5 T were excluded to ensure consistent image quality and alignment with clinical practice, where 1.5 and 3 T are most common. Data extractionCharting the data A standardized data charting form was developed in Microsoft Excel to systematically extract relevant information from the included studies. This form was applied for two animal studies and was refined based on team discussions to ensure effectiveness and comprehensiveness. The data extraction process captured a wide range of information, including study characteristics (author, year, country, and study design), participant attributes (sample size and sex), and MRI characteristics (scanner manufacturer and DKI parameters) in Table 1. Additionally, DKI parameters were evaluated for intervertebral disc degeneration and their effects on nucleus pulposus and annulus fibrosis. Table 1. Study framework and variables.
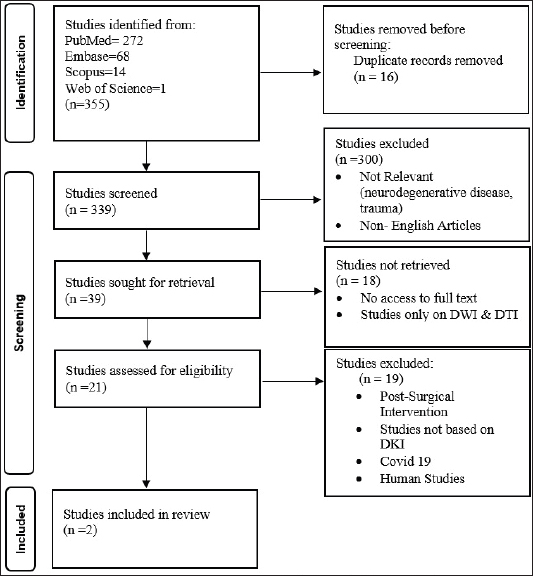
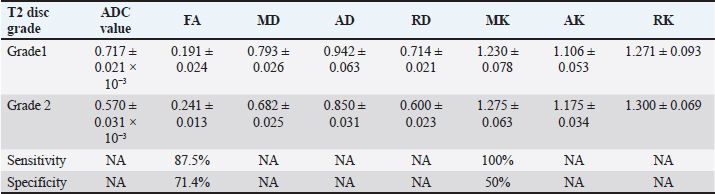
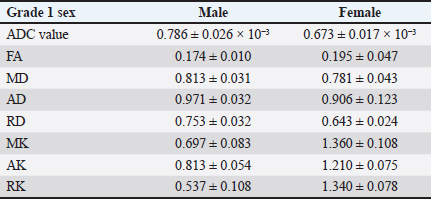
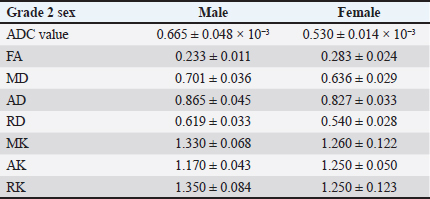
Collating, summarizing, and reporting the resultsThe extracted data were analyzed using a narrative synthesis approach. The findings were organized based on the key themes and concepts that emerged from the data. We also created tables and figures to present the key characteristics and findings of the included studies. In synthesizing the results, we focused on identifying patterns and trends in the relationship between the DKI parameters and the degenerative spine and highlighting any inconsistencies or gaps in the current evidence base. ResultsSearch results and study characteristicsA comprehensive literature review was conducted to examine studies investigating the relationship between DKI parameters and degenerative spinal disease in mammals. The search encompassed multiple prominent databases, yielding 355 articles. After removing 16 duplicate studies, 339 articles were screened, of which 300 were excluded due to their focus on neurodegenerative diseases, trauma, or being non-English. This left 39 articles eligible for full-text retrieval, but only 21 full texts were obtained. Of these, 18 were excluded as they focused on diffusion-weighted imaging and diffusion tensor imaging (DTI) rather than DKI. Further screening of the 21 articles resulted in the exclusion of 19 more, due to reasons such as post-surgical interventions, COVID-19-related studies, or lack of animal participants. Ultimately, two studies met the final review criteria, as depicted in Figure 1. The two studies included in the review explored the application of DKI in evaluating spinal cord microstructural changes associated with degenerative spinal disease in animal models. Analysing the structural parameters of DKIIn a study by Li et al. (2017, 2019), Table 2 presents a comparative analysis of DKI parameters for intervertebral discs categorized by T2 disc grades 1 and 2 according to the Pfirrmann grading system. The results indicate that grade 2 discs exhibit significantly lower apparent diffusion coefficient (ADC) values (0.570 ± 0.031 × 10−³) compared to grade 1 discs (0.717 ± 0.021 × 10−³), suggesting restricted diffusion associated with degeneration. Conversely, grade 2 discs show increased FA (0.241 ± 0.013) and MK (1.275 ± 0.063), reflecting enhanced directional diffusion and greater complexity in the diffusion process. Additionally, all diffusivity measures (MD, AD, and RD) decreased in grade 2 discs, indicating significant structural changes. The sensitivity of FA (87.5%) and MK (100%) highlights their effectiveness in identifying grade 2 discs, although the specificity values suggest a risk of false positives, particularly for MK. An assessment of the accuracy of DKI parameters across gendersThe study conducted by Li et al. (2017, 2019) which is depicted in Tables 3 and 4 presents a gender-based evaluation of DKI parameters for intervertebral discs categorized as grade 1 and grade 2. In grade 1 discs, males exhibited higher values for ADC (0.786 ± 0.026 × 10−³), MD (0.813 ± 0.031), AD (0.971 ± 0.032), and RD (0.753 ± 0.032) compared to females, while females showed significantly higher MK values (1.360 ± 0.108) and better-defined microstructural organization, as indicated by higher FA (0.195 ± 0.047). In grade 2 discs, ADC values were lower for both genders, with males at 0.665 ± 0.048 × 10−³ and females at 0.530 ± 0.014 × 10−³, reflecting increased diffusion restriction. Males maintained higher FA (0.233 ± 0.011) but lower MD (0.701 ± 0.036) compared to females (FA: 0.283 ± 0.024; MD: 0.636 ± 0.029). Additionally, males exhibited greater AD (0.865 ± 0.045) and RD (0.619 ± 0.033), while MK values were similar across genders (males: 1.330 ± 0.068; females: 1.260 ± 0.122). However, AK and RK values were higher in males (AK: 1.170 ± 0.043; RK: 1.350 ± 0.084) compared to females (AK: 1.250 ± 0.050; RK: 1.250 ± 0.123), indicating differing microstructural alterations associated with degeneration. Overall, these findings highlight the significant gender-based differences in DKI parameters across both disc grades. DiscussionAssessment of degenerative changes using DKI parametersThe main objective of the review is to provide a comprehensive summary of the existing evidence regarding microstructural changes in the degenerative spine and their assessment using DKI parameters. The degenerative spine can have a substantial impact on the mobility, balance, and overall quality of life of mammals. The review examines the significance of various DKI parameters in diagnosing these microstructural changes within the intervertebral disc space of rats. The following section will provide an in-depth analysis of the relationship between the spine, intervertebral disc space, and DKI parameters.
Fig. 1. Prisma chart. Anatomical significance of the spine in ratsThe spine plays a crucial role in various functions such as walking, running, climbing, balance, communication, and thermoregulation, making it essential to understand its anatomy for insights into health and behavior. The spinal column in rats consists of 60–61 vertebrae, each comprising of body, arch, and mechanism for attaching muscles and movement of those muscles (Harrison et al., 2013; Olude et al., 2013). The proximal vertebrae are the largest and most robust, that supports the muscles and skin. In contrast, the distal vertebrae are smaller and more flexible, allowing for a greater range of tail movement (Pankiv et al., 2021). The vertebrae are interconnected by an intervertebral disc, that consists of nucleus pulposus and annulus fibrosis, which allows flexibility and movement and works on biomechanical forces (Chan et al., 2011). The spine also protects the spinal cord, which transmits signals from the brain to the body, while contributing significantly to overall posture and locomotion by providing attachment points for muscles, ligaments, and ribs (Pankiv et al., 2021). Impact of spinal degeneration on rat modelDegenerative conditions in rats are similar to those in humans, particularly in the case of intervertebral disc degeneration, which results in a loss of height, disc protrusion into the spinal canal, and calcium deposits in the spine that make it less flexible and more rigid. Additionally, degeneration of the facet joints can cause abnormal mechanical and inflammation, resulting in joint pain and reduced mobility (Kim et al., 2011). This degeneration significantly impacts the rat’s movement and behavior. Pain and stiffness results in decreased mobility due to pain and a hunched posture. These lead to altered gait, reduced activity levels, decreased grooming, social withdrawal, increased irritability, and weight loss due to stress-induced decreased appetite (Giladi et al., 2013; Ruiz Santiago et al., 2022). Table 2. Comparative analysis of DKI parameters by T2 disc grades.
Table 3. Gender-based evaluation of DKI parameters in grade 1 discs.
Table 4. Gender-based evaluation of DKI parameters in grade 2.
Understanding these mechanisms and impacts is crucial for developing effective treatments and translating findings to human conditions. Comparative analysis of structural parameters of DKILi et al. (2017, 2019), evaluated the intervertebral discs classified as grade 1 and grade 2 using a five-grade Pfirrmann degenerating grading system. Significant microstructural differences were observed in the nucleus pulposus of the grade 2 disc compared to grade 1. The decreased ADC value in the grade 2 disc indicated restricted diffusion typically associated with degeneration, while increased FA value (0.241 ± 0.013) and MK value (1.275 ± 0.063) in Table 2 suggested alterations in fiber orientation and complexity within the microstructural environment. Interestingly, no significant differences were found in the disc between the grades in terms of height, MD, RD, AD, RK, and AK. These findings highlight the potential of FA and MK as sensitive markers for early microstructural changes in disc degeneration (Li et al., 2017). As compared to the study that they conducted in 2019, where they performed an interventional procedure and found the differences in T2 grades at different time intervals (Li et al., 2019). Therefore, they propose that by optimizing patient management strategies, DKI can be pivotal in monitoring individuals with disc degeneration without resorting to surgical interventions. Evaluating DKI parameters with ROC analysisLi et al. (2017, 2019) plotted a ROC curve graph where they found out FA exhibited the highest AUC of 0.790, with a sensitivity of 87.5% and specificity of 71.4%, making it the most reliable parameter for distinguishing between the Grade 1 and Grade 2 discs. Comparatively, MK exhibited an AUC of 0.684, sensitivity of 100%, and specificity of 50%, indicating that it was highly effective at detecting grade 2 degeneration, but its lower specificity suggests more false positives. Despite this, MK still is a valuable complementary marker alongside FA for early diagnosis. On the other hand, ADC values showed lower diagnostic accuracy with an AUC of 0.259, suggesting that, ADC alone may not be a sufficient parameter for accurately diagnosing the degenerative disease. Hence it stated the DKI parameters showed the superior diagnostic performance of DKI metrics over traditional diffusion parameters. These findings underscore the potential of DKI parameters as a more sensitive and reliable tool for the early detection and characterization of intervertebral disc degeneration (Li et al., 2017). Assessing DKI parameter accuracy across gendersLi et al. (2017, 2019), the study suggested that there was a significant difference in the nucleus pulposus of intervertebral disc in male rats as compared to the female rats in Tables 3 and 4. In addition, Li et al. (2017, 2019), suggested there was a decrease in ADC and RD values decreased as compared to MK, AK, and RK values significantly increased in the male group as compared to female the group. However, there was no significant differences were found in the parameters of FA, MD, and AD (Li et al., 2017). Li et al. (2017) found no significant differences in the DKI parameters of grade 2 discs between the male and female rats except for the ADC values. Compared to the study conducted by Li et al. (2019) where they recruited only female rats. Moreover, the findings suggested that male and female rats exhibit distinct microstructural characteristics highlighting the necessity for standardized animal models in disc degeneration. Time-dependent variations in imaging metrics post-punctureThe study conducted by Li et al. (2017, 2019), suggested that there were temporal changes in imaging parameters post-puncture provided insights into the progression of disc degeneration. At 3 hours post-puncture, there was an increase in MD, AD, and RD values compared to normal disc. By 48 hours post-puncture, further increases in ADC, MD, AD, and RD values were observed, coupled with a significant decrease in FA, MK, AK, and RK. The variation peaked at 3 days post-puncture, highlighting a critical window for observing maximal changes in disc microstructure. Subsequent reversals in trends at 7 and 10 days, with significant differences in MD, AD, RD, and RK compared to the 3-day group, suggest a dynamic healing or adaptation process post-injury (Li et al., 2019). Other findingsIn the context of degenerative spine conditions in rats, Li et al. (2017, 2019), addressed the use of DKI to evaluate microstructural changes induced by annulus needle puncture. The study found that DKI parameters were sensitive to changes caused by puncture and correlated with histological changes, suggesting that DKI can provide a quantitative assessment of disc degeneration. While not directly focused on degenerative spine conditions in rats, other studies have demonstrated the utility of DKI in detecting microstructural changes in different contexts (Li et al., 2019). For instance, Singhal et al. (2023), showed that DKI and DTI could detect microstructural changes in cervical spondylosis patients before conventional MRI. In summary, the application of DKI in the evaluation of degenerative spine conditions in rats, as demonstrated in Li et al. (2017, 2019), indicates that DKI is a sensitive imaging modality for detecting microstructural changes associated with disc degeneration (Li et al., 2019). The findings from related research further support the potential of DKI in the broader context of degenerative diseases and musculoskeletal disorders. Thus suggesting that DKI could be a valuable tool. The findings highlight the sensitivity of DKI in detecting early microstructural degenerative changes, in the spine surpassing conventional diffusion techniques and DTI. The studies reported that DKI captures subtle, non-Gaussian diffusion patterns, enabling early detection of the degenerative spine. Existing research indicates that the applications of DKI extend beyond spinal pathologies, with potential utility in evaluating musculoskeletal, neurodegenerative, and oncological disorders. DKI can provide detailed information about tissue heterogeneity and structural characteristics, making it a valuable tool in these diverse clinical contexts. This positions DKI as a promising diagnostic tool for earlier diagnosis and more accurate treatment planning, potentially improving patient outcomes in degenerative diseases (Li et al., 2017, 2019; Singhal et al., 2023). LimitationsFirst, the small sample sizes in the reviewed studies may limit the generalizability of their findings, necessitating larger studies to validate the results across broader populations. Additionally, there was significant variability in imaging protocols among the studies, including differences in MRI field strength, DKI parameters, and analytical techniques, making it challenging to compare findings and draw definitive conclusions. Second, the lack of standardized criteria for classifying degenerative changes and interpreting DKI parameters highlights the need for clear guidelines to improve the consistency and reproducibility of research findings. Furthermore, most existing studies focus on early stage degeneration, leaving the effectiveness of DKI in detecting advanced stages or differentiating between various degenerative changes less explored. Finally, interventional studies on the lumbar spine in humans are not feasible, as the results may differ significantly, making it difficult to establish clear conclusions. ConclusionThe integration of DKI into clinical and preclinical studies offers a sophisticated method for understanding and diagnosing degenerative spinal conditions. DKI assesses tissue integrity by quantitatively capturing the non-Gaussian diffusion behavior of water molecules. This capability is crucial for the early detection, monitoring, and treatment planning of degenerative spine diseases, which can significantly improve patient outcomes and advance the field of spinal health. The application of DKI enhances our ability to detect subtle microstructural changes that were not visible with conventional MRI techniques, making it a valuable tool in the ongoing effort to better understand and manage spinal pathologies. The promising results of DKI underscore its potential to revolutionize the approach to diagnosing and treating degenerative spine conditions, highlighting the need for continued research and application in both clinical and preclinical settings. Degenerative spine disease affects humans and animals and can significantly impact mobility, posture, and overall health. Imaging methods such as X-rays and T2-weighted MRI, are not effective enough to detect early microstructural changes in the spine. DKI provides enhanced diagnostic capabilities by measuring the non-Gaussian diffusion of water molecules and detecting the subtle microstructural changes that would have been missed in conventional imaging methods. DKI parameters, such as MD, MK, AK, and RK, were effective in identifying early microstructural changes in degenerative spine conditions, especially in animal models. Based on the findings of the study, MK and FA provide a more sensitive diagnostic tool than traditional methods for DKI metrics. Furthermore, the review highlights the importance of DKI in early diagnosis, potentially reducing the need for invasive surgical intervention. AcknowledgmentsThe author would like to express their sincere gratitude to their colleagues, Mrs. Sneha Ravichandran and Mr. Dilip Shettigar. FundingThis study did not receive any funding. Conflict of interestThere are no conflicts of interest. Authors’ contributionsConceptualization, W.D..; methodology, N.A.; W.D.; validation, N.A.; R.K.; and W.D.; formal analysis N.A.; A.P.; data curation, N.A.; W.D.; writing—original draft preparation, N.A.; writing—review and editing, N.A.; W.D.; S.S.; visualization, R.K.; V.K.; A.P.; supervision, S.S.; V.K.; W.D. All authors have read and agreed to the published version of the manuscript. Data availabilityAll data are presented in the article. ReferencesAbdalkader, M., Guermazi, A., Engebretsen, L., Roemer, F.W., Jarraya, M., Hayashi, D., Crema, M.D. and Mian, A.Z. 2020. MRI-detected spinal disc degenerative changes in athletes participating in the Rio de Janeiro 2016 Summer Olympics games. BMC Musculoskelet. Disord. 21(1), 45. Baethge, C., Goldbeck-Wood, S. and Mertens, S. 2019. SANRA—a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 4(1), 5. Bammer, R. 2003. Basic principles of diffusion-weighted imaging. Eur. J. Radiol. 45(3), 169–184. Barcellona, M.N., McDonnell, E.E., Samuel, S. and Buckley, C.T. 2022. Rat tail models for the assessment of injectable nucleus pulposus regeneration strategies. JOR Spine. 5(3): e1216. Budjan, J., Sauter, E.A., Zoellner, F.G., Lemke, A., Wambsganss, J., Schoenberg, S.O. and Attenberger, U.I. 2018. Diffusion kurtosis imaging of the liver at 3 Tesla: in vivo comparison to standard diffusion-weighted imaging. Acta Radiol. 59(1), 18–25. Chan, W.C.W., Sze, K.L., Samartzis, D., Leung, V.Y.L. and Chan, D. 2011. Structure and biology of the intervertebral disk in health and disease. Orthop. Clin. North Am. 42(4), 447–464. Che, Y.J., Guo, J.B., Liang, T., Chen, X., Zhang, W., Yang, H.L. and Luo, Z.P. 2019. Controlled immobilization-traction based on intervertebral stability is conducive to the regeneration or repair of the degenerative disc: an in vivo study on the rat coccygeal model. Spine J. 19(5), 920–930. Fukunaga, I., Hori, M., Masutani, Y., Hamasaki, N., Sato, S., Suzuki, Y., Kumagai, F., Kosuge, M., Hoshito, H., Kamagata, K., Shimoji, K., Nakanishi, A., Aoki, S. and Senoo, A. 2013. Effects of diffusional kurtosis imaging parameters on diffusion quantification. Radiol. Phys. Technol. 6(2), 343–348. Giladi, N., Horak, F.B. and Hausdorff, J.M. 2013. Classification of gait disturbances: distinguishing between continuous and episodic changes. Mov Disord. 28(11), 1469–1473. Hansen, B. 2019. An introduction to kurtosis fractional anisotropy. AJNR Am J Neuroradiol. 40(10), 1638–1641. Harrison, M., O’Brien, A., Adams, L., Cowin, G., Ruitenberg, M.J., Sengul, G. and Watson, C. 2013. Vertebral landmarks for the identification of spinal cord segments in the mouse. NeuroImage. 68, 22–29. Issy, A.C., Castania, V., Castania, M., Salmon, C.E.G., Nogueira-Barbosa, M.H., Bel, E. Del and Defino, H.L.A. 2013. Experimental model of intervertebral disc degeneration by needle puncture in Wistar rats. Braz. J. Med. Biol. Res. 46(3), 235–244. Kim, J.S., Kroin, J.S., Li, X., An, H.S., Buvanendran, A., Yan, D., Tuman, K.J., van Wijnen, A.J., Chen, D. and Im, H.J. 2011. The rat intervertebral disk degeneration pain model: relationships between biological and structural alterations and pain. Arthritis Res. Ther. 13(5), R165. Li, L., Zhou, Z., Li, J., Fang, J., Qing, Y., Tian, T., Zhang, S., Wu, G., Scotti, A., Cai, K. and Zhu, W. 2019. Diffusion kurtosis imaging provides quantitative assessment of the microstructure changes of disc degeneration: an in vivo experimental study. Eur. Spine J. 28(5), 1005–1013. Li, L., Zhu, W., Chen, W., Fang, J. and Li, J. 2017. The study of the intervertebral disc microstructure in matured rats with diffusion kurtosis imaging. Magn. Reson. Imaging. 42, 101–106. MacKenzie, S.J., Yi, J.L., Singla, A., Russell, T.M. and Calancie, B. 2015. Innervation and function of rat tail muscles for modeling cauda equina injury and repair. Muscle Nerve. 52(1), 94–102. Olude, M.A., Mustapha, O.A., Ogunbunmi, T.K. and Olopade, J.O. 2013. The vertebral column, ribs, and sternum of the african giant rat (Cricetomys gambianus Waterhouse). Sci. World J. 2013(1), 973537 Pankiv, M.V., Paltov, Ye. V. and Masna, Z.Z. 2021. Structural components of the normal thoracic and lumbar vertebrae in rats. JEHS. 11(1), 163–169. Rider, S.M., Mizuno, S. and Kang, J.D. 2019. Molecular mechanisms of intervertebral disc degeneration. Spine Surg. Relat. Res. 3(1), 1–11. Ruiz Santiago, F., Láinez Ramos-Bossini, A.J., Wáng, Y.X.J., Martínez Barbero, J.P., Espinosa, J.G. and Martínez Martínez, A. 2022. The value of magnetic resonance imaging and computed tomography in the study of spinal disorders. Quant. Imaging Med. Surg. 12(7), 3947–3986. dos Santos, D.R., de Araújo, N.P., Teixeira, R.K.C., de Bentes, L.G. B., Giubilei, D.B., de Chaves, R.H. F., Gonçalves, A.A., Yasojima, E.Y. and de Barros, R.S.M.. 2022. Anatomical description of the ventral and dorsal cervical rootlets in rats: a microsurgical study. Acta Cir. Bras. 37(3). e37030. Singhal, S., Saran, S., Saxena, S., Bhadoria, A.S. and Grimm, R. 2023. Role of diffusion kurtosis imaging in evaluating microstructural changes in spinal cord of patients with cervical spondylosis. Eur. Spine J. 32(3), 986–993. Wen, J., Sun, D., Tan, J. and Young, W. 2015. A consistent, quantifiable, and graded rat lumbosacral spinal cord injury model. J. Neurotrauma. 32(12), 875–892. | ||
| How to Cite this Article |
| Pubmed Style Barnes NA, Dkhar W, Kadavigere R, Sukumar S, K. V, Pradhan A. A narrative review of diffusion kurtosis MRI parameters in diagnosing degenerative spine diseases in animal models. Open Vet. J.. 2024; 14(12): 3181-3188. doi:10.5455/OVJ.2024.v14.i12.3 Web Style Barnes NA, Dkhar W, Kadavigere R, Sukumar S, K. V, Pradhan A. A narrative review of diffusion kurtosis MRI parameters in diagnosing degenerative spine diseases in animal models. https://www.openveterinaryjournal.com/?mno=220923 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i12.3 AMA (American Medical Association) Style Barnes NA, Dkhar W, Kadavigere R, Sukumar S, K. V, Pradhan A. A narrative review of diffusion kurtosis MRI parameters in diagnosing degenerative spine diseases in animal models. Open Vet. J.. 2024; 14(12): 3181-3188. doi:10.5455/OVJ.2024.v14.i12.3 Vancouver/ICMJE Style Barnes NA, Dkhar W, Kadavigere R, Sukumar S, K. V, Pradhan A. A narrative review of diffusion kurtosis MRI parameters in diagnosing degenerative spine diseases in animal models. Open Vet. J.. (2024), [cited January 25, 2026]; 14(12): 3181-3188. doi:10.5455/OVJ.2024.v14.i12.3 Harvard Style Barnes, N. A., Dkhar, . W., Kadavigere, . R., Sukumar, . S., K., . V. & Pradhan, . A. (2024) A narrative review of diffusion kurtosis MRI parameters in diagnosing degenerative spine diseases in animal models. Open Vet. J., 14 (12), 3181-3188. doi:10.5455/OVJ.2024.v14.i12.3 Turabian Style Barnes, Neil Abraham, Winniecia Dkhar, Rajagopal Kadavigere, Suresh Sukumar, Vaishali K., and Abhimanyu Pradhan. 2024. A narrative review of diffusion kurtosis MRI parameters in diagnosing degenerative spine diseases in animal models. Open Veterinary Journal, 14 (12), 3181-3188. doi:10.5455/OVJ.2024.v14.i12.3 Chicago Style Barnes, Neil Abraham, Winniecia Dkhar, Rajagopal Kadavigere, Suresh Sukumar, Vaishali K., and Abhimanyu Pradhan. "A narrative review of diffusion kurtosis MRI parameters in diagnosing degenerative spine diseases in animal models." Open Veterinary Journal 14 (2024), 3181-3188. doi:10.5455/OVJ.2024.v14.i12.3 MLA (The Modern Language Association) Style Barnes, Neil Abraham, Winniecia Dkhar, Rajagopal Kadavigere, Suresh Sukumar, Vaishali K., and Abhimanyu Pradhan. "A narrative review of diffusion kurtosis MRI parameters in diagnosing degenerative spine diseases in animal models." Open Veterinary Journal 14.12 (2024), 3181-3188. Print. doi:10.5455/OVJ.2024.v14.i12.3 APA (American Psychological Association) Style Barnes, N. A., Dkhar, . W., Kadavigere, . R., Sukumar, . S., K., . V. & Pradhan, . A. (2024) A narrative review of diffusion kurtosis MRI parameters in diagnosing degenerative spine diseases in animal models. Open Veterinary Journal, 14 (12), 3181-3188. doi:10.5455/OVJ.2024.v14.i12.3 |