| Review Article | ||
Open Vet. J.. 2025; 15(9): 3943-3960 Open Veterinary Journal, (2025), Vol. 15(9): 3943-3960 Research Article Infectious laryngotracheitis: A serious threat to poultry healthMaya Nurwartanti Yunita1*, Aswin Rafif Khairullah2, Muharam Saepulloh2, Bodhi Agustono1, Tabita Dameria Marbun3, Sarasati Windria4, Azhar Burhanuddin1, Ikechukwu Benjamin Moses5, Andi Thafida Khalisa6, Bantari Wisynu Kusuma Wardhani7, Riza Zainuddin Ahmad2, Bima Putra Pratama8, Ima Fauziah2, Dea Anita Ariani Kurniasih9, Muhammad Khaliim Jati Kusala2 and Syahputra Wibowo101Faculty of Health, Medicine, and Life Sciences (FIKKIA), Universitas Airlangga, Surabaya, Indonesia 2Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Department of Animal Science, Kyungpook National University, Sangju, Korea 4Department of Biomedical Science, Faculty of Medicine, Universitas Padjajaran, Bandung, Indonesia 5Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 6Faculty of Military Pharmacy, Universitas Pertahanan, Bogor, Indonesia 7Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 8Research Center for Agroindustry, National Research and Innovation Agency (BRIN), South Tangerang, Indonesia 9Research Center for Public Health and Nutrition, National Research and Innovation Agency (BRIN), Bogor, Indonesia 10Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Bogor, Indonesia *Corresponding Author: Maya Nurwartanti Yunita. Faculty of Health, Medicine, and Life Sciences (FIKKIA), Universitas Airlangga, Surabaya, Indonesia. Email: mayanurwantanti [at] fkh.unair.ac.id Submitted: 13/03/2025 Revised: 20/07/2025 Accepted: 14/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
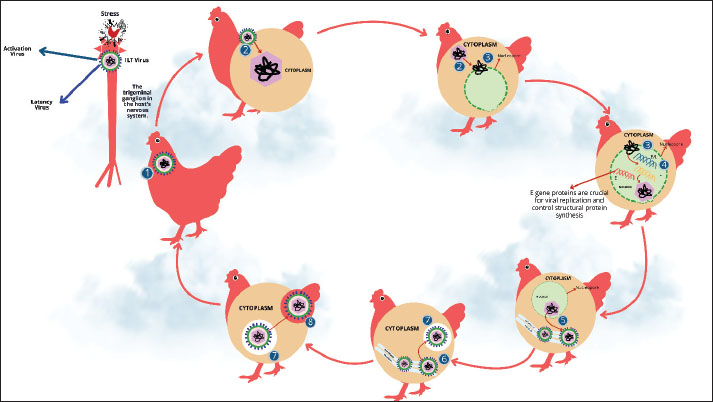
ABSTRACTInfectious laryngotracheitis (ILT) is a highly contagious disease of the upper respiratory tract in chickens. The Gallid alpha herpesvirus type 1, also commonly known as ILT virus (ILTV), causes ILT. Although ILT was first reported in the United States in 1925, it has also been reported in other countries/regions of the world, including Australia, Asia, and Europe. The outcome of infection can be influenced by several factors, including the host’s age, exposure route, pathogenicity of the challenging virus, and initial viral load. In infected chickens, rough lesions are detected on the conjunctiva and throughout the respiratory system, but they are most commonly seen in the trachea and larynx. Other typical signs of the illness in poultry birds are expectoration of bloody mucus, severe dyspnea, coughing, gasping, and rales. Avian cell lines and embryodized chicken eggs are commonly used to isolate ILTV. Three types of ILT are associated with this infection: acute, chronic, and peracute. Chickens contract ILTV through the eyes and upper respiratory tracts. The main pathway by which ILTV is spread in poultry is through direct or indirect contact with sick/infected poultry birds, such as chickens and turkeys. No medication has been proven to be successful in curtailing clinical signs or lesion severity. Vaccination can prevent ILTV infection. Several biological and ecological characteristics of ILTV make its eradication from intensive poultry production locations quite likely. ILT is deemed a serious concern for poultry health, including its significant economic impact on the poultry industry. Therefore, this review highlights important comprehensive information regarding the impact of ILT in poultry, a major source of protein. In addition, a deeper understanding of the causes, signs, diagnosis, treatment, and prevention of ILT in poultry birds was comprehensively discussed. Keywords: Chickens, Disease, ILT, Vaccine, Virus. IntroductionRespiratory viral infections have significantly and negatively impacted the poultry industry worldwide for many years(El-Shemy et al., 2024). Considering that the respiratory tract is the main site of infection, which provides a suitable environment for the virus to spread quickly and widely, the disease can have serious effects on heavily populated flocks of fowl (Yehia et al., 2023). Infectious laryngotracheitis (ILT), a highly contagious upper respiratory tract disease in chickens, has been considered a major problem for poultry welfare and health (Gowthaman et al., 2020). The virus that causes ILT is known as Gallid alpha herpesvirus type 1 (GaHV-1) or commonly known as ILTV. This virus belongs to the Alphaherpesvirinae subfamily and the Herpesviridae family (Gowthaman et al., 2014). ILT was initially identified in the United States in 1925 and has since been reported in other nations, making it a severe illness, particularly in regions with high chicken production and concentrations, such as China, South America, Europe, Australia, North America, and Southeast Asia (Ou and Giambrone, 2012). Only specific areas of the nation or even specific production sites with varying age ranges (industrial or backyard flocks) are epidemiologically known to harbor ILTV-infected chicken flocks (Gowthaman et al., 2020). Periodically, severe outbreaks of ILT sickness still happen whenever ILTV strains can spread from flocks of chickens that are consistently affected to flocks that are not (Hidalgo, 2003). Irregular or emergency-only ILT vaccination in various countries increases the uncertainty about the state of ILT sickness. The disease can affect chickens of any age; however, the most common symptoms are seen in adults (Shankar, 2008). The respiratory system and the eyes are the main transmission pathways (Parra et al., 2016). Signs of this illness are usually visible in the larynx and trachea; it also commonly affects the conjunctiva and respiratory tract (Carnaccini et al., 2022). Clinical signs include a sharp rise in the average daily mortality in impacted flocks. ILTV might take about 6 to 14 days to incubate, and lesions caused by laryngeal and tracheal epithelial cell infection can result in mucus accumulation, tracheal hemorrhage, dyspnea, and even death (Crespo et al., 2007). Three primary types of ILT have been identified in chickens: acute, chronic, and peracute forms (Kaur, 2021). The severity of the illness depends on several factors, such as the pathogenicity/virulence of the virus, the amount of herd immunity, co-infection with other pathogens, stressors, and the age of the susceptible chicken population (Gowthaman et al., 2016). The International Epizootic Office has listed ILT as a serious viral respiratory illness on List B. Although chickens are thought to be the main host, pheasants and peafowl have also been seen to contract the disease naturally (Crawshaw and Boycott, 1982). Other species that seem to be immune to the disease include crows, pigeons, ducks, starlings, and sparrows. More economically excruciating is that the poultry industry has suffered significant financial losses as a result of ILT outbreaks, especially due to high acute mortality rate, decreased egg production, stunted growth, vaccination-associated expenses, biosecurity, and treatment to prevent secondary infections by other poultry pathogens (Tsiouris et al., 2021). In this review, important and latest information focusing on the significant impact of ILT on poultry health and a deeper understanding of the causes, signs, diagnosis, treatment, and prevention of ILT were discussed. HistoryILT disease was first reported in the United States in 1925 and has since been reportedin Australia, England, and Europe (Gowthaman et al., 2020). This illness was first known as ILT, laryngotracheitis, and fowl diphtheria (Pajić et al., 2022). This illness was also known as infectious bronchitis (Gowthaman et al., 2020; Shao et al., 2025). In 1931, the Special Committee on Poultry Diseases of the American Veterinary Medical Association decided to call it ILT (García and Zavala, 2019). In the 1930s, Hudson and Beaudette (1932) of Cornell University developed an effective vaccine to prevent ILT, making it the first significant viral poultry disease to be successfully prevented. Brandly and Bushnell (1934) developed a chicken vaccination technique based on the delivery of virulent viruses to the cloaca. Baudette (1937) was the first to demonstrate that a filterable virus caused ILT. EpidemiologyILT is still a significant illness that has been documented in most nations. Outbreaks have been reported in Greece (Tsiouris et al., 2021), the USA (Wolfrum, 2020), Canada (Elshafiee et al., 2022), China (Wu et al., 2022), Brazil (Parra et al., 2016), Egypt (Ibrahim et al., 2021), Ethiopia (Adam et al., 2025), and Australia (Nazir et al., 2020). The sickness has been reported in at least 100 countries between 2000 and 2013 (Menendez et al., 2014). Molecular tests recently confirmed the first report from Iraq in the Al-Diwaniyah area (Alaraji et al., 2019). The majority of cases included mild clinical manifestations of ILT, although 88 cases of ILTV were confirmed in California alone during the 2007–2017 period (Blakey et al., 2019). In 2018, three ILT outbreaks were documented in Windhoek, Namibia, in 2018, leading to significant mortality among commercial laying hens and broilers (Molini et al., 2019). Between 2020 and 2024, further ILTV circulation cases were documented in China (Hong et al., 2024), Ethiopia (Birhan et al., 2022), Bangladesh (Kamal et al., 2024), Switzerland (Hermann et al., 2024), and Turkey (Müştak and Müştak, 2024) between 2020 and 2024. ILT outbreaks have increased globally due to several factors, such as high flock density, shorter production cycles, keeping chickens of different ages and purposes in the same region, inadequate vaccination, and biosecurity violations (Salhi et al., 2021). Since its discovery in the mid-1920s, ILT has continued to pose a significant concern and negatively affect the global poultry industry. Although ILTV infection can affect chickens of any age between 8 days and 4 years, chickens older than 3 weeks are most vulnerable (Preis et al., 2013). ILT outbreaks frequently occur in many regions of the world as a result of very intensive chicken production, the mixing of many poultry species in the same geographic area, and biosecurity violations (Pajić et al., 2022). Morbidity and mortality are influenced by viral load, ILTV strain virulence, and coexisting infections with other respiratory pathogens (Thilakarathne et al., 2020c). The effects of ILT in the field may be exacerbated by concomitant respiratory conditions such as Mycoplasma gallisepticum, Mycoplasma synoviae, infectious coryza, and other immunosuppressive conditions such as mycotoxicosis, chicken anemia virus, reticuloendotheliosis virus, and Marek’s disease virus (Rojs et al., 2021). Herds that have received insufficient vaccinations may experience sporadic cases of ILTV due to mistaken vaccine administration or biosecurity lapses (Vagnozzi et al., 2010). When younger vaccinated flocks are introduced to the farm, flocks that have not had enough vaccinations may be exposed to ILTV (Hidalgo, 2003). The characteristics of severe epizootic forms include rapid dissemination, substantial morbidity (90%–100%), and mortality ranging from 5% to 70% (average 10%–20%) (García et al., 2013). The characteristics of mild epizootic forms range from very low mortality (0.1%–2%) to low morbidity (<5%) (Ou et al., 2012). In dense poultry regions, where there is a constant reservoir, ILTV vaccine and field strains develop virulence, and the viruses then establish themselves in the field and produce epidemics (Mossad et al., 2022). Areas with high chicken production densities frequently suffer significant financial losses, with a total mortality rate as high as 70% (Bagust et al., 2000). Farms without a history of ILT may not encounter as many outbreaks as places that have hosted infected herds (Volkova et al., 2012). Similar to other herpes viruses, ILTV can develop latency in the trigeminal ganglion (TRG) of the central nervous system, following an acute infection that lasts for seven days (Theil et al., 2003). Stressful situations during transfer, the start of laying, and flock mixing help the virus to reactivate (Ebrahimi et al., 2021). Generally, intermittent and undetectable reactivation with productive replication in the tracheal epithelium causes viral shedding and infection transfer to vulnerable chickens (Zeng et al., 2023). A significant factor in the development of latency is the identification of long-term tracheal carriage (about 2%) in chickens recuperating from acute ILT infection (Thilakarathne et al., 2020c). According to recent experimental research, ILTV genomes have been detected in the kidneys, lungs, trachea, and Harderian glands for up to 28 days after infection (Neff et al., 2008). Due to virus latency, backyard flocks are a major source of infection for commercial chicken flocks (Tesfaye et al., 2019). Additionally, research has shown that unvaccinated flocks have a high seroprevalence of ILTV (72%), which may indicate that backyard poultry contribute to the disease’s epidemiology (Hernandez-Divers et al., 2008). Morphology and chemical composition of the virusesThe ILTV belongs to the Alphaherpesvirinae subfamily of the Herpesviridae family (Gowthaman et al., 2020). The taxonomy of this virus is GaHV-1 (Fuchs et al., 2007). Icosahedral virus particles with a shape resembling that of the herpes simplex virus are visible in an ILTV-infected chicken embryo cell culture electron micrograph (Lee et al., 2012). Watrach et al. (1963) described the hexagonal ILTV nucleocapsid as having a diameter of 80–100 nm. With icosahedral symmetry, the nucleocapsid comprises 162 long and hollow capsomeres. The diameter of the entire virus particle, including the asymmetrical envelope enclosing the nucleocapsid, is between 195 and 250 nm (Taha et al., 2017). Viral glycoprotein spikes are represented as tiny projections on the envelope surface. Similar to the DNA of other herpes viruses, the nucleic acid of ILTV has a buoyant density of 1.704 g/ml (Maini et al., 2024). The guanine-to-cytosine ratio of ILTV DNA is 45% (Loncoman et al., 2017). The 150-kb linear double-stranded molecule that makes up the DNA genome comprises distinct long and short portions surrounded by inverted repeats (Fuchs et al., 2007). Viral glycoproteins trigger humoral and cellular immune responses, just like other herpes viruses. Five primary envelope glycoproteins are known to exist, and their molecular weights are 60, 90, 115, 160, and 205 kD (York and Fahey, 1991). They are the main immunogens of ILTV. Sequencing has been performed on the distinct LTV glycoproteins gB, gD, gC, gK, gX, and gp60 (Bagust et al., 2000). Virus replicationTo start an infection, viruses first bind to cell receptors and then fuse their envelope with the host cell’s plasma membrane (Lv et al., 2019). Once the nucleocapsid is released into the cytoplasm and transported to the nuclear membrane, the viral DNA is freed from it and enters the nucleus through the nuclear pores (Fay and Panté, 2015). The viral DNA is transcribed and replicated in the nucleus (Ziemann et al., 1998). Like other alphaherpesvirus, ILTV DNA transcription occurs in a highly controlled and sequential cascade (Yi et al., 2024). Most approximately 70 proteins expressed by viruses are structural proteins, although some are enzymes and DNA-binding proteins that control viral DNA replication (Fuchs et al., 2005). The rolling circle technique of viral DNA replication produces concatemers, which are subsequently broken down into monomeric units and bundled into nucleocapsids that have previously been formed in the nucleus (Lo Piano et al., 2011), as depicted in Figure 1. The inner lamella of the nuclear membrane allows the DNA-containing nucleocapsid to migrate across it and acquire an envelope (Newcomb et al., 2017). After migrating through the endoplasmic reticulum, the coated particles then gather in cytoplasmic vacuoles. Either cell lysis or vacuole membrane fusion and exocytosis can release enveloped virions (Palomino-Tapia et al., 2023). This portrayal serves as a depiction of the ILTV infection and replication cycle within the host cell, as highlighted in Figure 1.
Fig. 1. Stress triggers dormant virus activation in the TRG. Once activated, the virus enters the replication phase (cycle process), which involves (1) viral attachment; (2) transport of the tegument and nucleocapsid into the cytoplasm; (3) release of viral DNA from the nucleocapsid into the nucleus via nuclear pores; (4) expression of three gene categories (E), early late (E/L), and late (L) are expressed to control viral replication and structural protein synthesis; (4–6) the nucleocapsids containing viral DNA acquire an envelope by budding from the inner lamellae of the nuclear membrane and are then transported to the cytoplasm; (7–8) release of the virions from these vacuoles through exocytosis or cell lysis. Chemical and physical resistance to the virusesChloroform and ether are examples of organic solvents (lipolytic chemicals) that impact enveloped ILTV infection (Ou and Giambrone, 2012). ILTV infection can be maintained in diluents such as glycerol or nutritional broth at 4°C for several months (Vagnozzi et al., 2012). Heat has been shown to quickly inactivate ILTV infection when subjected to 55°C for 15 minutes or 38°C for 48 hours (Bindari et al., 2020). However, a different study discovered that 1% of ILTV infections were still present after an hour at 56°C (Ebrahimi et al., 2021). According to Kamal et al. (2024), ILTV was eliminated in the chorioallantoic membrane (CAM)after 5 hours at 25°C or in the tracheal tissue of chicken carcasses within 44 hours at 37°C. However, according to other studies, ILTV in tracheal exudate and chicken carcasses can endure for 10–100 days at temperatures between 13°C and 23°C (Blacker et al., 2011). Inactivating ILTV using a 1% or 3% cresol solution takes less than a minute (Giambrone et al., 2008). The disinfection of laboratory bench surfaces using halogenated detergent mixes or commercial iodophors is simple (Artasensi et al., 2021). Using 5% hydrogen peroxide mist as a fumigant for chicken house equipment resulted in the complete elimination of ILTV infection (Neighbor et al., 1994). Antigenicity and virulence of different virus strainsDifferent ILTV strains have different virulence for chickens, pathogenicity for chicken embryos, and plaque sizes and shapes on the CAM of embryonated chicken eggs, as well as in cell culture (Gowthaman et al., 2020). The virulence of naturally occurring ILTV strains varies, ranging from low-virulence variants that cause moderate to nonexistent infections to highly virulent strains that cause substantial morbidity and mortality in chickens exposed to ILTV (Nazir et al., 2020). ILTV strains seem to be antigenically homogenous based on cross-protection tests, immunofluorescence assays, and virus neutralization (Lee et al., 2014). However, the discovery that heterologous antisera do not effectively neutralize certain strains has raised the possibility of slight antigenic diversity among strains. Distinguishing between ILTV strains with varying degrees of pathogenicity, particularly modified live vaccination viruses and wild-type (WT) viruses, is an important practical problem. Numerous techniques have been investigated for identifying ILTV, such as DNA hybridization assays, viral DNA restriction endonuclease studies, and pathogenicity studies for chicken embryos (García et al., 2013). Virulence and mortality patterns are closely related, and evaluating mortality patterns in chicken embryos has been proposed as a biological way to distinguish ILTV strains (Mossad et al., 2022). Distinct ILTV strains can be distinguished by cleaving the viral DNA with restriction endonucleases and separating the DNA pieces by electrophoresis (Han and Kim, 2001). ILTV DNA restriction endonuclease analysis has been used in epidemiological investigations of field outbreaks to distinguish between modified live vaccination viruses and WT viruses (Chacón and Ferreira, 2009). Reciprocal DNA-DNA hybridization using cloned DNA fragments can distinguish ILTV strains (Kotiw et al., 1986). Virus latencyReisolation of the virus from the seventh week after infection in repeated tracheal swabs and at 2 months after infection in tracheal organ cultures (TOCs) has demonstrated that ILTV, like other herpesviruses, creates a latent infection (Thilakarathne et al., 2020a). The TRG is the major site of latent ILTV (Mo and Mo, 2025). TRG provides the main sensory innervation to the upper respiratory tract tissues, and viral migration via the neurons is highly suspected (Su et al., 2022). Extratracheal virus dissemination to the trigeminal ganglia was observed in 40% of chickens exposed to the virulent Australian strain of ILTV 4–7 days after tracheal exposure (Tran et al., 2024). Additionally, research has indicated that latent ILTV was reactivated from the trigeminal ganglia 2, 5, or 10 months after herd vaccination (Thilakarathne et al., 2019). Williams et al. (1992) demonstrated that adult laying hens displayed viral DNA in the trigeminal ganglia at 31, 46, and 61 days after intratracheal inoculation with the field strain ILTV whenassessed by polymerase chain reaction (PCR). Davidson et al. (2016) documented that latently infected chicks reexcrete ILTV after the stress of rehoning and the start of reproduction. PathogenesisNumerous factors, such as the host’s age, exposure route, pathogenicity of the challenge virus, and initial viral load, may affect the outcome of an infection (Rouse and Sehrawat, 2010). ILTV replication occurs only in respiratory tissues when viremia is absent (Beltrán et al., 2017). Although outbreaks have been observed in hens as young as 3 weeks, the disease can infect and damage chicks of almost any age. The sickness often first appears in chicks at approximately 4 weeks of age (Dufour-Zavala, 2008). Peak virus replication in the tracheal epithelium occurs between 2 and 5 days post-infection (PI), according to independent experimental studies employing aerosol, intratracheal, intranasal, and intraconjunctival injection, as well as a chicken-to-chicken transmission model (Bagust and Johnson, 1995). The trachea of chickens vaccinated with eye drops contained viral DNA from the live attenuated vaccine 18–28 days after immunization, suggesting that the virus remains in the trachea at very low concentrations after the acute replication period (Thilakarathne et al., 2019). Infections can impact not only the trachea but also other mucous membranes, such as the conjunctiva, respiratory sinuses, air sacs, and lungs (Blakey et al., 2019). Mortality is not necessarily associated with tracheal pathology, even if the degree of microscopic lesions in the trachea correlates with greater viral replication in the trachea (Kirkpatrick et al., 2006). The tracheal and conjunctival tropism of ILTV strains varies significantly (Tran et al., 2024). A live-attenuated vaccine can be successfully administered to chickens as early as one day of age; nevertheless, the likelihood of serious vaccination reactions is high (Maekawa et al., 2019). The pathogenic effects of viruses that extend beyond the trachea to the lungs of very young chickens (1–3 days of age) may be the cause of the higher sensitivity observed in younger chicks (Gaghan et al., 2023). One of the main ways that herpes viruses, including ILTV, avoid host protection is by transforming into a dormant stage following infection (Pajić et al., 2022). Bagust et al. (2000) were the first to report the extratracheal dissemination of ILTV to the TGG. Chicken trachea were exposed to the virulent Australian ILTV strain for 4–7 days before TOCs were used to isolate the virus from their trigeminal ganglia. William et al. (1992) used polymerase chain reaction to confirm that the TRG is the primary site of ILTV latency Han et al. (2002) reported that challenge and vaccine virus strains started to move extratracheally to the trigeminal ganglia as early as 2 days after infection. Explants of the tracheal organs from recovered chickens showed evidence of virus reactivation 3–16 months PI (Thilakarathne et al., 2019). The use of organ culture explants from different tracheal regions has shown that latent sites are dispersed throughout the trachea, in addition to the trigeminal ganglia (Thilakarathne et al., 2020b). Chickens that appeared to be in good health showed spontaneous and intermittent virus shedding in repeated tracheal swabs taken 2–4 months after experimental infection with a vaccine strain or mildly pathogenic strain (Davidson et al., 2016). Another investigation documented the re-excretion of ILTV from chickens after stress from rehoming and the start of reproduction (Coppo et al., 2013). Immunosuppressive medication (such as cyclophosphamide or dexamethasone) treatment did not successfully reactivate latent ILTV infection, in contrast to reactivation trials with other alphaherpesvirus (Beltrão et al., 2012). Immune responseActive immunityNot all immune responses produced after an ILTV infection result in ILT resistance. Early research on the humoral response to ILTV infection revealed that virus-neutralizing antibodies could be found 5–7 days PI, peaked at 21 days PI, and then decreased to low levels over the course of the following few months (Sabir et al., 2019). Antibodies that neutralize viruses can be found for 1 year. Tracheal secretions show local antibodies starting around day 7 PI and peaking between days 10 and 28 (Gopakumar et al., 2025). Separate investigations have found no correlation between antibody titers and herd immune state, even if they are linked to the stage of ILTV infection in serum and mucosal surfaces (Godoy et al., 2013). Experiments using bursectomize chickens revealed a weak association between the humoral immune response and protection against ILT (Elshafiee et al., 2022). Bursal-dependent reactions did not significantly hinder virus multiplication in vaccinated chickens. This implies that the primary mechanism underlying ILT resistance is local cell-mediated immunity (CMI) responses in the trachea (Okino et al., 2014). The ability of spleen cells and peripheral blood leukocytes from congenic immune donors to transfer ILT resistance emphasizes the importance of the CMI response (Ou and Giambrone, 2012). Like other alphaherpesvirus that infect mammals, ILTV glycoprotein G is a viral chemokine-binding protein (Van de Walle et al., 2007). The tracheal mucosa of chickens inoculated with the gG deletion mutant exhibited more CD8+ T lymphocytes and lower serum neutralizing antibody levels than those inoculated with WT or gG revertant viruses (Krunkosky et al., 2020). Researchers hypothesize that by binding to chemokines, gG shifts the host immune response away from the cell-mediated arm toward the antibody-mediated immune response to generate conditions suitable for viral multiplication. The elements that make up the cell-mediated immune response that an ILTV infection triggers remain unclear. Nevertheless, preliminary research indicates a delayed-type hypersensitivity reaction to ILTV infection (Fuchs et al., 2007). Analysis of the transcriptional profile of ILTV-infected chicken embryo lung cells revealed notable variations in the expression of genes involved in inflammation, CMI, and antigen presentation compared with uninfected controls (Lee et al., 2010). Passive immunityEarly research has shown that while maternal antibodies to ILTV are transferred to chicksthrough the egg, this does not prevent infection or vaccination (Coppo et al., 2013). A recent study confirmed the transfer of maternal ILTV antibodies from chickens to their 1-day-old offspring (Pitcovski et al., 2017). PathologyRough lesions are detected on the conjunctiva and throughout the respiratory system in chickens infected with ILTV, but they are most commonly seen in the trachea and larynx (Gowthaman et al., 2020). In severe cases, the infection begins with mucoid inflammation and progresses to degeneration, necrosis, and bleeding (Parra et al., 2016). Mucoid casts that extend the entire tracheal length are a common sign of diphtheritic changes (Davidson, 2019). In some cases, mucus and necrotic tissue may be found in the blood, or blood casts from severe bleeding may be found in the tracheal lumen (Preis et al., 2014). The lungs and air sacs may become inflamed when the inflammation travels down the bronchi. The larynx and upper trachea may have varying degrees of bleeding in milder forms of the disease, and gross lesions in the trachea may be moderate mucoid tracheitis (Sellers et al., 2004). The only other symptoms are conjunctival edema and congestion with watery eyes (Villarreal et al., 2004). Chickens with severe cases of the disease experience conjunctivitis and nasal discharge, watery eyes, frothy eye secretions, and infraorbital sinus enlargement (Chacón et al., 2025). The disease stage affects the microscopic alterations. Two days after PI, initial microscopic alterations in the tracheal mucosa, including increased goblet cells and inflammatory cell infiltration of the mucosa, are visible (Zhang et al., 2024). As the viral infection worsens, the respiratory and conjunctival epithelial cells swell and become edematous. After 3 days, plasma cells, lymphocytes, and histiocytes settle in the mucosa and submucosa to form multinucleated cells, or syncytia (Gopakumar et al., 2025). Typically, inclusion bodies only occur in tracheal and conjunctival epithelial cells during the first 5 days of infection and vanish when the illness worsens because of necrosis and desquamation of the epithelium (Carnaccini et al., 2022). The mucosal surface will either have no epithelial layer or a thin layer of basal cells due to cell loss and desquamation (Kammon et al., 2020). Due to severe epithelial degradation and desquamation, blood vessels in the lamina propria may protrude into the tracheal lumen, and blood capillaries may burst and become exposed, resulting in bleeding (El-Saied et al., 2021). DiagnosisPathological diagnosisTypical clinical signs of acute ILT include nasal discharge, conjunctivitis, panting, hand-pump-type respiration, expectoration of bloody mucus, and dyspnea. With a mortality rate ranging from 5% to 70%, postmortem lesions include catarrhal to hemorrhagic tracheitis, fibrinopurulent to caseous exudate, or obstruction in the larynx and trachea (Pajić et al., 2022). Chickens with mild forms of the disease have a mortality rate of 2% and exhibit mild conjunctivitis, catarrhal tracheitis, and decreased egg production (Salhi et al., 2021). Histopathological findings of multinucleated syncytial cells with intranuclear eosinophilic inclusion bodies and the presence of inflammatory cells, such as heterophils, lymphocytes, and macrophages, in the larynx, trachea, and conjunctival mucosa are diagnostic features of ILTV infection (Beltrán et al., 2019). Isolation and identification of ILTVILTV is commonly isolated using avian cell lines and embryodized chicken eggs (Islam et al., 2010). The CAM shed from embryonated chicken eggs can develop opaque plaques with sunken cores or spot-like lesions of varying sizes when implanted with a virus-infected tissue suspension (Ou and Giambrone, 2012). Depending on virulence and the degree of infectiousness in the homologous host, embryonic mortality occurs between 2 and 8 days after inoculation, and opaque plaques can be observed on ILTV-infected CAMs as early as 2 days after inoculation (Parra et al., 2016). The virus multiplies more effectively when it infects more homologous hosts because infected embryos typically survive longer. The following cell lines are employed to propagate ILTV: adult chicken kidney (CK) cells, chicken embryonic fibroblast cell lines, chicken embryonic lung (CEL) cells, and chicken embryonic kidney (CEK) cells (Gowthaman et al., 2020). The most favored cell lines among them are CEL and CK cells. Cell-associated ILTV strains have occasionally been produced using the chicken embryo fibroblast cell line; however, this has not always been successful. This is because it is not appropriate for the isolation of primary viruses (Maekawa et al., 2021a). Cell cultures can exhibit cytotoxic effects as early as 4–6 hours after infection. These effects include increased refractoriness, cell swelling, chromatin displacement, nucleolar rounding, and multinucleated large cell (syncytia) development. Syncytia and certain isolated cells can have intranuclear inclusion bodies as early as 12 hours after infection, and the proportion of cells with viral inclusions peaks between 30 and 36 hours after infection. ILTV-infected CEK cell lines often exhibit large cytoplasmic vesicles, which eventually degenerate into basophilic masses (Gowthaman et al., 2020). Additionally, ILTV replication is allowed in avian leukocyte cultures made from buffy chicken feathers, wherein infected cells exhibit nuclear clumping and syncytia formation 72 hours after infection (Krunkosky et al., 2020). Leghorn is a continuous cell line obtained from a chemically generated chicken liver tumor. It allows ILTV replication if the virus has been accepted in the leghorn male hepatoma (LMH) cell line at least once; however, it is not suitable for primary isolation (Gopakumar et al., 2024). Nonetheless, the LMH cell line is frequently employed to investigate the ILTV genome and create recombinant ILTV (Contreras et al., 2020). Conjunctival organ cultures (COCs) and TOCs derived from day-old chick embryos or chicks are other systems that are frequently used for diagnosis and to investigate host-pathogen interactions (Reddy et al., 2014). For a long time, TOC has been widely employed for pathogenesis investigations, diagnostics, and host-pathogen interaction of several avian diseases, including ILTV. The vitality of TOC was evaluated by measuring ciliary beating and removal of latex beads following infection (Reddy et al., 2014). The host-virus interactions have also been studied using COC; ILTV-infected cells were detected by immunofluorescence with ILTV-specific antibodies, and distinctive cytopathic effects were detected (Gopakumar et al., 2024). ImmunodiagnosisThe following methods are commonly used for serological diagnosis: enzyme-linked immunosorbent assay using different glycoproteins, agar-gel immunodiffusion, virus neutralization assay using ILTV-specific antibodies, and immunofluorescence (Abebe et al., 2024). Monoclonal and polyclonal antibodies can be used for immunohistochemistry, radioimmunoprecipitation, immunoelectron microscopy, immunofluorescence, and western blot studies against ILTV proteins and other viral glycoproteins, including gC, gG, gE, and gJ (Fuchs et al., 2007). Molecular diagnosisReal-time quantitative PCR is the recommended molecular method for confirming and measuring the amount of virus present in biological materials (Zhao et al., 2013; Zhang et al., 2024). The restriction length polymorphism (RFLP) profiles of the entire viral genome are used to distinguish between vaccine strains and WT ILTV strains (Kim et al., 2013). Nonetheless, ILTV strains have also been characterized and distinguished by PCR amplification of many areas (infected cell polypeptide 4 (ICP4); glycoproteins C, E, G, and X; thymidine kinase; and other glycoprotein coding regions of ILTV followed by RFLP analysis (Kardoğan and İnce, 2024). Developments in DNA sequencing technology have enabled the separation of WT and vaccine virus isolates for epidemiological and phylogenetic investigations, in addition to the use of probe-based assays, such as the TaqMan single-nucleotide polymorphism genotyping assay, to examine the recombination events of Laryngotracheitis Virus Tissue in natural hosts (Loncoman et al., 2017). However, molecular diagnostic procedures such as real-time quantitative PCR and traditional PCR are frequently employed to determine the viral load and validate the etiology (Creelan et al., 2006). As a whole, the diagnosis of ILT can be made based on gross and microscopic findings, immunohistochemistry detection of viral antigens in tissues, electron microscopy identification of viral particles, detection of cytopathic features in cultured cells, or embryopathology in chicken embryos (Gowthaman et al., 2020). Although this molecular technique is very useful for diagnosis, it cannot tell the difference between live and non-living virions (Menendez et al., 2014). Consequently, interpreting PCR results carefully is important because positive results only show viral nucleic acid and not an active infection. Differential diagnosisOther respiratory infections in poultry that can result in comparable clinical signs and lesions must be distinguished from ILTV (Hidalgo, 2003). These include diseases caused by Aspergillus species, Newcastle disease virus, avian influenza virus, chicken adenovirus, and infectious bronchitis virus, as well as the diphtheritic form of avian poxvirus (Davidson et al., 2015). Clinical symptomsClinical signsILTV takes anywhere from 6 to 14 days to incubate (Rojs et al., 2021). According to earlier experimental research, ILTV shedding starts 2 days after infection and 4 days before clinical signs appear (Parra et al., 2016). The clinical course of ILT might range from 11 days to 6 weeks, depending on the disease’s type (Kirkpatrick et al., 2006). A sharp rise in the average daily mortality rate in the afflicted herd is a clinical sign (Gowthaman et al., 2014). Factors such as flock immune status, stress, co-infection with other diseases, and chicken age affect disease severity (Gowthaman et al., 2016). Three types of ILT are associated with this infection: peracute, acute, and chronic. Peracute formThe illness is distinguished by its high death rate, which can exceed 50%, and its rapid and abrupt spread. Affected chickens frequently exhibit moderate to severe conjunctivitis, swollen eyelids, and increased lacrimation (Beltrán et al., 2019). Chickens in good physical health may occasionally die before showing any symptoms (Preis et al., 2013). Clinical symptoms include panting and dyspnea accompanied by head and neck extension. The chicken also makes cracking, gurgling, and coughing noises as it tries to clear the blocked trachea of debris and clotted blood (Blakey et al., 2019). Additionally, clotted blood can be observed on the walls, floor, feed grass, and cage of the chicken coop. Affected chickens typically die within 3 days (Pajić et al., 2022). Acute formAlthough typical dyspnea is frequently observed in the acute form of ILT, it does not occur as abruptly or with as much severity as in the peracute form. Affected chickens first exhibit anorexia and then become lethargic (Tsiouris et al., 2021). Between 4 and 6 days after infection, the internal core temperature increases, and the total leukocyte count reveals mild to severe heterophilia and lymphopenia (Carnaccini et al., 2022). A high-pitched voice, moist rales, and prolonged gasps with the mouth open are signs of tracheal blockage caused by blood clots and exudate (Gowthaman et al., 2020). Additionally, afflicted chickens may exhibit nasal discharge, sinusitis, and purulent conjunctivitis with foamy exudate at the inner canthus of the eyes (Ou and Giambrone, 2012). Mortality ranges from 10% to 30%, and morbidity can reach 100% within a period of up to 15 days (Parra et al., 2016). Laying flocks exhibit varying degrees of egg production; some may completely stop producing eggs, but this may eventually return to normal levels (Salhi et al., 2021). Chronic formThe symptoms of mild or chronic ILT are similar to those of other respiratory tract infections and include malaise, coughing, wet hoarseness, head shaking, squinting, infraorbital sinus enlargement (almond-shaped eyes), decreased egg production (up to 10%), and weight loss (Carnaccini et al., 2022). Mortality is often maintained at less than 2%;however, morbidity can rise to 5% (Preis et al., 2013). TransmissionChickens contract ILTV through their eyes and upper respiratory tracts (Yegoraw et al., 2021). Another infection route could be through ingestion, but the nasal epithelium must then be exposed. The sources of ILTV include contaminated dust, litter, beetles, drinking water, fomites, clinically sick chickens, and latently infected carriers (Brown et al., 2020). Figure 2 illustrates these various transmission routes and describes the direct and indirect transmission pathways. Transmission is most often from acutely ill chickens. It is less likely to spread by coming into contact with clinically recovered carrier chickens (Bagust and Johnson, 1995). Severe viral replication occurs once ILTV infects the vulnerable chickens’ upper respiratory system (Mo and Mo, 2025). The virus often remains in tracheal tissue and secretions for 6–8 days after infection. The virus can remain at very low levels for up to 10 days after infection (Yegoraw et al., 2021), as depicted in Figure 2 (the infected orange host represents direct transmission to naïve). Conversely, the survival carrier possibly transfers to a susceptible host.
Fig. 2. Mapping the ILTV transmission. The ILTV latency targets the TRG (Thilakarathne et al., 2019). The ILTV of the TRG was reactivated 15 months after vaccination (Parra et al., 2016). DNA was found in the TRG of mature laying hens challenged with virulent ILTV using polymerase chain reaction (Davidson et al., 2016). ILTV DNA can be found in the TRG 2 days after eye drop vaccination (Coppo et al., 2013). ILTV is reactivated and transferred to vulnerable chickens during stressful times, such as the beginning of laying or rehoning (Hein et al., 2021). According to recent research, ILTV can survive in biofilms found in drinking water and then spread to vulnerable poultry (Ou and Giambrone, 2012). Mechanical transmission also occurs through contaminated equipment and litter, as illustrated by the storage drums and footprints in Figure 2, emphasizing the role of environmental contamination in spreading ILTV. Bacteria produce a sticky substance called biofilm, which can make microorganisms resistant to some common cleaning agents (Sharma et al., 2023). Both hydrogen peroxide and sodium hydrogen sulfate can deactivate ILTV in water channels (Ou and Giambrone, 2012). Littering and the use of contaminated equipment can result in mechanical transmission (Tsiouris et al., 2021). ILTV may spread through dark-colored insects found in poultry farms. Windborne transmission between fields is a major factor in the spread of ILTV (Johnson et al., 2005). Investigations have shown that dark-colored beetles in chicken coops infected with ILTV carried live virus for at least 42 days following the disease outbreak (Ou et al., 2012). Cats, dogs, and crows are other potential infection sources (Gowthaman et al., 2020). tissue culture origin (TCO) and chicken embryo origin (CEO) vaccines provide models for ILTV transmission and replication through the eye drop route (García et al., 2013). The trachea and conjunctiva are the primary sites of viral replication. It is possible to isolate the vaccine virus again and find viral DNA in exposed chickens as soon as 7 days after the initial exposure (Yegoraw et al., 2021). Risk factorsThe main way that ILTV is spread in poultry is through direct or indirect contact with sick chickens (Maekawa et al., 2021b). The danger of transmission is increased in crowded poultry habitats, such as farms with inadequate ventilation, because this virus is typically carried through the air (Ou and Giambrone, 2012). Additionally, the virus can spread through the use of tainted vehicles or equipment, including drinking water, food containers, and other maintenance tools (Groves et al., 2019). Poultry stressors, such as high density, drastic temperature swings, or dietary and health disruptions, can weaken the immune system and facilitate infection, which is another element that exacerbates transmission (Gržinić et al., 2023). Contact with migratory or wild chickens that are infected but do not exhibit clinical signs also increases the risk of transmission (Yegoraw et al., 2021). TreatmentNo medication has been proven to be successful in ameliorating clinical signs or lesions severity. If an ILT diagnosis is made early in an outbreak, unaffected chickens may receive sufficient protection before being exposed (Gowthaman et al., 2020). VaccinationThe ILT vaccine protects against challenges in just 1 week. The main immunological response to ILTV in chickens is not humoral immunity. Studies confirm the role of CMI in ILTV resistance. The experiment suppressed the humoral immune response by surgically bursectomizing chickens with cyclofospamide. A protective CMI response is developed in vaccinated bursectomize chickens in response to a severe ILTV challenge (Bagust and Johnson, 1995). This demonstrates that humoral immunity is less significant than CMI (Vagnozzi et al., 2012). Vaccination can prevent ILTV infection. However, the ILT vaccination virus can produce latently infected carrier chickens (Salhi et al., 2021). Unvaccinated herds can contract the virus from these latent carriers (Adam et al., 2025). Therefore, ILT vaccination should beadministered only in endemic regions. Attenuated and modified TCO or CEO viruses are currently the most commonly used ILT vaccine strains (García, 2017). The immunity of chickens at 10 weeks after vaccination did not significantly differ from that of the chickens treated with TCO and CEO vaccines. However, the CEO vaccination outperformed the TCO vaccine in terms of protection when administered to chicks aged >20 weeks (Vagnozzi et al., 2010). Live vaccinations can be administered via aerosol spray, ocular drops, or drinking water. There are certain issues with the drinking water route since the quality of the water differs throughout poultry houses, and the chickens might not obtain enough virus in the target organ (nasal epithelial cells) (Gržinić et al., 2023). As a result, these chickens can experience a rolling (continuous) reaction and not establish protective immunity (Davidson et al., 2016). However, some chickens may respond severely to the spray route because the minute droplets may deeply enter the respiratory system and cause high dosages (Parra et al., 2016). Modified live vaccinations increase pathogenicity by transferring from chicken to chicken. The lively modified ILT vaccine underwent 35 generations of serial in vivo transmission. This vaccination strain caused serious clinical signs in challenged chickens after the sixth infection. Additionally, there were no alterations between the isolates according to restriction endonuclease analysis of the viral genome between the original and late transmissions. The CEO vaccine is more likely to make chickens more virulent than the TCO vaccine (Kotiw et al., 1995). PCR RFLP analysis was used to investigate ILTV isolates that were gathered from all over the world. They showed that the vaccination strain shares a close relationship with some existing virulent field isolates (Oldoni et al., 2008). The vaccination strain was the source of the field isolates following reverse transmission in chickens. The recent commercialization of recombinant vaccines includes the addition of a modified turkey herpesvirus (HVT) and a partial ILTV gene into the fowlpox genome (Zai et al., 2022). A recombinant fowlpox vaccine containing the ILTV glycoprotein B (gB) gene provides protection against virulent strains (Chen et al., 2020). The ILTV gB and UL 32 genes found in another recombinant fowlpox virus demonstrated some effectiveness in offering defense against encounters with virulent strains (García, 2017). The United States has two approved commercial recombinant ILT vaccinations. Ceva Santé Animale (Biomune Company, Lenexa, KS) made the first construct by inserting the ILTV gB and UL 32 genes into a fowlpox virus vector. The second method was made by Intervet (Intervet, Inc. Millsboro, DE), which cloned the ILTV gI and gD genes into HVT (Ou and Giambrone, 2012). Clinical symptoms decreased when 18-day-old embryos were injected with this approved commercial recombinant ILT vaccination in vo, but viral replication did not decrease following challenge (Johnson et al., 2010). This recombinant ILTV vaccination does not cause latent infection and virulent reversion. Although these recombinant vaccines are safer than previously created live vaccines, their usage has been restricted due to their higher cost and injection requirement (Brisse et al., 2020). Numerous studies have attempted to create novel gene deletion-based ILT vaccine candidates. When the virulence genes are removed, certain ILTVs can still elicit an immunological response without causing latency or clinical symptoms. Recombinant viruses with removed gJ, TK, and UL0 genes exhibit attenuation and can be employed to produce vaccines (Okamura et al., 1994; Schnitzlein et al., 1995; Veits et al., 2003). Three-week-old pathogen-free chicks administered ILTV without gG via eye drops or drinking water developed sufficient immunity to challenge (Ravikumar et al., 2022). Consequently, it might be applied to mass vaccination. More research is necessary to ascertain whether this gG-deficient vaccination is protective in commercial chicken farms (Elshafiee et al., 2022). Additionally, a non-essential ILTV gene was removed to evaluate its potential as a vaccine. The green fluorescent protein is introduced into the lost area of the UL50 gene, and gN and gM are eliminated in the ILT mutant, which has five distinct ORFs A–E removed (Fuchs et al., 2000). ILTV that has had its gC deleted is less virulent and may be used as a marker vaccination (Fuchs et al., 2005). Gene-deleted and recombinant ILTV are viable options for differentiating vaccinated chickens from sick chickens in the field (Basavarajappa et al., 2014). ILTV is a viral vector used in recombinant vaccines to carry the highly pathogenic avian influenza virus (HPAIV) H5 or H7 gene (Lüschow et al., 2001; Veits et al., 2003). This recombinant ILTV can protect chickens from HPAIV and ILT. A novel ILTV vector that inserts the HPAI H5 gene into the deleted region of the UL50 gene was recently created. This recombinant virus shields chickens from homologous and heterologous H5N1 and H5N2 viruses (Pavlova et al., 2009a, b). A DNA vaccine for the ILTV gB gene is being developed. In comparison to a monocistronic vector of the gB gene alone, the bicistronic vector of the gB gene and chicken IL-18 provided superior protection for chickens against ILTV challenge (Chen et al., 2010). The use of chicken IL-18 plasmid DNA and ILTV gB gene plasmid DNA vaccination as adjuvants induces a T helper-1 immune response, protecting chickens against virulent ILTV challenge (Chen et al., 2011). EradicationSeveral biological and ecological characteristics of ILTV make its eradication from intensive poultry production locations quite likely (García, 2017). These characteristics include the high level of host specificity of ILTV, its antigenic homogeneity and genomic stability, and its vulnerability to virus infection outside of chickens (Mo and Mo, 2025). Chickens serve as the primary host species and reservoir host; wildlife reservoirs are either nonexistent or have little ecological value in the context of ILTV (Gowthaman et al., 2020). Since backyard flocks and fancier fowl are probably ILTV reservoirs, all of these chickens must be identified and included in any eradication campaign (Bagust and Johnson, 1995). Recent studies have shown that vaccination viruses can persist outside hosts for longer than previously believed (García et al., 2013). Vaccine laryngotracheitis causes the majority of illness outbreaks in commercial chickens in Brazil (Santos et al., 2022). Some live-attenuated ILTV vaccinations can produce virulent viruses that cause outbreaks of serious illness (Perez-Contreras et al., 2021). Therefore, improving the current ILT immunization procedures will be necessary to eradicate ILTV. This entails enhancing the safety and protective immunity of potential future vaccine candidates, refining mass vaccination strategies, and creating diagnostic tools that can instantly detect latently infected chickens (Ravikumar et al., 2022). ControlUnprotected chickens should not come into contact with vaccinated or recovered field virus-infected chickens. To prevent and control ILTV infection, it is also critical to dispose of contaminated fomites (Mo and Mo, 2025). Better biosecurity and management practices are required to contain ILTV outbreaks. Biosecurity includes procedures and methods to prevent infection and disease spread through humans, insects, wild birds, or other animals (Ebrahimi et al., 2021). According to a study, the virus was lowered below detectable levels by heating litter for 24 hours at 38°C, using a commercial litter treatment, and internal composting for 5 days (Giambrone et al., 2008). ILT control requires prompt diagnosis, suitable immunization protocols, and collaboration between the public and private sectors (Salhi et al., 2021). There was an “LT vaccine” outbreak on broiler farms in California in 2005. Despite boosting biosecurity and vaccines on these farms, the corporation was unable to prevent chickens from contracting ILT. A depopulation, prolonged downtime, and stringent biosecurity approach was implemented to eradicate ILT on these farms (Chin et al., 2009). Recently, geospatial information systems have been utilized to offer information on biosecurity, quarantine, vaccine, and processing plant routes to control outbreaks. Governmental organizations, businesses, farmers, and veterinarians must collaborate to create outbreak control programs (Dufour-Zavala, 2008). ConclusionIn conclusion, ILT severely impacts poultry health and industry globally, leading to significant economic losses due to high mortality rates and reduced poultry bird productivity. Understanding the causes, symptoms, and management of ILT is crucial for mitigating its effects on poultry farming. AcknowledgmentsThe authors thank the Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga. Author’s contributionsBA, MS, ARK, and BWKW drafted the manuscript. DAAK, IBM, ATK, and SW revised and edited the manuscript. MNY, TDM, BPP, and RZA participated in the preparation and critical checking of the manuscript. IF, SW, AB, and MKJK edited the references. All authors have read and approved the final version of the manuscript. Conflict of interestThe authors declare no conflict of interest. FundingThe authors did not receive any funding for this study. Data availabilityAll references are open access, so data can be obtained from the internet. ReferencesAbebe, S., Ferrara, G., Getachew, B., Hirpa, E. and Moje, N. 2024. Serological and molecular investigation of infectious laryngotracheitis virus in chickens from Robe town, Southeastern Ethiopia. Animals 14(22), 3227. Adam, O., Oladele, O.A., Yimam, T.M., Getachew, B., Deresse, G., Birhanu, K., Legesse, A., Tefera, T.A. and Bitew, M. 2025. Serological and molecular detection of infectious laryngotracheitis virus in chickens in Central Gondar Zone, Ethiopia. Front. Vet. Sci. 12(1), 1517373. Alaraji, F., Hammadi, H., Abed, A.A. and Khudhair, Y.I. 2019. Molecular detection and phylogenetic tree of infectious laryngotracheitis virus in layers in Al-Diwaniyah province, Iraq. Vet. World 12(4), 605–608. Artasensi, A., Mazzotta, S. and Fumagalli, L. 2021. Back to basics: choosing the appropriate surface disinfectant. Antibiotics (Basel). 10(6), 613. Bagust, T.J. and Johnson, M.A. 1995. Avian infectious laryngotracheitis: virus‐host interactions in relation to prospects for eradication. Avian Pathol. 24(3), 373–391. Bagust, T.J., Jones, R.C. and Guy, J.S. 2000. Avian infectious laryngotracheitis. Rev. Sci. Tech. 19(2), 483–492. Basavarajappa, M.K., Kumar, S., Khattar, S.K., Gebreluul, G.T., Paldurai, A. and Samal, S.K. 2014. A recombinant Newcastle disease virus (NDV) expressing infectious laryngotracheitis virus (ILTV) surface glycoprotein D protects against highly virulent ILTV and NDV challenges in chickens. Vaccine 32(28), 3555–3563. Baudette, F.R. 1937. Infectious laryngotracheitis. Poult. Sci. 16(2), 103–105. Beltrán, G., Hurley, D.J., Gogal, R.M., Sharif, S., Read, L.R., Williams, S.M., Jerry, C.F., Maekawa, D.A. and García, M. 2019. Immune responses in the eye-associated lymphoid tissues of chickens after ocular inoculation with vaccine and virulent strains of the respiratory infectious laryngotracheitis virus (ILTV). Viruses 11(7), 635. Beltrán, G., Williams, S.M., Zavala, G., Guy, J.S. and García, M. 2017. The route of inoculation dictates the replication patterns of the infectious laryngotracheitis virus (ILTV) pathogenic strain and chicken embryo origin (CEO) vaccine. Avian Pathol. 46(6), 585–593. Beltrão, N., Egochega, R.F., Furian, T.Q., Rodenbusch, C.R., Fallavena, L.C.B., Pasquali, G. and Canal, C.W. 2012. A sensitive nested-polymerase chain reaction protocol to detect infectious laryngotracheitis virus. Acta Sci. Vet. 40(3), 1055. Bindari, Y.R., Walkden-Brown, S.W. and Gerber, P.F. 2020. Methods to prevent PCR amplification of DNA from non-viable virus were not successful for infectious laryngotracheitis virus. PLoS One 15(5), e0232571. Birhan, M., Syoum, A., Ibrahim, S.M., Fentahun, T., Mohammed, A., Berhane, N., Bitew, M., Gelaye, E., Atanaw, M.B., Getachew, B., Dessalegn, B., Abat, A.S., Berrie, K., Adamu, K. and Abayneh, T. 2022. Serological evidence of infectious laryngotracheitis infection and associated risk factors in chickens in Northwestern Ethiopia. Sci. World J. 1(1), 6096981. Blacker, H.P., Kirkpatrick, N.C., Rubite, A., O’Rourke, D. and Noormohammadi, A.H. 2011. Epidemiology of recent outbreaks of infectious laryngotracheitis in poultry in Australia. Aust. Vet. J. 89(3), 89–94. Blakey, J., Stoute, S., Crossley, B. and Mete, A. 2019. Retrospective analysis of infectious laryngotracheitis in backyard chicken flocks in California, 2007–2017, and determination of strain origin by partial ICP4 sequencing. J. Vet. Diagn. Invest. 31(3), 350–358. Brandly, C.A. and Bushnell, L.D. 1934. A report of some investigations of infectious laryngotracheitis. Poult. Sci. 13(4), 212–217. Brisse, M., Vrba, S.M., Kirk, N., Liang, Y. and Ly, H. 2020. Emerging concepts and technologies in vaccine development. Front. Immunol. 11(1), 583077. Brown, L., Premaratna, D., Segal, Y. and Beddoe, T. 2020. Air sampling for detection of infectious laryngotracheitis (ILT) in commercial poultry flocks. BMC Res. Notes 13(1), 556. Carnaccini, S., Palmieri, C., Stoute, S., Crispo, M. and Shivaprasad, H.L. 2022. Infectious laryngotracheitis of chickens: pathologic and immunohistochemistry findings. Vet. Pathol. 59(1), 112–119. Chacón, J.L. and Ferreira, A.J. 2009. Differentiation of field isolates and vaccine strains of infectious laryngotracheitis virus by DNA sequencing. Vaccine 27(48), 6731–6738. Chacón, J.L., Chacón, R.D., Hagemann, H.L., Astolfi-Ferreira, C.S., Nunes, C., Sesti, L., Alva, B. and Ferreira, A.J.P. 2025. Molecular characterization of the infectious laryngotracheitis virus (ILTV) involved in poultry outbreaks reveals the virus origin and estimated spreading route. Viruses 17(2), 213. Chen, H.Y., Zhang, H.Y., Li, X.S., Cui, B.A., Wang, S.J., Geng, J.W. and Li, K. 2011. Interleukin-18-mediated enhancement of the protective effect of an infectious laryngotracheitis virus glycoprotein B plasmid DNA vaccine in chickens. J. Med. Microbiol. 60(Pt 1), 110–116. Chen, H.Y., Zhao, L., Wei, Z.Y., Cui, B.A., Wang, Z.Y., Li, X.S., Xia, P.A. and Liu, J.P. 2010. Enhancement of the immunogenicity of an infectious laryngotracheitis virus DNA vaccine by a bicistronic plasmid encoding glycoprotein B and interleukin-18. Antiviral Res. 87(2), 235–241. Chen, S., Xu, N., Ta, L., Li, S., Su, X., Xue, J., Du, Y., Qin, T. and Peng, D. 2020. Recombinant fowlpox virus expressing gB gene from predominantly epidemic infectious laryngotracheitis virus strain demonstrates better immune protection in SPF chickens. Vaccines (Basel). 8(4), 623. Chin, R.P., García, M., Corsiglia, C., Riblet, S., Crespo, R., Shivaprasad, H.L., Rodríguez-Avila, A., Woolcock, P.R. and França, M. 2009. Intervention strategies for laryngotracheitis: impact of extended downtime and enhanced biosecurity auditing. Avian Dis. 53(4), 574–577. Contreras, A.P., van der Meer, F., Checkley, S., Joseph, T., King, R., Ravi, M., Peters, D., Fonseca, K., Gagnon, C.A., Provost, C., Ojkic, D. and Abdul-Careem, M.F. 2020. Analysis of whole-genome sequences of infectious laryngotracheitis virus isolates from poultry flocks in Canada: evidence of recombination. Viruses 12(11), 1302. Coppo, M.J.C., Noormohammadi, A.H., Browning, G.F. and Devlin, J.M. 2013. Challenges and recent advancements in infectious laryngotracheitis virus vaccines. Avian Pathol. 42(3), 195–205. Crawshaw, G.J. and Boycott, B.R. 1982. Infectious laryngotracheitis in peafowl and pheasants. Avian Dis. 26(2), 397–401. Creelan, J.L., Calvert, V.M., Graham, D.A. and McCullough, S.J. 2006. Rapid detection and characterization from field cases of infectious laryngotracheitis virus by real-time polymerase chain reaction and restriction fragment length polymorphism. Avian Pathol. 35(2), 173–179. Crespo, R., Woolcock, P.R., Chin, R.P., Shivaprasad, H.L. and García, M. 2007. Comparison of diagnostics techniques in an outbreak of infectious laryngotracheitis from meat chickens. Avian Dis. Dig. 2(4), e3–e3. Davidson, I. 2019. Biotic concerns in generating molecular diagnosis matrixes for 4 avian viruses with emphasis on Marek’s disease virus. J. Virol. Methods 274(1), 113708. Davidson, I., Raibstein, I. and Altory, A. 2015. Differential diagnosis of fowlpox and infectious laryngotracheitis viruses in chicken diphtheritic manifestations by mono and duplex real-time polymerase chain reaction. Avian Pathol. 44(1), 1–4. Davidson, I., Raibshtein, I., Altori, A. and Elkin, N. 2016. Infectious laryngotracheitis virus (ILTV) vaccine intake evaluation by detection of virus amplification in feather pulps of vaccinated chickens. Vaccine 34(13), 1630–1633. Dufour-Zavala, L. 2008. Epizootiology of infectious laryngotracheitis and presentation of an industry control program. Avian Dis. 52(1), 1–7. Ebrahimi, M.M., Shahsavandi, S., Yousefi, A.R. and Ebrahimi, N. 2021. Isolation, identification and chemical inactivation of infectious laryngotracheitis virus for use as a vaccine candidate. Acta Virol. 65(1), 33–41. El-Saied, M., El-Mahdy, M., El-Din Sakr, E., Bastami, M. and Shaalan, M. 2021. Anatomopathological, ultrastructural, immunohistochemical and molecular characterization of infectious laryngotracheitis outbreaks in poultry farms in Egypt (2018–2020). Braz. J. Vet. Pathol. 14(2), 88–98. El-Shemy, A.A., Amer, M.M., Hassan, H.M. and Elaish, M. 2024. Epidemiological distribution of respiratory viral pathogens in marketable vaccinated broiler chickens in five governorates in the Nile Delta, Egypt, from January 2022 to October 2022. Vet. World 17(2), 303–312. Elshafiee, E.A., Isham, I.M., Najimudeen, S.M., Perez-Contreras, A., Barboza-Solis, C., Ravi, M. and Abdul-Careem, M.F. 2022. Host responses following infection with Canadian-origin wildtype and vaccine revertant infectious laryngotracheitis virus. Vaccines10(5), 782. Fay, N. and Panté, N. 2015. Nuclear entry of DNA viruses. Front. Microbiol. 6(1), 467. Fuchs, W., Mettenleiter, T.C., Teifke, J.P., Werner, O. and Ziemann, K. 2000. The non-essential UL50 gene of avian infectious laryngotracheitis virus encodes a functional dUTPase which is not a virulence factor. J. Gen. Virol. 81(Pt 3), 627–638. Fuchs, W., Veits, J., Helferich, D., Granzow, H., Teifke, J.P. and Mettenleiter, T.C. 2007. Molecular biology of avian infectious laryngotracheitis virus. Vet. Res. 38(2), 261–279. Fuchs, W., Wiesner, D., Veits, J., Teifke, J.P. and Mettenleiter, T.C. 2005. In vitro and in vivo relevance of infectious laryngotracheitis virus gJ proteins that are expressed from spliced and nonspliced mRNAs. J. Virol. 79(2), 705–716. Gaghan, C., Browning, M., Fares, A.M., Abdul-Careem, M.F., Gimeno, I.M. and Kulkarni, R.R. 2023. In ovo vaccination with recombinant herpes virus of the Turkey-laryngotracheitis vaccine adjuvanted with CpG-oligonucleotide provides protection against a viral challenge in broiler chickens. Viruses 15(10), 2103. García, M. 2017. Current and future vaccines and vaccination strategies against infectious laryngotracheitis (ILT) respiratory disease of poultry. Vet. Microbiol. 206(1), 157–162. García, M. and Zavala, G. 2019. Commercial vaccines and vaccination strategies against infectious laryngotracheitis: what we have learned and knowledge gaps that remain. Avian Dis. 63(2), 325–334. García, M., Volkening, J., Riblet, S. and Spatz, S. 2013. Genomic sequence analysis of the United States infectious laryngotracheitis vaccine strains chicken embryo origin (CEO) and tissue culture origin (TCO). Virology 440(1), 64–74. Giambrone, J.J., Fagbohun, O. and Macklin, K.S. 2008. Management practices to reduce infectious laryngotracheitis virus in poultry litter. J. Appl. Poult. Res. 17(1), 64–68. Godoy, A., Icard, A., Martinez, M., Mashchenko, A., García, M. and El-Attrachea, J. 2013. Detection of infectious laryngotracheitis virus antibodies by glycoprotein-specific ELISAs in chickens vaccinated with viral vector vaccines. Avian Dis. 57(2 Suppl), 432–436. Gopakumar, G., Coppo, M.J., Diaz-Méndez, A., Hartley, C.A. and Devlin, J.M. 2025. Host-virus interactions during infection with a wild-type ILTV strain or a glycoprotein G deletion mutant ILTV vaccine strain in an ex vivo system. Microbiol. Spectr. 13(2), e0118324. Gopakumar, G., Diaz-Méndez, A., Coppo, M.J.C., Hartley, C.A. and Devlin, J.M. 2024. Transcriptomic analyses of host-virus interactions during in vitro infection with wild-type and glycoprotein g-deficient (ΔgG) strains of ILTV in primary and continuous cell cultures. PLoS One 19(10), e0311874. Gowthaman, V., Koul, M. and Kumar, S. 2016. Avian infectious laryngotracheitis: a neglected poultry health threat in India. Vaccine 34(36), 4276–4277. Gowthaman, V., Kumar, S., Koul, M., Dave, U., Murthy, T.R.G.K., Munuswamy, P., Tiwari, R., Karthik, K., Dhama, K., Michalak, I. and Joshi, S.K. 2020. Infectious laryngotracheitis: etiology, epidemiology, pathobiology, and advances in diagnosis and control – a comprehensive review. Vet. Quart. 40(1), 140–161. Gowthaman, V., Singh, S.D., Dhama, K., Barathidasan, R., Mathapati, B.S., Srinivasan, P., Saravanan, S. and Ramakrishnan, M.A. 2014. Molecular detection and characterization of infectious laryngotracheitis virus (Gallid herpesvirus-1) from clinical samples of commercial poultry flocks in India. Virusdisease 25(3), 345–349. Groves, P.J., Williamson, S.L., Sharpe, S.M., Gerber, P.F., Gao, Y.K., Hirn, T.J. and Walkden-Brown, S.W. 2019. Uptake and spread of infectious laryngotracheitis vaccine virus within meat chicken flocks following drinking water vaccination. Vaccine 37(35), 5035–5043. Gržinić, G., Piotrowicz-Cieślak, A., Klimkowicz-Pawlas, A., Górny, R.L., Ławniczek-Wałczyk, A., Piechowicz, L., Olkowska, E., Potrykus, M., Tankiewicz, M., Krupka, M., Siebielec, G. and Wolska, L. 2023. Intensive poultry farming: a review of the impact on the environment and human health. Sci. Total Environ. 858, 160014. Han, M.G. and Kim, S.J. 2001. Comparison of virulence and restriction endonuclease cleavage patterns of infectious laryngotracheitis viruses isolated in Korea. Avian Pathol. 30(4), 337–344. Han, M.G., Kweon, C.H., Mo, I.P. and Kim, S.J. 2002. Pathogenicity and vaccine efficacy of a thymidine kinase gene deleted infectious laryngotracheitis virus expressing the green fluorescent protein gene. Arch. Virol. 147(5), 1017–1031. Hein, R., Koopman, R., García, M., Armour, N., Dunn, J.R., Barbosa, T. and Martinez, A. 2021. Review of poultry recombinant vector vaccines. Avian Dis. 65(3), 438–452. Hermann, S., Stevens, M.J.A., Sigrist, B., Bilic, I., Albini, S. and Wolfrum, N. 2024. Unveiling the genetic landscape of infectious laryngotracheitis virus in Switzerland: evidence for vaccine-like and wild-type strains. Virology 600(1), 110217. Hernandez-Divers, S.M., Villegas, P., Jimenez, C., Hernandez-Divers, S.J., Garcia, M., Riblet, S.M., Carroll, C.R., O’Connor, B.M., Webb, J.L., Yabsley, M.J., Williams, S.M. and Sanchez, S. 2008. Backyard chicken flocks pose a disease risk for neotropic birds in Costa Rica. Avian Dis. 52(4), 558–566. Hidalgo, H. 2003. Infectious laryngotracheitis: a review. Braz. J. Poult. Sci. 5(3), 157–168. Hong, X., Zhang, H., Zhang, X., Zhang, X.P. and Zhang, T. 2024. A meta-analysis for prevalence of infectious laryngotracheitis in chickens in mainland China in 1981–2022. BMC Vet. Res. 20(1), 142. Hudson, C.B. and Beaudette, F.R. 1932. Infection of the Cloaca with the virus of infectious bronchitis. Science 76(1958), 34–34. Ibrahim, S.F., Zayan, K.A. and Saad, A.E. 2021. Molecular characterization and isolation of Infectious laryngotracheitis virus (ILTV) strains causing outbreaks in layer chicken farms of Qalyubia Province, Egypt. Benha Vet. Med. J. 40(2), 126–130. Islam, M., Khan, M., Islam, M., Hassan, J., Affroze, S. and Islam, M. 2010. Isolation and characterization of infectious laryngotracheitis virus in layer chickens. Bangl. J. Vet. Med. 8(2), 123–130. Johnson, D.I., Vagnozzi, A., Dorea, F., Riblet, S.M., Mundt, A., Zavala, G. and García, M. 2010. Protection against infectious laryngotracheitis by in ovo vaccination with commercially available viral vector recombinant vaccines. Avian Dis. 54(4), 1251–1259. Johnson, Y.J., Gedamu, N., Colby, M.M., Myint, M.S., Steele, S.E., Salem, M. and Tablante, N.L. 2005. Wind-borne transmission of infectious laryngotracheitis between commercial poultry operations. Int. J. Poult. Sci. 4(5), 263–267. Kamal, M.M., Sadekuzzaman, M., Parvin, K., Haque, M.E., Hayat, S., Islam, M.A., Khatun, M.M., Siddique, M.P., Nahar, S.S., Khasruzzaman, A.K.M., Hossain, M.T. and Islam, M.A. 2024. Characterization of infectious laryngotracheitis virus isolated from commercial layer chickens in Bangladesh during the year 2021–2022. J. Adv. Vet. Anim. Res. 11(2), 398–407. Kammon, A., Shabba, J., Asheg, A. and Abouzeed, Y. 2020. Isolation, serological and molecular detection of infectious laryngotracheitis virus (ILTV) in chickens in Libya. Appro. Poult. Dairy Vet. Sci. 7(4), 655–665. Kardoğan, O. and İnce, S.S. 2024. Molecular characterization and phylogenetic analysis of infectious laryngotracheitis virus isolates from commercial chicken flocks in Turkey. Arch. Virol. 169(11), 231. Kaur, J. 2021. Infectious laryngotracheitis in avian species: a review. Pharm. Innov. 10(6), 450–454. Kim, H.R., Kang, M.S., Kim, M.J., Lee, H.S. and Kwon, Y.K. 2013. Restriction fragment length polymorphism analysis of multiple genome regions of Korean isolates of infectious laryngotracheitis virus collected from chickens. Poult. Sci. 92(8), 2053–2058. Kirkpatrick, N.C., Mahmoudian, A., Colson, C.A., Devlin, J.M. and Noormohammadi, A.H. 2006. Relationship between mortality, clinical signs and tracheal pathology in infectious laryngotracheitis. Avian Pathol. 35(6), 449–453. Kotiw, M., Sheppard, M., May, J.T. and Wilks, C.R. 1986. Differentiation between virulent and avirulent strains of infectious laryngotracheitis virus by DNA: DNA hybridization using a cloned DNA marker. Vet. Microbiol. 11(4), 319–330. Kotiw, M., Wilks, C.R. and May, J.T. 1995. The effect of serial in vivo passage on the expression of virulence and DNA stability of an infectious laryngotracheitis virus strain of low virulence. Vet. Microbiol. 45(1), 71–80. Krunkosky, M., García, M., Beltran, G., Williams, S.M., Hurley, D.J. and Gogal Jr., R.M. 2020. Ocular exposure to infectious laryngotracheitis virus alters leukocyte subsets in the head-associated lymphoid tissues and trachea of 6-week-old White Leghorn chickens. Avian Pathol. 49(4), 404–417. Lee, J., Bottje, W.G. and Kong, B.W. 2012. Genome-wide host responses against infectious laryngotracheitis virus vaccine infection in chicken embryo lung cells. BMC Genomics 13(1), 143. Lee, J.Y., Song, J.J., Wooming, A., Li, X., Zhou, H., Bottje, W.G. and Kong, B.W. 2010. Transcriptional profiling of host gene expression in chicken embryo lung cells infected with laryngotracheitis virus. BMC. Genomics 11(1), 445. Lee, S.W., Markham, P.F., Coppo, M.J., Legione, A.R., Shil, N.K., Quinteros, J.A., Noormohammadi, A.H., Browning, G.F., Hartley, C.A. and Devlin, J.M. 2014. Cross-protective immune responses between genotypically distinct lineages of infectious laryngotracheitis viruses. Avian Dis. 58(1), 147–152. Lo Piano, A., Martínez-Jiménez, M.I., Zecchi, L. and Ayora, S. 2011. Recombination-dependent concatemeric viral DNA replication. Virus. Res. 160(1–2), 1–14. Loncoman, C.A., Hartley, C.A., Coppo, M.J.C., Vaz, P.K., Diaz-Méndez, A., Browning, G.F., García, M., Spatz, S. and Devlin, J.M. 2017. Genetic diversity of infectious laryngotracheitis virus during in vivo coinfection parallels viral replication and arises from recombination hot spots within the genome. Appl. Environ. Microbiol. 83(23), e01532. Lüschow, D., Werner, O., Mettenleiter, T.C. and Fuchs, W. 2001. Protection of chickens from lethal avian influenza A virus infection by live-virus vaccination with infectious laryngotracheitis virus recombinants expressing the hemagglutinin (H5) gene. Vaccine 19(30), 4249–4259. Lv, Y., Zhou, S., Gao, S. and Deng, H. 2019. Remodeling of host membranes during herpesvirus assembly and egress. Protein Cell 10(5), 315–326. Maekawa, D., Riblet, S.M., Newman, L., Koopman, R., Barbosa, T. and García, M. 2019. Evaluation of vaccination against infectious laryngotracheitis (ILT) with recombinant herpesvirus of turkey (rHVT-LT) and chicken embryo origin (CEO) vaccines applied alone or in combination. Avian Pathol. 48(6), 573–581. Maekawa, D., Riblet, S.M., Whang, P., Alvarado, I. and García, M. 2021a. A cell line adapted infectious laryngotracheitis virus strain (BΔORFC) for in ovo and hatchery spray vaccination alone or in combination with a recombinant HVT-LT vaccine. Avian Dis. 65(3), 500–507. Maekawa, D., Riblet, S.M., Whang, P., Hurley, D.J. and Garcia, M. 2021b. Activation of cytotoxic lymphocytes and presence of regulatory T cells in the trachea of non-vaccinated and vaccinated chickens as a recall to an infectious laryngotracheitis virus (ILTV) challenge. Vaccines 9(8), 865. Maini, G., Cianci, G., Ferraresi, M., Gentili, V. and Bortolotti, D. 2024. DNA-based technology for herpesvirus detection. DNA 4(4), 553–581. Menendez, K.R., García, M., Spatz, S. and Tablante, N.L. 2014. Molecular epidemiology of infectious laryngotracheitis: a review. Avian Pathol. 43(2), 108–117. Mo, J. and Mo, J. 2025. Infectious laryngotracheitis virus and avian metapneumovirus: a comprehensive review. Pathogens 14(1), 55. Molini, U., Aikukutu, G., Khaiseb, S., Kahler, B., Van Der Westhuizen, J., Cattoli, G. and Dundon, W.G. 2019. Investigation of infectious laryngotracheitis outbreaks in Namibia in 2018. Trop. Anim. Health Prod. 51(7), 2105–2108. Mossad, Z., Moussa, S.A., Saied, M., Fathy, M.M. and Zanaty, A.M. 2022. Molecular and genetic detection of infectious laryngeotracheitis disease virus in broiler farms after a disease outbreak in Egypt. Virusdisease 33(4), 404–412. Müştak, I.B. and Müştak, H.K. 2024. Circulation and molecular characterization of infectious laryngotracheitis virus in poultry flocks with respiratory disorders in Turkey, 2018-2022. Avian Dis. 68(2), 112–116. Nazir, S., Yegoraw, A.A., Charlesworth, R.P.G., Williamson, S., Sharpe, S., Walkden-Brown, S.W. and Gerber, P.F. 2020. Marked differences in virulence of three Australian field isolates of infectious laryngotracheitis virus in meat and layer chickens. Avian Pathol. 49(6), 600–610. Neff, C., Sudler, C. and Hoop, R.K. 2008. Characterization of western European field isolates and vaccine strains of avian infectious laryngotracheitis virus by restriction fragment length polymorphism and sequence analysis. Avian Dis. 52(2), 278–283. Neighbor, N.K., Newberry, L.A., Bayyari, G.R., Skeeles, J.K., Beasley, J.N. and McNew, R.W. 1994. The effect of microaerosolized hydrogen peroxide on bacterial and viral poultry pathogens. Poult. Sci. 73(10), 1511–1516. Newcomb, W.W., Fontana, J., Winkler, D.C., Cheng, N., Heymann, J.B. and Steven, A.C. 2017. The primary enveloped virion of herpes simplex virus 1: its role in nuclear egress. mBio 8(3), e00825. Okamura, H., Sakaguchi, M., Honda, T., Taneno, A., Matsuo, K. and Yamada, S. 1994. Construction of recombinant infectious laryngotracheitis virus expressing the LacZ Gene of E. coli with thymidine kinase gene. J. Vet. Med. Sci. 56(4), 799–801. Okino, C.H., Dos Santos, I.L., Fernando, F.S., Alessi, A.C., Wang, X. and Montassier, H.J. 2014. Inflammatory and cell-mediated immune responses in the respiratory tract of chickens to infection with avian infectious bronchitis virus. Viral Immunol. 27(8), 383–391. Oldoni, I., Rodríguez-Avila, A., Riblet, S. and García, M. 2008. Characterization of infectious laryngotracheitis virus (ILTV) isolates from commercial poultry by polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP). Avian Dis. 52(1), 59–63. Ou, S.C. and Giambrone, J.J. 2012. Infectious laryngotracheitis virus in chickens. World J. Virol. 1(5), 142–149. Ou, S.C., Giambrone, J.J. and Macklin, K.S. 2012b. Detection of infectious laryngotracheitis virus from darkling beetles and their immature stage (lesser mealworms) by quantitative polymerase chain reaction and virus isolation. J. Appl. Poult. Res. 21(1), 33–38. Pajić, M., Knežević, S., Djurdjević, B., Polaček, V., Todorović, D., Petrović, T. and Lazić, S. 2022. Diagnosis of infectious laryngotracheitis outbreaks on layer hen and broiler breeder farms in Vojvodina, Serbia. Animals 12(24), 3551. Palomino-Tapia, V.A., Zavala, G., Cheng, S. and Garcia, M. 2023. Attenuation of a field strain of infectious laryngotracheitis virus in primary chicken culture cells and adaptation to secondary chicken embryo fibroblasts. Poultry 2(4), 516–530. Parra, S., Nuñez, L. and Ferreira, A. 2016. Epidemiology of avian infectious laryngotracheitis with special focus to South America: an update. Rev. Bras. Cienc. Avic. 18(4), 551–562. Pavlova, S.P., Veits, J., Keil, G.M., Mettenleiter, T.C. and Fuchs, W. 2009a. Protection of chickens against H5N1 highly pathogenic avian influenza virus infection by live vaccination with infectious laryngotracheitis virus recombinants expressing H5 hemagglutinin and N1 neuraminidase. Vaccine 27(5), 773–785. Pavlova, S.P., Veits, J., Mettenleiter, T.C. and Fuchs, W. 2009b. Live vaccination with an H5-hemagglutinin-expressing infectious laryngotracheitis virus recombinant protects chickens against different highly pathogenic avian influenza viruses of the H5 subtype. Vaccine 27(37), 5085–5090. Perez-Contreras, A., Barboza-Solis, C., Najimudeen, S.M., Checkley, S., Meer, F.V.D., Joseph, T., King, R., Ravi, M., Peters, D., Fonseca, K., Gagnon, C.A., Ojkic, D. and Abdul-Careem, M.F. 2021. Pathogenic and transmission potential of wildtype and chicken embryo origin (CEO) vaccine revertant infectious laryngotracheitis virus. Viruses 13(4), 541. Pitcovski, J., Pitcovski, E., Goldenberg, D. and Shahar, E. 2017. Pair-epitopes vaccination: enabling offspring vaccination in the presence of maternal antibodies. Avian Pathol. 46(6), 581–584. Preis, I.S., Braga, J.F.V., Couto, R.M., Brasil, B.S.A.F., Martins, N.R.S. and Ecco, R. 2013. Outbreak of infectious laryngotracheitis in large multi-age egg layer chicken flocks in Minas Gerais, Brazil. Pesq. Vet. Bras. 33(5), 591–596. Preis, I.S., Fiúza, A.T.L., Silva, C.C., Braga, J.F.V., Couto, R.M.I., Martins, N.R. da S. and Ecco, R. 2014. Pathological, immunohistochemical, and molecular findings in commercial laying hens and in backyard chickens naturally infected with the infectious laryngotracheitis virus. Braz. J. Poult. Sci. 16(4), 359–366. Ravikumar, R., Chan, J. and Prabakaran, M. 2022. Vaccines against major poultry viral diseases: strategies to improve the breadth and protective efficacy. Viruses 14(6), 1195. Reddy, V.R.A.P., Steukers, L., Li, Y., Fuchs, W., Vanderplasschen, A. and Nauwynck, H.J. 2014. Replication characteristics of infectious laryngotracheitis virus in the respiratory and conjunctival mucosa. Avian Pathol. 43(5), 450–457. Rojs, O.Z., Dovč, A., Krapež, U., Žlabravec, Z., Račnik, J. and Slavec, B. 2021. Detection of laryngotracheitis virus in poultry flocks with respiratory disorders in slovenia. Viruses 13(4), 707.Rouse, B.T. and Sehrawat, S. 2010. Immunity and immunopathology to viruses: what decides the outcome?. Nat. Rev. Immunol. 10(7), 514–526. Sabir, A.J., Adams, T.E., O’Rourke, D., Devlin, J.M. and Noormohammadi, A.H. 2019. Investigation into the correlation between systemic antibodies to surface glycoproteins of infectious laryngotracheitis virus (iltv) and protective immunity. Vet. Microbiol. 228(1), 252–258. Salhi, O., Messaï, C.R., Ouchene, N., Boussaadi, I., Kentouche, H., Kaidi, R. and Khelef, D. 2021. Indicators and risk factors of infectious laryngotracheitis in layer hen flocks in Algeria. Vet. World 14(1), 182–189. Santos, W.H.M., Oliveira, L.B.D., Leão, P.A., Hergot, I.G., Wenceslau, R.R., Rocha, C.M.B.M.D., Ferreira, H.L., Resende, M., Martins, N.R.S., Spatz, S.J. and Ecco, R. 2022. A five-year surveillance study of vaccination schedules using viral-vectored vaccines against infectious laryngotracheitis in a high-density layer region. Pesq. Vet. Bras. 42(1), e07037. Schnitzlein, W.M., Winans, R., Ellsworth, S. and Tripathy, D.N. 1995. Generation of thymidine kinase-deficient mutants of infectious laryngotracheitis virus. Virology 209(2), 304–314. Sellers, H.S., García, M., Glisson, J.R., Brown, T.P., Sander, J.S. and Guy, J.S. 2004. Mild infectious laryngotracheitis in broilers in the southeast. Avian Dis. 48(2), 430–436. Shankar, B.P. 2008. Common respiratory diseases of poultry. Vet. World 1(7), 217–219. Shao, G., Fu, J., Pan, Y., Gong, S., Song, C., Chen, S., Feng, K., Zhang, X. and Xie, Q. 2025. Development of a recombinant infectious bronchitis virus vaccine expressing infectious laryngotracheitis virus multiple epitopes. Poult. Sci. 104(1), 104578. Sharma, S., Mohler, J., Mahajan, S.D., Schwartz, S.A., Bruggemann, L. and Aalinkeel, R. 2023. Microbial biofilm: a review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms 11(6), 1614. Su, Y., Barr, J., Jaquish, A., Xu, J., Verheyden, J.M. and Sun, X. 2022. Identification of lung innervating sensory neurons and their target specificity. Am. J. Physiol-Lung. Cellular Mol. Physiol. 322(1), L50–L63. Taha, Z.H., Allawe, A.B., Kadhum, M.J. and Abbas, A.A. 2017. Isolation and sequencing of field isolates of infectious laryngotracheitis virus in Iraq. J. Entomol. Zool. Stud. 5(5), 882–886. Tesfaye, A., Sahle, M., Sori, T., Kassa, T., Garoma, A., Koran, T., Dima, C., Guyassa, C., Hilu, H., Guta, S. and Tadesse, F. 2019. Infectious laryngotracheitis virus in commercial and backyard chicken production systems in central and south Ethiopia (First report) ILT in Ethiopian poultry production. J. Appl. Poultry Res. 28(4), 1324–1329. Theil, D., Derfuss, T., Paripovic, I., Herberger, S., Meinl, E., Schueler, O., Strupp, M., Arbusow, V. and Brandt, T. 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am. J. Pathol. 163(6), 2179–2184. Thilakarathne, D.S., Coppo, M.J.C., Hartley, C.A., Diaz-Méndez, A., Quinteros, J.A., Fakhri, O., Vaz, P.K. and Devlin, J.M. 2019. Attenuated infectious laryngotracheitis virus vaccines differ in their capacity to establish latency in the trigeminal ganglia of specific pathogen free chickens following eye drop inoculation. PLoS One 14(3), e0213866. Thilakarathne, D.S., Hartley, C.A., Diaz-Méndez, A., Coppo, M.J.C. and Devlin, J.M. 2020a. Development and application of a combined molecular and tissue culture-based approach to detect latent infectious laryngotracheitis virus (ILTV) in chickens. J. Virol. Methods 277(1), 113797. Thilakarathne, D.S., Hartley, C.A., Diaz-Méndez, A., Quinteros, J.A., Fakhri, O., Coppo, M.J.C. and Devlin, J.M. 2020b. Latency characteristics in specific pathogen-free chickens 21 and 35 days after intra-tracheal inoculation with vaccine or field strains of infectious laryngotracheitis virus. Avian Pathol. 49(4), 369–379. Thilakarathne, D.S., Noormohammadi, A.H., Browning, G.F., Quinteros, J.A., Underwood, G.J., Hartley, C.A., Coppo, M.J., Devlin, J.M. and Diaz-Méndez, A. 2020c. Pathogenesis and tissue tropism of natural field recombinants of infectious laryngotracheitis virus. Vet. Microbiol. 243(1), 108635. Tran, T.T., Andronicos, N. and Gerber, P.F. 2024. Expression of immune genes and leukocyte population in the conjunctiva, harderian gland and trachea of chickens inoculated with a live vaccine and a field strain infectious laryngotracheitis virus. Poultry 3(4), 399–408. Tsiouris, V., Mavromati, N., Kiskinis, K., Mantzios, T., Homonnay, Z.G., Mato, T., Albert, M., Kiss, I. and Georgopoulou, I. 2021. A case of infectious laryngotracheitis in an organic broiler chicken farm in Greece. Vet. Sci. 8(4), 64. Vagnozzi, A., García, M., Riblet, S.M. and Zavala, G. 2010. Protection induced by infectious laryngotracheitis virus vaccines alone and combined with Newcastle disease virus and/or infectious bronchitis virus vaccines. Avian Dis. 54(4), 1210–1219. Vagnozzi, A., Zavala, G., Riblet, S.M., Mundt, A. and García, M. 2012. Protection induced by commercially available live-attenuated and recombinant viral vector vaccines against infectious laryngotracheitis virus in broiler chickens. Avian Pathol. 41(1), 21–31. Van De Walle, G.R., May, M.L., Sukhumavasi, W., Von Einem, J. and Osterrieder, N. 2007. Herpesvirus chemokine-binding glycoprotein G (gG) efficiently inhibits neutrophil chemotaxis in vitro and in vivo. J. Immunol. 179(6), 4161–4169. Veits, J., Lüschow, D., Kindermann, K., Werner, O., Teifke, J.P., Mettenleiter, T.C. and Fuchs, W. 2003. Deletion of the non-essential UL0 gene of infectious laryngotracheitis (ILT) virus leads to attenuation in chickens, and UL0 mutants expressing influenza virus haemagglutinin (H7) protect against ILT and fowl plague. J. Gen. Virol. 84(Pt 12), 3343–3352. Villarreal, L.Y.B., Brandão, P.E.B., Chacón, J.L.V., Doretto, L., Ito, N., Gama, N.S., Ishizuka, M.M., Luchese, A., Buchala, F., Astolfi-Ferreira, C.S. and Ferreira, A.J.P. 2004. Detection and molecular characterization of infectious laryngotracheitis virus in laying hens in Brazil. Braz. J. Poult. Sci. 6(4), 253–256. Volkova, V., Thornton, D., Hubbard, S.A., Magee, D., Cummings, T., Luna, L., Watson, J. and Wills, R. 2012. Factors associated with introduction of infectious laryngotracheitis virus on broiler farms during a localized outbreak. Avian Dis. 56(3), 521–528. Watrach, A.M., Hanson, L.E. and Watrach, M.A. 1963. The structure of infectious laryngotracheitis virus. Virology 21(4), 601–608. Williams, R.A., Bennett, M., Bradbury, J.M., Gaskell, R.M., Jones, R.C. and Jordan, F.T.W. 1992. Demonstration of sites of latency of infectious laryngotracheitis virus using the polymerase chain reaction. J. Gen. Virol. 73(Pt 9), 2415–2420. Wolfrum, N. 2020. Infectious laryngotracheitis: an update on current approaches for prevention of an old disease. J. Anim. Sci. 98(Suppl 1), S27–S35. Wu, M., Zhang, Z., Su, X., Lu, H., Li, X., Yuan, C., Liu, Q., Teng, Q., Geri, L. and Li, Z. 2022. Biological characteristics of infectious laryngotracheitis viruses isolated in China. Viruses 14(6), 1200. Yegoraw, A.A., Assen, A.M., Gerber, P.F. and Walkden-Brown, S.W. 2021. Transmission of infectious laryngotracheitis virus vaccine and field strains: the role of degree of contact and transmission by whole blood, plasma and poultry dust. Vet. Res. 52(1), 91. Yehia, N., Salem, H.M., Mahmmod, Y., Said, D., Samir, M., Mawgod, S.A., Sorour, H.K., Abdelrahman, M.A.A., Selim, S., Saad, A.M., El-Saadony, M.T., El-Meihy, R.M., Abd El-hack, M.E., El-Tarabily, K.A. and Zanaty, A.M. 2023. Common viral and bacterial avian respiratory infections: an updated review. Poult. Sci. 102(5), 102553. Yi, C., Li, G., Mu, Y., Cui, S., Zhang, D., Xu, Q., Liang, C., Wang, M., Zhou, S., Zhou, H., Zhong, M. and Zhang, A. 2024. Isolation, identification, molecular and pathogenicity characteristics of an infectious laryngotracheitis virus from Hubei province, China. Poultry Sci. 103(2), 103271. York, J.J. and Fahey, K.J. 1991. Vaccination with affinity‐purified glycoproteins protects chickens against infectious laryngotracheitis herpesvirus. Avian Pathol. 20(4), 693–704. Zai, X., Shi, B., Shao, H., Qian, K., Ye, J., Yao, Y., Nair, V. and Qin, A. 2022. Recombinant Turkey herpesvirus expressing H9N2 HA gene at the HVT005/006 site induces better protection than that at the HVT029/031 site. Viruses 14(11), 2495. Zeng, Z., He, Y., Wang, Z., Yao, L., Li, L., Shang, Y., Wang, H., Zhang, R., Shao, H., Luo, Q. and Wen, G. 2023. Characterization of a recombinant thermostable Newcastle disease virus (NDV) expressing glycoprotein gB of infectious laryngotracheitis virus (ILTV) protects chickens against ILTV challenge. Viruses 15(2), 500. Zhang, X., Tang, L., Duan, L., Yang, R., Liu, K., Zhao, J., Zhao, Y. and Zhang, G. 2024. Molecular characteristics and pathogenicity analysis of infectious laryngotracheitis virus isolated in China from 2015 to 2019. Poult. Sci. 104(2), 104751. Zhao, Y., Kong, C., Cui, X., Cui, H., Shi, X., Zhang, X., Hu, S., Hao, L. and Wang, Y. 2013. Detection of infectious laryngotracheitis virus by real-time PCR in naturally and experimentally infected chickens. PLoS. One 8(6), e67598. Ziemann, K., Mettenleiter, T.C. and Fuchs, W. 1998. Infectious laryngotracheitis herpesvirus expresses a related pair of unique nuclear proteins which are encoded by split genes located at the right end of the UL genome region. J. Virol. 72(8), 6867–6874. | ||
| How to Cite this Article |
| Pubmed Style Yunita MN, Khairullah AR, Saepulloh M, Agustono B, Marbun TD, Windria S, Burhanuddin A, Moses IB, Khalisa AT, Wardhani BWK, Ahmad RZ, Pratama BP, Fauziah I, Kurniasih DAA, Kusala MKJ, Wibowo S. Infectious laryngotracheitis: A serious threat to poultry health. Open Vet. J.. 2025; 15(9): 3943-3960. doi:10.5455/OVJ.2025.v15.i9.3 Web Style Yunita MN, Khairullah AR, Saepulloh M, Agustono B, Marbun TD, Windria S, Burhanuddin A, Moses IB, Khalisa AT, Wardhani BWK, Ahmad RZ, Pratama BP, Fauziah I, Kurniasih DAA, Kusala MKJ, Wibowo S. Infectious laryngotracheitis: A serious threat to poultry health. https://www.openveterinaryjournal.com/?mno=247301 [Access: January 11, 2026]. doi:10.5455/OVJ.2025.v15.i9.3 AMA (American Medical Association) Style Yunita MN, Khairullah AR, Saepulloh M, Agustono B, Marbun TD, Windria S, Burhanuddin A, Moses IB, Khalisa AT, Wardhani BWK, Ahmad RZ, Pratama BP, Fauziah I, Kurniasih DAA, Kusala MKJ, Wibowo S. Infectious laryngotracheitis: A serious threat to poultry health. Open Vet. J.. 2025; 15(9): 3943-3960. doi:10.5455/OVJ.2025.v15.i9.3 Vancouver/ICMJE Style Yunita MN, Khairullah AR, Saepulloh M, Agustono B, Marbun TD, Windria S, Burhanuddin A, Moses IB, Khalisa AT, Wardhani BWK, Ahmad RZ, Pratama BP, Fauziah I, Kurniasih DAA, Kusala MKJ, Wibowo S. Infectious laryngotracheitis: A serious threat to poultry health. Open Vet. J.. (2025), [cited January 11, 2026]; 15(9): 3943-3960. doi:10.5455/OVJ.2025.v15.i9.3 Harvard Style Yunita, M. N., Khairullah, . A. R., Saepulloh, . M., Agustono, . B., Marbun, . T. D., Windria, . S., Burhanuddin, . A., Moses, . I. B., Khalisa, . A. T., Wardhani, . B. W. K., Ahmad, . R. Z., Pratama, . B. P., Fauziah, . I., Kurniasih, . D. A. A., Kusala, . M. K. J. & Wibowo, . S. (2025) Infectious laryngotracheitis: A serious threat to poultry health. Open Vet. J., 15 (9), 3943-3960. doi:10.5455/OVJ.2025.v15.i9.3 Turabian Style Yunita, Maya Nurwartanti, Aswin Rafif Khairullah, Muharam Saepulloh, Bodhi Agustono, Tabita Dameria Marbun, Sarasati Windria, Azhar Burhanuddin, Ikechukwu Benjamin Moses, Andi Thafida Khalisa, Bantari Wisynu Kusuma Wardhani, Riza Zainuddin Ahmad, Bima Putra Pratama, Ima Fauziah, Dea Anita Ariani Kurniasih, Muhammad Khaliim Jati Kusala, and Syahputra Wibowo. 2025. Infectious laryngotracheitis: A serious threat to poultry health. Open Veterinary Journal, 15 (9), 3943-3960. doi:10.5455/OVJ.2025.v15.i9.3 Chicago Style Yunita, Maya Nurwartanti, Aswin Rafif Khairullah, Muharam Saepulloh, Bodhi Agustono, Tabita Dameria Marbun, Sarasati Windria, Azhar Burhanuddin, Ikechukwu Benjamin Moses, Andi Thafida Khalisa, Bantari Wisynu Kusuma Wardhani, Riza Zainuddin Ahmad, Bima Putra Pratama, Ima Fauziah, Dea Anita Ariani Kurniasih, Muhammad Khaliim Jati Kusala, and Syahputra Wibowo. "Infectious laryngotracheitis: A serious threat to poultry health." Open Veterinary Journal 15 (2025), 3943-3960. doi:10.5455/OVJ.2025.v15.i9.3 MLA (The Modern Language Association) Style Yunita, Maya Nurwartanti, Aswin Rafif Khairullah, Muharam Saepulloh, Bodhi Agustono, Tabita Dameria Marbun, Sarasati Windria, Azhar Burhanuddin, Ikechukwu Benjamin Moses, Andi Thafida Khalisa, Bantari Wisynu Kusuma Wardhani, Riza Zainuddin Ahmad, Bima Putra Pratama, Ima Fauziah, Dea Anita Ariani Kurniasih, Muhammad Khaliim Jati Kusala, and Syahputra Wibowo. "Infectious laryngotracheitis: A serious threat to poultry health." Open Veterinary Journal 15.9 (2025), 3943-3960. Print. doi:10.5455/OVJ.2025.v15.i9.3 APA (American Psychological Association) Style Yunita, M. N., Khairullah, . A. R., Saepulloh, . M., Agustono, . B., Marbun, . T. D., Windria, . S., Burhanuddin, . A., Moses, . I. B., Khalisa, . A. T., Wardhani, . B. W. K., Ahmad, . R. Z., Pratama, . B. P., Fauziah, . I., Kurniasih, . D. A. A., Kusala, . M. K. J. & Wibowo, . S. (2025) Infectious laryngotracheitis: A serious threat to poultry health. Open Veterinary Journal, 15 (9), 3943-3960. doi:10.5455/OVJ.2025.v15.i9.3 |