| Research Article | ||
Open Vet. J.. 2024; 14(12): 3552-3562 Open Veterinary Journal, (2024), Vol. 14(12): 3552-3562 Research Article Robust anticancer efficacy of Naja haje venom-loaded silica nanoparticles against triple-negative breast cancer xenografts in a preclinical rat modelNabil A. Soliman*, Amr A. Shalaby, Heba Allah Mohamed, Sara M. Abdelkarem Alashqar and Mohamed Ahmed AmmarZoology Department, Faculty of Science, Zagazig University, Sharkia, Egypt *Corresponding Author: Nabil A. Soliman. Zoology Department, Faculty of Science, Zagazig University, Sharkia, Egypt. Email: nabilsoliman54 [at] yahoo.com Submitted: 16/10/2024 Accepted: 24/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
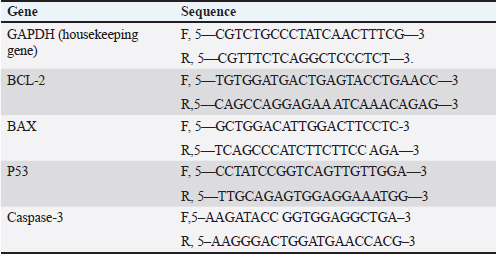
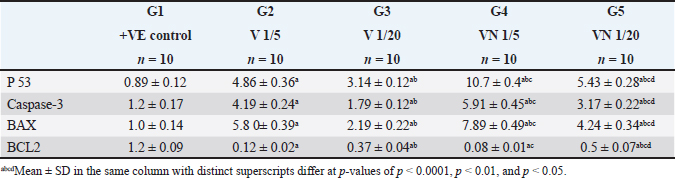
AbstractBackground: Breast cancer, a prevalent disease affecting women globally, is particularly aggressive and has limited treatment options. Aim: Snake venom, containing active chemicals, has shown potential in medicine. Methods: The study investigates the anticancer effect of Egyptian cobra Naja haje venom alone and in combination with Nanoparticles (NP) on TNBC in vivo. The study involved dividing experimental animals into five groups, each with 10 rats, each treated with different doses of crude venom, G2 and G3, respectively. The study involved loading venom onto NP-based delivery systems, measuring inflammatory cytokines and tumor markers, extracting RNA, real-time qRT-PCR gene expression, and histopathological examination of breast tissue. Results: The study involved administering Naja haje crude venom at higher (1/5 LD50) and lower (1/20 LD50) dose levels in groups G2 and G3, respectively. Conclusion: The study found that venom treatment in groups G4 and G5 significantly improved inflammatory cytokine and tumor markers levels, increased expression of tumor-suppressor genes, and increased apoptosis and necrosis. Keywords: Triple negative breast cancer, Naja haje venom, Anticancer, Inflammatory cytokines, NPs. IntroductionBreast cancer (BC) is the second most common cause of cancer-related mortality among women. (Chan and Moris, 2006). As reported by (Shah et al., 2014) classifications for BC have been created in an effort to treat patients more successfully. Triple-negative breast cancer (TNBC), is an extremely truculent subtype of BC (Zhu et al., 2023). It is more common in young women compared to other BC subtypes, and it is linked to higher cancer risk and death (Dent et al., 2007). On the surface of TNBC, there is no expression of the progesterone, estrogen, or HER2 receptors (Borri and Granaglia, 2021). Considering this fact, TNBC represents a unique and challenging entity within BC due to its aggressive behavior, limited treatment options, and poorer prognosis compared to other subtypes (Wein and Loi, 2017). Although chemotherapy is the main form of treatment for patients with TNBC, the effectiveness of chemotherapy for TNBC is still limited (Yi et al., 2021). Animal poisons and venoms from a variety of species, including snakes, scorpions, cone snails, bees, and wasps, have been extensively researched over time due to their potential as a significant source of bioactive chemicals (Harvey, 2014). A wealth of active pharmacological proteins and peptides have been reported to be found in animal venoms (Mohamed et al., 2019). For many years numerous studies have demonstrated the significant effect of cobra venom in providing relief from various types of pain, including that associated with cancer, neuralgia, and joint-related disorders (Liang et al., 2015). Predominately snake venom comprises various polypeptide toxins and enzymes manifesting diverse pharmacological and biological activities contra virus, bacteria, and tumor (Salama et al., 2018; Roy and Bharadvaja, 2021). Venom enzymes extracted from different snake species have recently been shown in a number of studies to have potential therapeutic benefits, and this has led to a growing interest in their application in the biomedical area (Cedro et al., 2018). Furthermore, snake venom has been utilized in varied communities based on its ability to decrease the cultivating of cancer cells, stifle cancer alternation and metastasis (Gomes et al., 2010). Its anticancer mechanisms rely on the immunosuppressive, cytotoxic, and antiproliferative strength of its proteins, which have the capability to promote cancer cells apoptosis (Mahmoud et al., 2019). The potential of snake venom in the treatment of cancer has been demonstrated by several studies. Cancer cells are cytotoxically affected by phospholipase A2, L-amino-acid oxidase, metalloprotease, disintegrin, and other peptides found in snake venom (Shanbhag, 2015). The majority of phospholipases A2 are found in elapids, especially Naja sp., and they have a variety of functional pharmacological effects, including immunological modulation, hemolysis, edoema, neurotoxicity, myotoxicity, cytotoxicity, anticoagulation, and anticancer activities (Kang et al., 2011). To increase the effectiveness of treatment, NPs may be engineered to specifically target cancer cells and deliver therapeutic substances like medications or contrast agents. This can be achieved by combining biological and engineering techniques (Sun et al., 2020). Several types of NPs such as solid, hollow, mesoporous, mesoporous core-shell, or hybrid forms have been widely applied as drug delivery agents for cancer diagnosis and treatment (Tiburcius et al., 2021). Chemical therapeutics-loaded nanoparticles (NP) have showed a lot of promise in the treatment of cancer. NPs can successfully raise medication concentrations in cancer tissues and work at the cellular level to improve antitumor efficacy when loaded with anticancer drugs (Barratt, 2003). Therefore, we examined the effects of Najahaje venom on the growth of TNBC cell line MDA-MB-231- bearing experimental rats in the current investigation, both alone and in conjunction with silica NPs (venom + NP). Materials and MethodsExperimental animalsThis experiment relied on fifty adult female Albino Wistar rats, aged between 7 and 9 weeks. The rats varied in weight from 100 to 120 g. The rats were obtained from the faculty of veterinary medicine, Zagazig University, Egypt. To provide suitable housing conditions, the rats were kept in plastic cages with ten rats per cage under controlled temperature and provided with standard rodent chow in the animal house, F aculty of Medicine, Zagazig University, Egypt. Tumor cellsThe triple negative BC cell line (MDA-MB-231) was acquired from the American type culture collection. Cells were cultured in RPMI replenished with 10% FBS, Penicillin 100 U/ml, and 100 mg/ML of streptomycin at criterion conditions (a humidified atmosphere at 37°C and 5% CO2). The tumor cells then were collected out for fecundation by washing with PBS twice pursued by compendious incubation in 0.25% trypsin and 0.02% EDTA (Neudert et al., 2003). Tumor cell’s inoculationTo prepare for the injection of tumor cells into the mammary fat pad, the rats underwent anesthesia using metofane, and a small incision measuring 5 mm was made in the skin on the lateral thorax. This allowed for the exposure of the mammary fat pad (M.F.P). Using a 27-gauge needle, a volume of 0.1 ml of cell inoculation was injected into the tissue. The growth of tumors in the mammary fat pad was monitored on a weekly basis for a duration of 6 weeks. Subsequently, the treatment process was initiated (Price et al., 1990). VenomDifferent ways of extracting venom were employed from Bothrops alternatus, Bothrops Neuwiedi, and Crotalus durissus. These methods included: a) spontaneous ejaculation during biting; b) hand massage on glands; and c) electrical stimulus to the muscle. For these animals, electrical stimulation produces superior outcomes (di Tada et al., 1978). In the laboratory of the Physiology Department of the Faculty of Science, Ain Shams University, Egypt, the venom of the Egyptian cobra, Naja haje, was extracted by spontaneous ejaculation when biting and lyophilized. Using the Meier and Theakston method, the deadly toxic dose (LD50) of the venom was found in (1986). Combination of snake venom with silica NPsSilica NPs and their combination with snake venom were prepared at the Nanotechnology Laboratory of Cairo University in Egypt. Cobra venom-silica nanocomposites were synthesized using sonochemical methods, wherein ultrasonic waves induced the formation and growth of micro-bubbles. These micro-bubbles generated extreme temperature and pressure both internally and externally. As the bubbles collapsed, the cobra venom molecules were exposed to these extreme conditions, leading to the nucleation of NPs. Rapid cooling then facilitated the synthesis of cobra venom loaded into mesoporous silica NPs (MSNs). In another step, 0.05 g of MSNs were dispersed in 100 ml of double-deionized water. This dispersion was combined with a solution of 0.1 g of cobra venom in 100 ml of double-deionized water, resulting in a mixture of 200 ml. The mixture was subjected to sonication for 3 hours using specific conditions, including a pulse time of 2 seconds, a rest time of 1 second, and a temperature maintained below 50°C with an amplitude of 75%. Experimental animal designLethality was assessed by injecting different doses of venom in 0.5 ml saline via the intraperitoneal route, resulting in an LD50 value of 0.568 mg/kg. The experiment included five groups, each consisting of ten rats, of which five groups had tumors. Group 1(+ve control): served as the positive control group (a MDA-MB231induced non treated group). Group 2 (1/5 LD50V), and Group 3 (1/20 LD50V) represented the treated group which included MDA-MB231 -induced mammary gland tumors for 6 weeks (Price et al., 1990), then intramuscular injected with a dose equivalent to 1/5 of the LD50 (0.1 mg/kg) for G1, and 1/20 of the LD50 (0.02 mg/kg) for G3 of Nh cobra venom for 4 weeks (twice weekly), (Markland et al., 2002). Group 4 (1/5 LD50V + NP) & Group 5 (1/20 LD50 V + NP) were similar to G2 and G3, however in addition to the intramuscular injection with Nh venom, the venom was combined with MSNs (Bhowmik et al., 2014). At the end of the experiment, the rats were euthanized under Na thiopental anesthesia to conclude the experiment. Assessment of biochemical markersThe collected blood samples were allowed to coagulate at ambient temperature for 30 minutes, followed by centrifugation at 3,000 rpm for 10 minutes. The separated serum was then analyzed for the concentrations of the tumor markers carcinoembryonic antigen (CEA) and cancer antigen 15.3 (CA15.3) by using reagent ELISA-kits obtained from Sino Gene Clon Biotech Co, China. Tumor necrosis factor alpha (TNF-α) was stately by Elisa kit with Cat. No E0082Hu and interleukin 6 (IL 6) by Bioassay Technology Laboratory ELISA kit with the cat. No E0090Hu. Real-time quantitative PCR analysisTotal RNA was isolated from the tissue samples using the Trizol (Invitrogen; Thermo Fisher Scientific, Inc.). Specifically, 30 mg of the whole tissue was homogenized in 1 ml of Trizol reagent. Subsequently, 200 μl of chloroform was added to the homogenate, the mixture was vortexed, incubated for 3 minutes, and then centrifuged at 12,000 g for 15 minutes at 4°C. The quality of the extracted RNA was assessed by analyzing the A260/A280 ratio using a NanoDrop® ND-1000 Spectrophotometer. For complementary DNA synthesis, a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems™, USA) was utilized. The real-time reverse transcription-polymerase chain reaction (RT-qPCR) was performed using a Mx3005P Real-Time PCR System (Agilent Stratagene, USA) and TOPreal™ qPCR 2X PreMIX (SYBR Green with low ROX) (Enzynomics, Korea). The real-time RT-qPCR amplification was performed using the following cycling parameters: an initial denaturation step at 95°C for 12 minutes, followed by 40 cycles of denaturation at 95°C for 20 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds. The gene-specific oligonucleotide primer sequences were designed and synthesized by Sangon Biotech (Beijing, China) (Table 1). The expression levels of the target genes (BAX- BCL-2, P53, Caspase-3 and BAX/BCL2 ratio) were normalized to the mRNA expression of the housekeeping gene, rat Gapdh. The results are presented as fold-changes compared to the control group, calculated using the 2-ΔΔCT method (Livak and Schmittgen, 2001). Histopathological essayAt the conclusion of the experiment, the mammary gland tissues were harvested from the sacrificed rats. The collected tissue samples were first fixed in a 4% buffered formaldehyde solution, and then embedded in paraffin wax for sectioning. Using a manually operated rotary microtome (model CUT 4055 or 4055F, R), the paraffin-embedded tissue samples were sectioned at a thickness of 5 micrometers. These thin tissue sections were then mounted onto glass microscope slides and stained using the hematoxylin and eosin histological staining technique (Bancroft and Gamble, 2008). Statistical analysisSoftware Statistical Product and Service Solutions version 19 was used for the collection, tabulation, and statistical analysis of all the data. The information was presented using the mean ± standard deviation (SD). A one-way analysis of variance test and a least significant difference analysis were used to evaluate the difference. p-value: value of p ≥ 0.05 was regarded as statistically insignificant, and a value of p < 0.001 as statistically significant. Ethical approvalOn June 26, 2024, the ZU-IACUC Committee and the International Animals and Use Committee accepted the updated protocol and gave it the approval number ZU-IACUC/1/F/143/2024. ResultsThe expression of tumor-suppressor gene P53, Caspase-3, pro-apoptotic gene BAX, and anti-apoptotic gene BCL-2 was observed in the cancerous group (G1) and all the provided treatment groups (G2,3,4&5). For P53, Caspase-3, and BAX genes there was a significant upregulation (p < 0.001) in all treated groups compared to the cancerous group (+ve control) which exhibited a significant downregulation in the level of previously mentioned genes. The best result of the elevation in the level of expression of (P53, Caspase-3, and BAX) between all groups was observed in the group that was cured with 1/5 LD50 of Nh venom loaded on NPs (G4), and by comparing between G2 and G3 that received the crude venom with different LD50 doses, the one that treated with higher dose (1/5 LD50) (G2) showed a significant increasing (p < 0.001) in (P53, Caspase-3, and BAX) genes than G3 that cured with lower concentrated dose (1/20 LD50) The anti-apoptotic gene BCL-2 levels were significantly elevated after inoculating of cancer cells in the mammary fat tissue in G1. When comparing the +VE control group (G1) with all treated groups, there was a significant (p < 0.001) lowering in the BCL-2 levels, reaching the best result in both groups that were treated with 1/5 LD50Nh venom +NP (G4), and group (2) that received 1/5 LD50Nh venom, where the previously mentioned groups showed non-significant (p > 0.5) difference in BCL-2 level of expression. Table 1. Primer sequence of genes used in this trial.
Table 2. Effect of 1/5, 1/20 LD50 of Nh venom and 1/5, 1/20 LD50 of Nhvenom + NP on the expression of P53, Caspase-3, BAX and BCL-2.
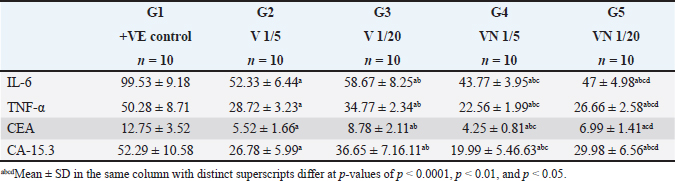
Table 3. Effect of 1/5, 1/20 LD50 of Nh venom and 1/5, 1/20 LD50 of NhV +NP on the level of Inflammatory cytokines (TNF-α & IL-6) and on CEA and CA15.3 in all studied groups.
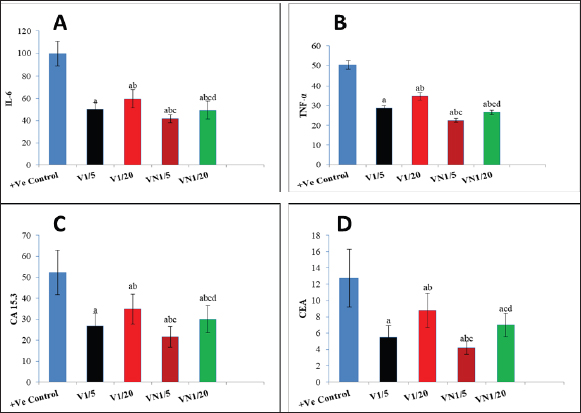
Regarding the Inflammatory cytokines (TNF-α and IL-6), In the cancerous group (G1) both TNF-α and IL-6 were significantly increased, and the administration of Nh venom in both (G2 and G3) resulted in substantial reduction (p < 0.001) compared to (G1) considering the higher venom dose (1/5) in G2 that exhibiting an even more pronounced effect leading to a significant reduction (p < 0.001) in both TNF-α and IL-6 when compared with G3. The combined treatment of (1/20) dose of venom with NPs in G5 resulted in a more effective and significant reduction (p < 0.001) in both TNF-α and IL-6 compared to G3 & G2 which received the same dose of crude venom. The higher venom dose (1/5) combined with NPs in G5 exhibited the best substantial reduction (p < 0.001) of TNF-α and IL-6 among all treatment groups. Concerning the levels of the cancer biomarkers CEA and CA15.3, both were elevated in the cancerous group (G1). The exposure to Nh venom in both G2&G3 with doses (1/5) and (1/20), respectively, exhibited a significant (p < 0.001) reduction in the previously mentioned parameters. Furthermore, the higher venom dose (1/5) in G2 gave significant downregulation (p < 0.001) in both levels of CEA & CA15.3 when compared with the lower venom dose (1/20) in G3, Also G2 showed significant downregulation (p < 0.001) in the level of CA15.3 when compared with G5 (1/20 VN), but for CEA levels, there was a nonsignificant difference (p > 0.05) between G2 and G5. The combination of the higher venom dose (1/5) with NPs in group G4 showed the most authentic and significant suppression (p < 0.001) of CEA and CA15.3 levels among all groups. On the other hand, G4 which represented the combination of lower venom dose (1/20) with NPs, showed a significant reduction when compared with the same dose (1/20), but for crude venom only in G2.
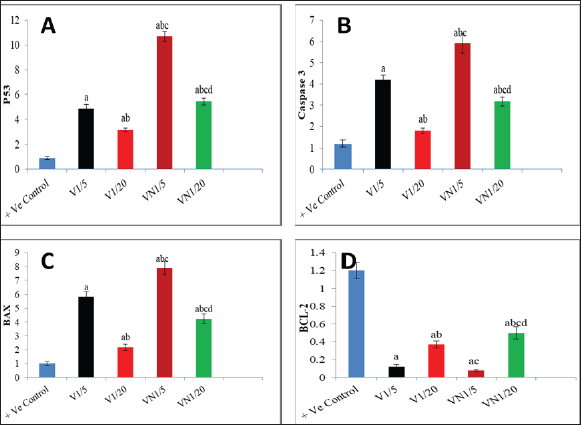
Fig. 1. Levels of genes expression (P53, Caspase3, BAX and BCL-2) in al studied groups, G1 (+Ve control), G2 (1/5 LD50V), G3 (1/20LD50V), G4 (1/5 LD50V + NP) and G5 (1/20 LD50V + NP) where (A) represents (P53), (B) represents (Caspase3), (C) (BAX) and (D) (BCL-2). aSignificant difference with group 1, bSignificant difference with group 2, csignificant difference with group 3, and dsignificant difference with group 4. The levels of CEA, CA15.3, and inflammatory cytokines (IL-6 and TNF-α) were observed in all the groups under study: G1 (+Ve control), G2 (1/5 LD50V), G3 (1/20 LD50V), G4 (1/5 LD50 V + NP), and G5 (1/20 LD50 V + NP), where (A) stands for IL-6, (B) for TNF-α, (C) for CEA, and (D) for (CA15.3). Groups 1 and 2 showed a significant difference, group 3 showed a significant difference, and group 4 showed a significant difference. DiscussionWorldwide, BC is the most frequent malignant disease that affects women. Despite the fact that BC incidence has increased substantially over the past few decades (Leong et al., 2010). TNBC refers to tumors that are devoid of ER, progesterone, or HER2 expression; these molecules are targets for therapeutic interventions (Malorni et al., 2012). Additionally, Chemotherapeutic resistance is one of the biggest hurdles to the efficient treatment of cancer. It frequently stops tumor cells from passing through phases of programmed cell death; apoptosis conducting in the survival of cancer cells and delaying the treatment process (Wilson et al., 2009). Consequently, focusing on the disrupted apoptotic signaling pathways has become a viable approach to tackle this persistent challenge in the treatment of cancer (Liu et al., 2023). It could potentially be conceivable to steer cancer cells toward self-destruction with therapeutic drugs that have the ability to either activate pro-apoptotic proteins or inhibit anti-apoptotic proteins. This would rescind resistance mechanisms, which frequently thwart the effectiveness of traditional cytotoxic therapies (Fymat, 2017). Snake venoms are increasingly considered as a potential source of biologically active compounds that hold promise for therapeutic purposes including the treatment of chronic diseases such as cancer (Li et al., 2018), Additionally it has been demonstrated that snake venom can induce cytotoxicity in various types of tumor cells (Bazaa et al., 2009). Previous studies have reported the promising anti-cancer activity of snake venoms in vivo as well as it has demonstrated that cobra venom can inhibit the growth of inoculated hepatocellular carcinoma cells in rat models (Sun et al., 2003).
Fig. 2. The levels of CEA, CA15.3, and inflammatory cytokines (IL-6 and TNF-α) were observed in all the groups under study. For more accuracy in the treatment process, a significant amount of cancer-related research has been conducted with the goal of creating a medication that more precisely targets tumor cells and raising drug concentration in cancer cells, for instance, NP-based drug delivery systems have featured numerous preferences in cancer treatment as rigorous targeting of cancerous cells, improve pharmacokinetic behavior of the drugs and reduction of side effects of drug resistance (Dadwal et al., 2018). In this study, we investigated the potential therapeutic efficacy of the Egyptian cobra, Naja haje venom either alone or in combination with silica nanoparticles on BC that was developed into adult female rats. The experimental BC model was established by inoculating the rats with the MDA-MB-231 TNBC cell line. Tumor markers are molecules that are released by both healthy and malignant cells. However, the levels of these markers are markedly elevated in the presence of cancer activity, reported by (Lohmann et al., 2018). Our results obtained from the tumor marker (CEA&CA15.3), following the subcutaneous inoculation of MDA-MB-231 in (+ve control group) that did not receive any treatment, revealed a significant elevation as a result of cancer development, which came in agreement with (Ebeling et al., 2002) and (Guo and Gau, 2022), Moreover, Wu et al., 2014 demonstrated that the majority of patients with BC suffering from increasing in both CA15.3 and CEA level. Lee et al., 2013 reported that increased tumor load has been shown to be strongly correlated with increasing serum levels of the tumor markers CA15.3 and CEA, the higher the concentrations of these biomarkers, the greater the risk of the cancer spreading and developing systemic metastases, which came in confirmation with our histopathological examination of the breast tissue in the same group where breast ducts were found to be dilated and lined by atypical epithelial cells exhibiting increased mitotic activity and prominent nucleoli. Similarly, Marangoni et al., 2007 depended on the inoculation of BC fragments into the fat pad of old female mice, resulting in the presence of necrotic areas in the ductal structure, inflammatory areas, and infiltrating ductal carcinoma, as well as, Angeline Kirubha et al., (2012), who depended on 7,12-Dimethyl benz(a)anthracene (DMBA) to evaluate mammary cancer, founded a ductal carcinoma and abnormal epithelial proliferative breast lesions situ plus central necrosis of rat mammary tissue, Along with that, Roy et al., (2019) who also demonstrated a atypical hyperplasia, proliferation of ductal epithelial lining in the mammary tissue of the cancerous rat group. There is a well-established correlation between the induction of cancer development and the chronic inflammation. This connection is primarily mediated through the action of inflammatory cytokines as TNF-α and IL-6 which not only promote the inflammation but also drive the epigenetic alternation in the promoter regions of tumor suppressor genes and cell cycle regulatory genes Hodge et al., 2005. Our TNF-α & IL-6 findings exhibited high levels of both cytokines in the inoculated cancerous group which is similar to Al-Hassan et al., 2012 who also reported elevated serum IL-6 and TNF-α within newly diagnosed BC patients. Analogous to Gulbahce-Mutlu et al., 2021 who announced that the DMBA-induced BC group had the highest IL-6 levels.
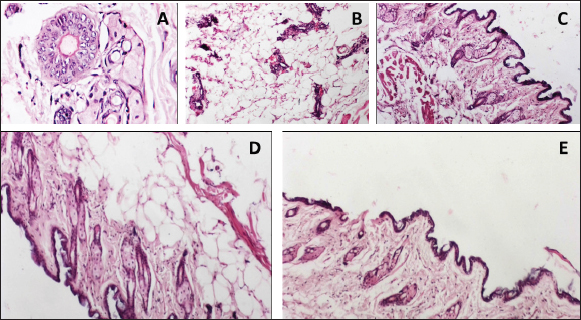
Fig. 3. Histopathological examination of mammary gland tissues in the studied groups. (A) Group (1) the cancerous group showed dilated breast duct lined by atypical epithelial cells with increased mitosis and prominent nucleoli. (B) Group (2) breast cancer group that received (1/5) LD50 of Nh venom showed dilated breast duct containing secretory material surrounded by moderate inflammation and fibrosis with obvious decrease of atypical cell lining. (C) Group (3) breast cancer group that received (1/20) LD50 of Nh venom revealed dilated breast duct containing secretory material surrounded by mild inflammation and fibrosis with obvious decrease of atypical cell lining. (D) Group (4) (1/5 LD50V) and (E) Group (5) (1/20 LD50V + NP) both showed near normal breast architecture with replacement of the abnormal ducts with adipose tissue. (TNF-α) and (IL-6) appear to play an essential role in diverse stages of tumor development, including formation, invasion, and metastasis. This is primarily assignable to their ability to activate a range of oncogenic transcription factors, such as the signal transducer and activator of transcription (STAT) proteins STAT1, STAT3, and STAT5, and induce epigenetic alterations in the promoter regions of tumor suppressor proteins and cell cycle regulatory genes causing inactivation of P53 a tumor suppressor gene (Su et al., 2019). P53 is involved in the progression of many biological processes such as cell cycle arrest and apoptosis through the transcriptional regulation of the corresponding genes as BAX and BCL-2 genes. BCL-2 is an anti-apoptotic protein that enhances survival via cytochrome c residue binding. Contrarily BAX is a pro-apoptotic protein participant in the release of cytochrome C and inducing the caspase-dependent apoptotic pathway (Um, 2016) and (Renault et al., 2017). At the molecular level and in consistency with our inoculated group with the tumorous cell line, the decreased activities of P53 in BC models and liver carcinoma are accompanied by downregulation of both BAX and caspase-3 levels of expression besides elevating in the BCL-2 levels as reported by (Roy et al., 2019) and (Changizi et al., 2021) which came in similarity with our findings for the previously mentioned genes. The present study comprehensively evaluated the antineoplastic efficacy of crude Naja haje (Nh) venom at two dose levels, 1/5 LD50 (high dose) and 1/20 LD50 (low dose), as well as the effect of Nh venom loaded on nanoparticles delivery systems at the corresponding LD50 doses. The results demonstrated that both the high-dose (1/5 LD50) and low-dose (1/20 LD50) of the crude Nh venom were effective in significantly suppressing the growth of TNBC implanted in the rat model. However, the high-dose (1/5 LD50) crude venom treatment showed a more pronounced anti-tumor activity compared to the low-dose (1/20 LD50) crude venom group. Captivatingly the 1/5 LD50 NhV + NP showed the most potent inhibitory effects on TNBC tumor development. These findings analogous to the seminal work by (Omran, 2003), which previously demonstrated a remarkable cytotoxic and anticancer properties of high doses of Najahaje venom against both breast and prostate cancer cell lines in contrast to the diminished effects shown at lower venom concentrations. Abe et al., 2002 reported that apoptosis induction by venom have been resulted in the inhibition of the tumor cells. Badr et al., 2014 revealed that Walterinnesia aegyptia venom, both alone and in combination with nanoparticles (NPs) increased the activities of caspase-3, caspase-8, and caspase-9 in human breast and prostate cancer cells. Furthermore, Shebl et al., 2012 illustrated the induction of apoptosis after treatment with Viperalebtina snake venom by elevating the expression of the pro-apoptotic p53 and BAX genes and in the contrary downregulation of the BCL-2 level of expression. These data are in harmony with a study reporting that crud cobra snake venom helps in suppressing human breast and liver carcinoma progression via increasing the expression of BAX and decreasing the expression of BCL-2 published by El-Sharkawi et al., 2015. All the previously mentioned studies came in agreement with El-Ghani and Amr 2020 who observed that levels of Caspase-3 was elevated in the group treated with Egyptian Nh venom similarly El hakim et al., 2011, illustrated the induction of mitochondrial apoptosis pathway and over expression of caspase-3 in the groups treated with Nh venom. The real-time RT- PCR results in our study demonstrated that the Nh and Nh + NP treatment activated P53, increased BAX and Caspase3 levels of expression, and decreased the BCL-2 gene level of expression which indicated of initiating apoptosis cascade and initiating the healing process in a response to the venom treatment. Consistent with the induction of apoptosis at molecular levels, the levels of tumor markers CEA & CA15.3 inflammatory cytokines TNF-α and IL-6 were noteworthy downregulated in all treatment groups with the preferable outcome in G4 that received 1/5 LD50 Nh + NP compared to either the cancerous group or to the other treatment groups. Aslam et al., 2024 found that Naja oxiana venom suppress inflammation by attenuating pro-inflammatory cytokines pathway, Cui et al., 2014 agreed to this report by demonstrated in a study depended on Naja naja atra venom to mend pulmonary fibrosis by downregulation of TNF-α. Snake venoms is a complex mixture of diverse bioactive constituents, such as growth factors, poisons, enzymes, activators, and inhibitors. Furthermore, the combined between the multifarious components of Naja haje (Nh) venom probably participate to the observed anti-tumor effects and the mechanistic pathways demonstrated in the present study. This study revealed the unique biological effects of Nh and Nh + NP with both high dose and low dose on Triple negative BC cell line inoculated in rat model which may permit these compounds to be utilized in treatments for breast cancer. Conflict of interestThe authors declare no competing interests. FundingNot applicable. Authors’ contributionsThe study was conceptualized and designed by all authors, with the experiment being supervised by N.A.S. and A.A.S.H.A.M. carried out the study’s practical components, conducted data analysis, S.M.A and M.A.A. wrote and revised the manuscript. All authors read and appropriate the manuscript. Data availabilityThe raw data are available and can be provided upon reasonable request from the corresponding author. ReferencesAl-Hassan, A.A., Al-Ghurabi, B. and Al-Karkhi, I. 2012. Prognostic value of proinflammatory cytokines in breast cancer. J. Biomol. Res. Ther. 1(104), 2. Al-Sadoon, M.K., Abdel-Maksoud, M.A., Rabah, D.M. and Badr, G. 2012. Induction of apoptosis and growth arrest in human breast carcinoma cells by a snake (Walterinnesiaaegyptia) venom combined with silica nanoparticles: crosstalk between Bcl2 and caspase 3. Cell Physiol. Biochem. 30(3), 653–665. Angeline Kirubha, S.P., Anburajan, M., Venkataraman, B., Akila, R., Sharath, D., and Raj, B. 2012. Evaluation of mammary cancer in 7, 12-dimethylbenz (a) anthracene-induced Wister rats by asymmetrical temperature distribution analysis using thermography: a comparison with serum CEA levels and histopathology. BioMed. Res. Int. 2012(1), 786417. Aslam, F., Jahan, N., Siddiqui, F., Alam, M., Jabeen, M., Ahmed, N., Mahmood, S. and Farooqui, W.A. 2024. Protein estimation and toxicity determination of two fractions of Pakistani origin Naja oxiana venom and its anti-inflammatory assay by ROS, TNF-α and IL-β. Preprints 2024. 2024030691. Badr, G., Sayed, D., Maximous, D., Mohamed, A.O. and Gul M. 2014. Increased susceptibility to apoptosis and growth arrest of human breast cancer cells treated by a snake venom-loaded silica nanoparticles. Cell Physiol. Biochem. 34(5), 1640–1651. Bancroft, J.D. and Gamble, M. 2008. Theory and practice of histological technique, 4th edition. New York, NY, London, San Francisco, Tokyo: Churchil Livingston. Barratt, G. 2003. Colloidal drug carriers: achievements and perspectives. CMLS, 60, 21–37. Bazaa, A., Luis, J., Srairi-Abid, N., Kallech-Ziri, O., Kessentini-Zouari, R., Defilles, C., Lissitzky, J.C., El Ayeb, M. and Marrakchi, N. 2009. MVL-PLA2, a phospholipase A2 from Macrovipera lebetina trans mediterranea venom, inhibits tumor cells adhesion and migration. Matrix Biol. 28,188–193. Bhowmik, T., PratimSaham, P., Kumar DasGupta, A. and Gomes, A. 2014. Influence of gold nanoparticle tagged snake venom protein toxin NKCT1 on Ehrlich ascites carcinoma (EAC) and EAC induced solid tumor bearing male albino mice. Curr. Drug Deliv. 11(5), 652–664. Borri, F. and Granaglia, A. 2021. Pathology of triple negative breast cancer. In Seminars in cancer biology. 72, 136–145. Cedro, R.C., Menaldo, D.L., Costa, T.R., Zoccal, K.F., Sartim, M.A., Santos-Filho, N.A. and Sampaio, S.V. 2018. Cytotoxic and inflammatory potential of a phospholipase A 2 from Bothrops jararaca snake venom. J. JVATiTD. 24:33. Chan, K. and Morris, G.J. 2006. Chemoprevention of breast cancer for women at high risk. In SON. 33(6), 642–646. Changizi, Z., Moslehi, A., Rohani, A.H. and Eidi, A. 2021. Chlorogenic acid induces 4T1 breast cancer tumor’s apoptosis via p53, Bax, Bcl-2, and caspase-3 signaling pathways in BALB/c mice. J. Biochem. Mol. Toxicol. 35(2), e22642. Cui, K., Kou, J.Q., Gu, J.H., Han, R., Wang, G., Zhen, X. and Qin, Z.H. 2014. Naja naja atra venom ameliorates pulmonary fibrosis by inhibiting inflammatory response and oxidative stress. BMC Complement Altern. Med., 14, 1–11. Dadwal, A., Baldi, A. and Kumar Narang, R. 2018. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 46(sup2), 295–305. Dent, R., Trudeau, M., Pritchard, K.I., Hanna, W.M., Kahn, H.K., Sawka, C.A. and Narod, S.A. 2007. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 13(15), 4429–4434. di Tada, I.E., Martori, R.A., Doucet, M.E. and Abalos, J.W. 1978. Venom yield with different milking procedures. Toxins. 3–7. Ebeling, F.G., Stieber, P., Untch, M., Nagel, D., Konecny, G.E., Schmitt, U.M. and Seidel, D.J.B.J. 2002. Serum CEA and CA 15-3 as prognostic factors in primary breast cancer. Br. J. Cancer. 86(8), 1217–1222. El Hakim, A.E., Gamal-Eldeen, A.M., Shahein, Y.E., Mansour, N.M.,Wahby, A.F. and Abouelella, A.M. 2011. Purification and characterization of a cytotoxic neurotoxin-like protein from Naja haje haje venom that induces mitochondrial apoptosis pathway. Arch. Toxicol. 85, 941–952. El Sharkawi, F.Z., Saleh, S.S. and El Sayed, A.F.M. 2015. Potential anti cancer activity of snake venom, bee venom and their components in liver and breast carcinoma. Int. J. Pharm. Sci. Res., 6(8), 3224. El-Ghani, S.F. and Amr, E.M. 2020. A comparative study on anticancer effect of crude venoms of the Egyptian Naja-haje and Viper Cerastescerastes on head and neck squamous cell carcinoma (In Vitro Study). Egypt. Dent. J. 66(1-January (Oral Med Oral Surg Oral Pathol Oral Radiol). 237–246. Fymat, A.L. 2017. Disrupting cell mitoses to provoke cancer self-destruction. CTOIJ. 5(1), 1–3. Gomes, A., Bhattacharya, S., Chakraborty, M., Bhattacharjee, P., Mishra, R. and Gomes, A. 2010. Anti-arthritic activity of Indian monocellate cobra (Naja kaouthia) venom on adjuvant induced arthritis. Toxicon. 55(2-3), 670–673. Gulbahce-Mutlu, E., Baltaci, S.B., Menevse, E., Mogulkoc, R. and Baltaci, A.K. 2021. The effect of zinc and melatonin administration on lipid peroxidation, IL-6 levels, and element metabolism in DMBA-induced breast cancer in rats. Biol. Trace Elem. Res. 199(3), 1044–1051. Guo, N. and Gao, J. 2022. Harmol alleviates dimethylhydrazine induced colon cancer by downregulating Bcl2/IL-6/TNF-α expression in association with p53 mediated apoptosis. Eur. J. Inflamm. 20,1–14. Harvey, A.L. 2014. Toxins and drug discovery. Toxicon. 92, 193–200. Hodge, D.R., Peng, B., Cherry, J.C., Hurt, E.M., Fox, S.D., Kelley, J.A. and Farrar, W.L. 2005. Interleukin 6 supports the maintenance of p53 tumor suppressor gene promoter methylation. Cancer Res. 65(11), 4673–4682. Kang, T.S., Georgieva, D., Genov, N., Murakami, M.T., Sinha, M., Kumar, R.P. and Kini, R.M. 2011. Enzymatic toxins from snake venom: structural characterization and mechanism of catalysis. FEBS J. 278(23), 4544–4576. Lee, J.S., Park, S., Park, J.M., Cho, J.H., Kim, S.I., and Park, B.W. 2013. Elevated levels of serum tumor markers CA 15-3 and CEA are prognostic factors for diagnosis of metastatic breast cancers. 141, 477–484. Leong, S.P., Shen, Z.Z., Liu, T.J., Agarwal, G., Tajima, T., Paik, N.S. and Foulkes, W.D. 2010. Is breast cancer the same disease in Asian and Western countries?. World J. Surg. 34, 2308–2324. Li, L., Huang, J. and Lin, Y. 2018. Snake venoms in cancer therapy: past, present and future. Toxins (Basel). 10(9), E346. Liang, Y.X., Zhang, Z.Y. and Zhang, R. 2015. Antinociceptive effect of najanalgesin from naja naja atra in a neuropathic pain model via inhibition of c-jun NH2-terminal kinase. Chin. Med. J. 128(17), 2340–2345. Liu, Z., Liu, S., Liu, B., Bian, Y., Yuan, M., Yang, C. and Lin, J. 2023. Fe (III)-naphthazarin metal–phenolic networks for glutathione-depleting enhanced ferroptosis–apoptosis combined cancer therapy. Small. 19(19), 2207825. Livak, K.J. and Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25,402–408. Lohmann, A.E., Dowling, R.J., Ennis, M., Amir, E., Elser, C., Brezden-Masley, C. and Chang, M.C. 2018. Association of metabolic, inflammatory, and tumor markers with circulating tumor cells in metastatic breast cancer. JNCI Cancer Spectr. 2(2), pky028. Mahmoud, G.H., Saber, S.A., El-Fiky, A.A. and Mohamed, A.F. 2019. In vitro evaluation of anticancer potential of Echis pyramidum venom (Viperidae) and related genetic and apoptotic profile alterations. EJHM. 76(4), 3891–3900. Malorni, L., Shetty, P.B., De Angelis, C., Hilsenbeck, S., Rimawi, M.F., Elledge, R. and Arpino, G. 2012. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res. Treat. 136, 795–804. Markland, F.S., Shieh, K., Zhou, Q., Golubkov, V., Sherwin, R.P., Richters, V. and Sposto, R. 2002. A novel snake venom disintegrin that inhibits human ovarian cancer dissemination and angiogenesis in an orthotopic nude mouse model. Pathophysiol Haemost Thromb. 31(3-6), 183–191. Marangoni, E., Vincent-Salomon, A., Auger, N., Degeorges, A., Assayag, F., de Cremoux, P., de Plater, L., Guyader, C., De Pinieux, G., Judde, J.G., Rebucci, M., Tran-Perennou, C., Sastre-Garau, X., Sigal-Zafrani, B., Delattre, O., Diéras, V. and Poupon, M.F. 2007. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin. Cancer Res. 13(13), 3989–3998. Meier, J. and Theakston, R.D.G. 1986. Approximate LD50 determinations of snake venoms using eight to ten experimental animals. Toxicon. 24(4), 395–401. Mohamed Abd El-Aziz, T., Soares, A.G. and Stockand, J.D. 2019. Snake venoms in drug discovery: valuable therapeutic tools for life saving. Toxins. 11(10), 564. Neudert, M., Fischer, C., Krempien, B., Bauss, F. and Seibel, M.J. 2003. Site-specific human breast cancer (MDA-MB-231) metastases in nude rats: model characterisation and in vivo effects of ibandronate on tumour growth. Int. J. Cancer Res. 107(3), 468–477. Omran, M.A.A. 2003. ln vitro Anticancer Effect of Scorpion Leiurusquinquestriatus and Egyptian Cobra Venom. J. Med. Sci. 3(1), 66–86. Price, J.E., Polyzos, A., Dan Zhang, R. and Daniels, L.M. 1990.Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 50(3), 717–721. Renault, T.T., Dejean, L.M. and Manon, S. 2017. A brewing understanding of the regulation of Bax function by Bcl-xL and Bcl-2. Mech Ageing Dev. 161, 201–210. Roy, A. and Bharadvaja, N. 2021. Venom-derived bioactive compounds as potential anticancer agents: a review. Int. J. Pept. Res. Ther. 27, 129–147. Roy, S., Sil, A. and Chakraborty, T. 2019. Potentiating apoptosis and modulation of p53, Bcl2, and Bax by a novel chrysin ruthenium complex for effective chemotherapeutic efficacy against breast cancer. J. Cell. Physiol. 234(4), 4888–4909. Salama, W.H., Ibrahim, N.M., El Hakim, A.E., Bassuiny, R.I., Mohamed, M.M., Mousa, F.M. and Ali, M.M. 2018. L-Amino acid oxidase from Cerastesvipera snake venom: Isolation, characterization and biological effects on bacteria and tumor cell lines. Toxicon. 150, 270–279. Shah, R., Rosso, K. and Nathanson, S.D. 2014. Pathogenesis, prevention, diagnosis and treatment of breast cancer. World J. Clin. Onco. 5(3), 283. Shanbhag, V.K. L. 2015. Applications of snake venoms in treatment of cancer. Asian Pacific Trop. Biomed. 5(4), 275–276. Shebl, R.I., Mohamed, A.F., Ali, A.E. and Amin, M.A. 2012. Cerastes cerastes and Vipera lebetina snake venoms apoptotic–stimulating activity to human breast cancer cells and related gene modulation. J. Cancer Sci. Ther. 4, 317–323. Su, H., Kang, Q., Wang, H., Yin, H., Duan, L., Liu, Y. and Fan, R. 2019. Changes in expression of p53 and inflammatory factors in patients with ulcerative colitis Erratum in/10.3892/etm. 2019.7994. Exp.Ther.Med . 17(4), 2451–2456. Sun, P., Ren, X.D., Zhang, H.W., Li, X.H.,Cai, S.H., Ye, K.H. and Li, X.K. 2003. Serum from rabbit orally administered cobra venom inhibits growth of implanted hepatocellular carcinoma cells in mice. WJG. 9(11), 2441. Sun, Q., Wu, J., Jin, L., Hong, L., Wang, F., Mao, Z. and Wu, M. 2020. Cancer cell membrane-coated gold nanorods for photothermal therapy and radiotherapy on oral squamous cancer. J. Mater. Chem. 8(32), 7253–7263. Tiburcius, S., Krishnan, K., Yang, J.H., Hashemi, F., Singh, G., Radhakrishnan, D. and Vinu, A. 2021. Silica-based nanoparticles as drug delivery vehicles for prostate cancer treatment. TCR. 21(6), 1535–1568. Um, H.D. 2016. Bcl-2 family proteins as regulators of cancer cell invasion and metastasis: a review focusing on mitochondrial respiration and reactive oxygen species. Oncotarget. 7(5), 5193. Wein, L. and Loi, S . 2017. Mechanisms of resistance of chemotherapy in early-stage triple negative breast cancer (TNBC). Breast. 34, S27–S30. Wilson, T.R., Johnston, P.G. and Longley, D.B. 2009. Anti-apoptotic mechanisms of drug resistance in cancer. Curr. cancer drug targets. 9(3), 307–319. Wu, S.G., He, Z.Y., Zhou, J., Sun, J.Y., Li, F.Y., Lin, Q. and Lin, H.X. 2014. Serum levels of CEA and CA15-3 in different molecular subtypes and prognostic value in Chinese breast cancer. The Breast. 23(1), 88–93. Yi, M., Li, T., Niu, M., Luo, S., Chu, Q. and Wu, K. 2021. Epidemiological trends of women’s cancers from 1990 to 2019 at the global, regional, and national levels: a population-based study. Biomark. Res. 9(1), 55. Zhu, S., Wu, Y., Song, B., Yi, M., Yan, Y., Mei, Q. and Wu, K. 2023. Recent advances in targeted strategies for triple-negative breast cancer. J. Hematol. Oncol. 16(1), 100. | ||
| How to Cite this Article |
| Pubmed Style Soliman NA, Shalaby AA, Mohamed HA, Alashqar SMA, Ammar MA. Robust anticancer efficacy of Naja haje venom-loaded silica nanoparticles against triple-negative breast cancer xenografts in a preclinical rat model. Open Vet. J.. 2024; 14(12): 3552-3562. doi:10.5455/OVJ.2024.v14.i12.37 Web Style Soliman NA, Shalaby AA, Mohamed HA, Alashqar SMA, Ammar MA. Robust anticancer efficacy of Naja haje venom-loaded silica nanoparticles against triple-negative breast cancer xenografts in a preclinical rat model. https://www.openveterinaryjournal.com/?mno=224749 [Access: January 12, 2026]. doi:10.5455/OVJ.2024.v14.i12.37 AMA (American Medical Association) Style Soliman NA, Shalaby AA, Mohamed HA, Alashqar SMA, Ammar MA. Robust anticancer efficacy of Naja haje venom-loaded silica nanoparticles against triple-negative breast cancer xenografts in a preclinical rat model. Open Vet. J.. 2024; 14(12): 3552-3562. doi:10.5455/OVJ.2024.v14.i12.37 Vancouver/ICMJE Style Soliman NA, Shalaby AA, Mohamed HA, Alashqar SMA, Ammar MA. Robust anticancer efficacy of Naja haje venom-loaded silica nanoparticles against triple-negative breast cancer xenografts in a preclinical rat model. Open Vet. J.. (2024), [cited January 12, 2026]; 14(12): 3552-3562. doi:10.5455/OVJ.2024.v14.i12.37 Harvard Style Soliman, N. A., Shalaby, . A. A., Mohamed, . H. A., Alashqar, . S. M. A. & Ammar, . M. A. (2024) Robust anticancer efficacy of Naja haje venom-loaded silica nanoparticles against triple-negative breast cancer xenografts in a preclinical rat model. Open Vet. J., 14 (12), 3552-3562. doi:10.5455/OVJ.2024.v14.i12.37 Turabian Style Soliman, Nabil A., Amr A. Shalaby, Heba Allah Mohamed, Sara M. Abdelkarem Alashqar, and Mohamed Ahmed Ammar. 2024. Robust anticancer efficacy of Naja haje venom-loaded silica nanoparticles against triple-negative breast cancer xenografts in a preclinical rat model. Open Veterinary Journal, 14 (12), 3552-3562. doi:10.5455/OVJ.2024.v14.i12.37 Chicago Style Soliman, Nabil A., Amr A. Shalaby, Heba Allah Mohamed, Sara M. Abdelkarem Alashqar, and Mohamed Ahmed Ammar. "Robust anticancer efficacy of Naja haje venom-loaded silica nanoparticles against triple-negative breast cancer xenografts in a preclinical rat model." Open Veterinary Journal 14 (2024), 3552-3562. doi:10.5455/OVJ.2024.v14.i12.37 MLA (The Modern Language Association) Style Soliman, Nabil A., Amr A. Shalaby, Heba Allah Mohamed, Sara M. Abdelkarem Alashqar, and Mohamed Ahmed Ammar. "Robust anticancer efficacy of Naja haje venom-loaded silica nanoparticles against triple-negative breast cancer xenografts in a preclinical rat model." Open Veterinary Journal 14.12 (2024), 3552-3562. Print. doi:10.5455/OVJ.2024.v14.i12.37 APA (American Psychological Association) Style Soliman, N. A., Shalaby, . A. A., Mohamed, . H. A., Alashqar, . S. M. A. & Ammar, . M. A. (2024) Robust anticancer efficacy of Naja haje venom-loaded silica nanoparticles against triple-negative breast cancer xenografts in a preclinical rat model. Open Veterinary Journal, 14 (12), 3552-3562. doi:10.5455/OVJ.2024.v14.i12.37 |