| Review Article | ||
Open Vet. J.. 2025; 15(9): 3961-3979 Open Veterinary Journal, (2025), Vol. 15(9): 3961-3979 Review Article Potential of growth factors and steroid hormones from cell cultures to improve in vitro fertilization in bovineSri Mulyati1*, Fedik Abdul Rantam2, Aswin Rafif Khairullah3, Imam Mustofa1, Adeyinka Oye Akintunde4, Riza Zainuddin Ahmad3, Ulvi Fitri Handayani5, Andi Thafida Khalisa6, Lili Anggraini5, Bima Putra Pratama7, Latifah Latifah5, Bantari Wisynu Kusuma Wardhani8, Dea Anita Ariani Kurniasih9 and Syahputra Wibowo101Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 4Department of Agriculture and Industrial Technology, Babcock University, Ikenne, Nigeria 5Research Center for Animal Husbandry, National Research and Innovation Agency (BRIN), Bogor, Indonesia 6Faculty of Military Pharmacy, Universitas Pertahanan, Bogor, Indonesia 7Research Center for Agroindustry, National Research and Innovation Agency (BRIN), Tangerang, Indonesia 8Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 9Research Center for Public Health and Nutrition, National Research and Innovation Agency (BRIN), Bogor, Indonesia 10Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Bogor, Indonesia *Corresponding Author: Sri Mulyati. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: sri-m [at] fkh.unair.ac.id Submitted: 29/03/2025 Revised: 25/07/2025 Accepted: 17/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
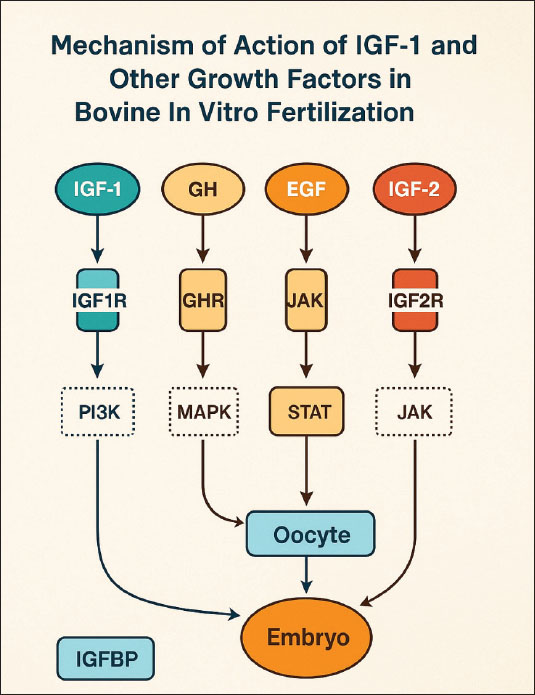
ABSTRACTThe application of biotechnology attempts to improve the efficiency of animal reproduction, specifically to produce cattle of high quality and quantity. One successful and efficient method in the field of reproduction is embryo transfer. The capacity of donor females to produce embryos limits the number of embryos that can be produced in vivo. In this instance, an alternate source of embryos is in vitro embryo production. Two mitogenic chemicals, estrogen and insulin-like growth factor-I (IGF-I), can be employed to promote and accelerate cell proliferation. The liver and other tissues release the polypeptide growth factor IGF-I, also known as somatomedin, in response to growth hormone stimulation. The molecular weight of the 70 amino acids that make up IGF-I is 7649 Da or 7.65 kDa. Estrogen, specifically estradiol, is a steroid hormone produced by developing follicles in the ovary, corpora lutea, and placenta. IGF-I and estrogen promote mitogenic activity in proliferating cells in paracrine, autocrine, and endocrine ways. IGF-I and estrogen can be produced by the liver and ovaries. Both the ovary and the liver are the primary producers of the sex steroid estrogen and IGF-I, respectively, and both may be produced using monolayer cell culture by supplementing them with either bovine serum albumin or fetal calf serum as precursor substances. IGF-I and the steroid sex hormones progesterone and estrogen in liver and cumulus cell monolayer culture may be used as a culture medium for embryo development. Keywords: Bovine IVF, Embryo development, IGF-1, Sex steroids, Cumulus cells. IntroductionThe main initiative of the Indonesian government, free nutritious meals, is scheduled to run from 2024 to 2029. The objectives of this program are to lower stunting rates, prevent malnutrition, and enhance the nutritional intake of schoolchildren (Sarjito, 2024). However, the absence of indigenous milk and beef production is the primary obstacle to the effectiveness of the free healthy meal program. Indonesia produces far fewer milk than is needed. The country will require 8.7 million tons of milk by 2024, but only 0.9 million tons will be produced (Marantha et al., 2024). This is because small-scale farmers in Indonesia who employ traditional farming practices hold most dairy cows. The application of biotechnology is one attempt to improve livestock reproduction efficiency (Gupta and Savaliya, 2012). In the realm of reproduction, embryo transfer is an effective technique for obtaining sufficient numbers of high-quality animals (Erdem et al., 2020). However, the caliber of the embryos created determines how well this technique works. The ability of donor females to create embryos limits the creation of embryos in vivo; therefore, in vitro embryo development is an option (Gualtieri et al., 2024). The process of in vitro fertilization, which involves bringing oocytes and spermatozoa together outside the mother’s body, is the focus of modern reproductive technology. The steps in this process include oocyte collection, oocyte maturation, spermatozoa collection, spermatozoa capacitation, and spermatozoa penetration into the oocyte zona pellucida until zygote development (Anifandis et al., 2014). Numerous studies have also been conducted on the addition of growth factors, such as insulin-like growth factor 1 (IGF-1), to oocyte maturation media and embryo culture (Arias et al., 2022; Gao-Ping et al., 2023; Kim et al., 2023). IGF-1 has a synergistic effect on the steroidogenesis process, oocyte maturation, and embryo development in vitro. When added to embryo culture conditions, it can also boost cumulus cell growth, oocyte nuclear maturation, and embryo cleavage rate, as well as accelerate the meiotic and mitotic processes (Fernandez-Gonzalez et al., 2021). IGF-1 can save embryos from dying in various species, including cattle (Carrillo-Gonzalez et al., 2024). The reproductive process and health of granulosa cells are also significantly supported by the hormones progesterone and estrogen, which are mostly produced by the ovaries (Skinner et al., 2008). Hepatocytes are stable cells with a considerable regeneration ability and a comparatively lengthy lifespan (Kholodenko and Yarygin, 2017). Tumor Necrosis Factor-α influences hepatocyte regeneration following partial hepatectomy (Michalopoulos, 2010). The growing ovarian follicle gives rise to the cumulus oophorus, a structural and functional unit that generates steroid hormones, IGF-1, and other substances (Turathum et al., 2021). The ovaries generate the steroid hormone estrogen, which is involved in the growth and maintenance of the structure of female reproductive organs (Xu et al., 2022). There have never been any studies that concentrate on hepatocyte and cumulus cell cultures capable of producing IGF-1 and steroid hormones (progesterone and estrogen). It is hoped that hepatocyte cultures and oophorus cumulus tissue, which can be obtained from slaughterhouses, will eventually be used to promote in vitro fertilization in cattle by producing ovarian steroid hormones and IGF-1 as growth materials. To meet demand and help children’s nutrition programs succeed, it is hoped that milk and beef production will increase domestically and employ suitable technology (Britt et al., 2018). Achieving these objectives may depend on biotechnology innovation in the livestock industry, specifically in the form of more effective reproductive techniques. The purpose of this review article is to increase understanding and offer a theoretical foundation for the utilization of hepatocyte and ovarian tissue from slaughterhouse cattle to create compounds that may be used to enhance the outcomes of in vitro conception in cattle. Mechanism of action of insulin-like growth factor-1 and other growth factorsIGF-1 is a peptide hormone that regulates the growth, differentiation, and metabolism of reproductive cells in mammals, including cattle (Al-Samerria and Radovick, 2021). In the context of bovine in vitro fertilization (IVF), IGF-1 mediates its effects by binding to the IGF1 receptor, a transmembrane receptor with tyrosine kinase activity (Werner, 2023). This interaction induces autophosphorylation of tyrosine residues in the cytoplasmic domain of IGF1R, which then activates key signal transduction pathways, namely phosphatidylinositol 3-kinase (PI3K)-Akt and mitogen-activated protein kinase (MAPK)/ERK (Voudouri et al., 2015). The PI3K-Akt pathway plays a role in increasing protein synthesis, energy metabolism, and preventing apoptosis in oocytes and early embryos, whereas the MAPK/ERK pathway regulates oocyte proliferation, differentiation, and maturation (Kalous et al., 2023). Activation of these two pathways significantly contributes to improved oocyte quality, increased fertilization rates, and improved embryo development in bovine IVF systems. The biological activity of IGF-1 in in vitro culture is also influenced by insulin-like growth factor binding proteins (IGFBPs), particularly IGFBP-3, which binds IGF-1 in in vitro culture, extending its half-life and regulating its bioavailability to target cells (Kim et al., 2022). Specific proteases mediate the release of IGF-1 from the IGFBP complex, ensuring the availability of this hormone during critical phases such as in vitro maturation and in vitro culture (Sato et al., 2017). This regulation is crucial to prevent excessive proliferation stimulation or undirected responses, which could reduce embryo quality. Figure 1 shows the mechanism of action of IGF-1 and other factors in bovine IVF.
Fig. 1. Mechanism of action of IGF-1 and other factors in bovine IVF. In addition to IGF-1, several other growth factors also play a synergistic role in supporting the success of bovine IVF. Although IGF-2 is more dominant in early embryonic development, it can interact with IGF1R and the IR to stimulate cell growth (Lewitt and Boyd, 2019). growth hormone (GH) acts both directly through the GHR by activating the JAK/STAT pathway and indirectly by increasing IGF-1 synthesis in somatic cells in culture media (Dehkhoda et al., 2018). Epidermal growth factor (EGF) activates the MAPK/ERK pathway through the epidermal growth factor receptor, which plays a role in cumulus expansion, oocyte nuclear maturation, and increasing embryonic developmental competence (Turathum et al., 2021). The combination of IGF-1, GH, and EGF stimulation can produce a synergistic effect that improves the efficiency of oocyte maturation and fertilization. Liver cell culture as an IGF-1 sourceIGF-1 is a polypeptide with a structure that resembles insulin (Bailes and Soloviev, 2021). As a modulator of the actions of growth hormones, IGF-1 plays a crucial role in the regulation of metabolism, stimulation of protein synthesis, and cell growth and development (Al-Samerria and Radovick, 2021). Additionally, IGF-1 promotes the development of bones, muscles, and organs (Ahmad et al., 2020). The complex process of IGF-1 synthesis in liver cells includes transcription, translation, PTM, and growth hormone activation (Poreba and Durzynska, 2020). The primary regulator of IGF-1 synthesis in hepatocytes, or liver cells, is GH, which is generated by the anterior pituitary gland (Al-Samerria and Radovick, 2021). Hormone-releasing hormones such as growth hormone-releasing hormone (GHRH) stimulate GH release, whereas somatostatin inhibits it (Pech-Pool et al., 2020). Once it has reached the liver cells, GH attaches itself to GH receptors on the hepatocyte membrane. As a result of this interaction, signal transduction pathways, including the proteins Signal Transducer and Activator of Transcription 5 (STAT5) and JAK2 (Janus kinase 2), are activated (Furth et al., 2011). STAT5 phosphorylation results from JAK2 activation. Following phosphorylation, STAT5 moves into the cell nucleus and attaches itself to a response element in the IGF-1 gene promoter (Khan et al., 2025). This process increases the transcription of the IGF-1 gene. Chromosome 19 contains the bovine IGF-1 gene (Werner, 2023). Although hepatocyte cultures may serve as a potential source of IGF-1, prior purification, standardization, and bioactivity validation are required to ensure its effectiveness and safety. Further studies are required to evaluate the feasibility of this approach. A transcription complex comprising RNA polymerase II and other transcription factors is formed at the start of the transcription process (Liu et al., 2013). The produced transcript is called IGF-1 messenger RNA (mRNA) (Kasprzak and Szaflarski, 2020). Intron splicing and the attachment of a 5’ cap and poly-A tail at the 3’ end are among the processing steps that the freshly generated mRNA will undergo (Huang et al., 2023). The stability of mRNA and its subsequent translation are dependent on this. Following processing, IGF-1 mRNA makes its way to the ribosome, where protein synthesis occurs (Iresjö et al., 2022). Ribosomes use amino acids carried by tRNA to convert the mRNA sequence into an IGF-1 polypeptide chain (Bastide and David, 2018). Following synthesis, the IGF-1 polypeptide undergoes post-translational modification, which results in the production of the active form of IGF-1 through protein cleavage (Bikle et al., 2015). Furthermore, transport proteins such as IGFBPs frequently bind to IGF-1, modifying its biological function and enhancing its stability in the bloodstream (LeRoith et al., 2021). IGF-1 production increases in nutrient-rich environments, particularly those high in glucose and amino acids, and decreases in nutrient-deficient environments (Caputo et al., 2021). Cattle undergoing IVF benefit greatly from the production and release of IGF-1, which is largely dependent on the liver (Velazquez et al., 2008). Studies using hepatic cell cultures, which are generated from liver tissue, have shown that IGF-1 is produced. These growth factors are secreted into the culture environment after being expressed by hepatic cells (Kang et al., 2012). IGF-1 generated from liver cell cultures can be introduced to oocyte and embryo culture media to assist several crucial processes in fertilization and embryo development (Carrillo-Gonzalez et al., 2024). IGF-1 aids in the growth and maturation of oocytes (Yang et al., 2019). IGF-1 can enhance the quality and pace of oocyte maturation when it is introduced to the culture medium of cow’s oocytes (Barrera et al., 2023). The success of IVF is increased when mature, high-quality oocytes have a higher chance of fertilizing (Fanton et al., 2023). IGF-1 can aid in the growth of embryos after fertilization (Mehtaet al., 2013). One of the primary roles of IGF-1 is to promote cell division and embryo growth, which raises the likelihood that an embryo will survive the culture process (Lin et al., 2003). When IGF-1 is present in the culture media, it is more likely that embryos will develop properly and be prepared for transfer into a surrogate cow’s uterus (Annes et al., 2023). Additionally, IGF-1 contributes to the development of the ideal culture conditions for oocytes and embryos (Fernandez-Gonzalez et al., 2021). Cultured liver cells can contribute extra elements that promote cellular development and proliferation, aid in the creation of superior culture media, and boost the effectiveness of the IVF procedure (Hu and Li, 2015). Other growth factors, including growth hormone and reproductive hormones, work in concert with IGF-1 (Blum et al., 2018). A sufficient amount of IGF-1 in culture can amplify the beneficial effects of other hormones, thereby improving embryonic cell division and fertilization (Tang et al., 2025). The liver and other organs release the polypeptide growth factor IGF-1, or somatomedin, in response to growth hormone stimulation. IGF-1 is made up of 70 amino acids and has a molecular weight of 7649 Daltons or 7.65 kDa (Laron, 2001). Its core structure is 60% similar to that of proinsulin. It also has domains A, B, and C (Macháčková et al., 2017). IGF-1 is a polypeptide structurally similar to proinsulin, sharing homologous A and B chains connected by a C peptide, but differing in specific domain arrangements (Poreba and Durzynska, 2020). IGF-1 is present in the circulation for less than 10 minutes, 10 minutes when it is bound to IGFBP-1 and IGFBP-2, and more than 6 hours when it is bound to IGFBP-3 (Rajpathak et al., 2009). The IGF-1 and IGFBP-3 protein complex functions endocrinely because of IGF-1’s high molecular weight, which allows it to enter the blood circulation system (Martín et al., 2021). IGF-1 in follicle cells has an endocrine function that involves stimulating type 1 receptor cells in theca and GCs (Hayes et al., 2024). The presence of estrogen mediated by IGF-1 through the IGF-1 receptor (IGF-1R) increases the expression of these receptors in GCs (Ogo et al., 2014). Integrins control IGF-1R to enable it to respond to ligands, and a connection between IGF-1R and ligand-bound Estrogen receptor is necessary for IGF-1 and estrogen to cross-talk (McDermott et al., 2025). In cattle, small antral follicles exhibit more IGF-1 receptors (Echternkamp et al., 2012). Gallelli et al. (2020) reported that the IGF-1 receptor concentration in developing follicles with a diameter of 0.8–8.0 mm does not change in llamas. The endocrine hormone IGF-1 is mostly produced in the liver and other organs, and it acts on target tissues in a paracrine/autocrine fashion (Talia et al., 2021). The hypothalamic-anterior pituitary axis controls IGF-1 secretion by stimulating GH, which is controlled by GHRH (Al-Samerria and Radovick, 2023). IGF-1 influences almost every cell in the human body, but it is particularly important for muscle, skin, bone, kidneys, nerves, cartilage, and lungs (Hellström et al., 2016). Furthermore, IGF-1 controls DNA synthesis in cells as well as cell growth and development, particularly in nerve cells (Talia et al., 2021). The hypothalamus and pituitary receive negative feedback if the IGF-1 concentration is high or near its peak, which prevents the liver from being stimulated by GH (Ma and Stanley, 2023). For the development and upkeep of target tissues, including bones, muscles, the neurological system, and the immunological system, GHRH indirectly has a beneficial impact (Martínez-Moreno et al., 2018). Figure 2 shows the IGF-1-GH flow diagram with the hypothalamic axis.
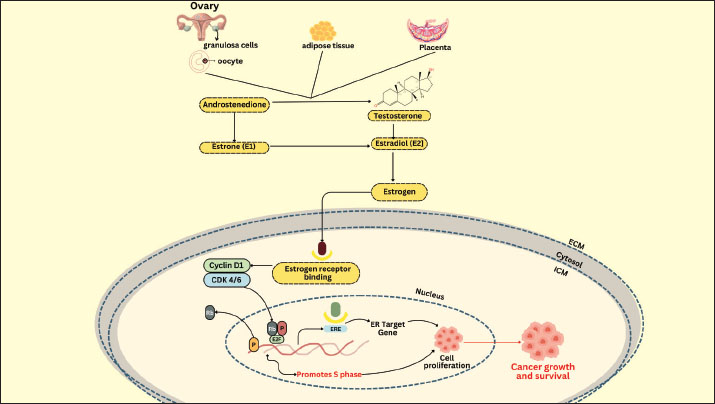
Fig. 2. Schematic of the relationship between IGF-1 and GH and the hypothalamic axis. The presence of estrogen and gonadotropins in cows enhances the role of IGF-1 in ovarian follicle cells in stimulating receptor cells in GCs (Baumgarten et al., 2017). IGF-1 receptor expression is elevated in tiny antral follicles. Furthermore, IGF-1 also contributes to the stimulation of granulosa cell proliferation and differentiation depending on the stage of follicle development (Zhu et al., 2022). It promotes the proliferation of granulosa cells from small follicles (diameter 1–3 mm) and stimulates the secretion of progesterone by granulosa cells from large follicle cells (diameter > 5 mm), but not in small follicles (Monniaux et al., 1994). IGF-1 has both autocrine and paracrine functions, mechanistically promoting mitogenic activity in proliferating tissue, such as the development of the corpus luteum from ovarian follicles (Zhou et al., 2021). IGF-1 stimulates the release of reproductive hormones and inhibits IGFBP activity, causing the ovaries to release more steroids. This shows that IGF-I can affect the hormone production of gonadotropin-dependent dominant follicles (Ipsa et al., 2019). According to Hull and Harvey (2014), IGF-1 promotes GC differentiation and proliferation in humans, pigs, rats, and sheep. It also promotes steroidogenesis in theca cells. Meanwhile, Lin et al. (2003) used stem cell line culture and evidence to show that IGF-1/IGFBP-1 boosted the number and development of blastocysts. Barrera et al. (2023) noted that IGF-1 and insulin can both inhibit apoptosis and accelerate blastocyst growth. Based on previous studies, it has been proposed that IGF-1 and sex steroids produced in liver and cumulus cell cultures play a role in supporting oocyte maturation and embryo development; however, their direct effect on IVF success remains a theoretical concept that requires further experimental validation (Velazquez et al., 2008; Silva et al., 2009; Li et al., 2014; Zhao et al., 2020; Turathum et al., 2021; Yang et al., 2022). According to Drummond (2006), steroids and growth factors are crucial in controlling ovarian follicle development. The first two distinctions are seen in cattle, where robust dominant follicles are better able to produce insulin-like growth factor and estradiol than subordinate follicles, which subsequently suffer from atresia (Fortune et al., 2001). IGF-1 and estradiol increase the proliferation of granulosa cells in vitro and maintain their survival by enhancing apoptosis resistance (Mani et al., 2010). Furthermore, IGF-1 and estradiol can strengthen the cell development cycle by increasing apoptosis resistance (Kasprzak and Szaflarski, 2020). According to Velazquez et al. (2009), IGF-1 is also crucial for follicle development, oocyte maturation, embryo viability, and apoptosis reduction in bovine embryos. According to Makarevich and Markkula (2002), IGF-1 is a mitogenic and anti-apoptotic chemical that can promote cell proliferation in oocyte maturation, production, and in vitro embryo culture. Liver cell culture techniques for IGF-1 productionHepatocytes predominantly synthesize liver-derived IGF-1 under the regulation of GH, making hepatocyte cultures a promising model for studying and potentially harvesting IGF-1 in vitro. Several key methodological considerations must be addressed to ensure hepatocyte viability, functionality, and sustained IGF-1 secretion. Hepatocyte isolation and culturePrimary hepatocytes can be isolated from rodent, bovine, or human liver tissue using a two-step collagenase perfusion technique (Green et al., 2017). This method preserves cell membrane integrity and maximizes post-isolation viability. Hepatocytes are typically seeded in monolayer cultures on collagen-coated surfaces once isolated, which mimic the native extracellular matrix and support attachment and polarity (LeCluyse et al., 2012). While monolayer systems are widely used due to their simplicity and accessibility, they often suffer from rapid dedifferentiation, which impairs IGF-1 synthesis (LeRoith et al., 2021). Supplementation with hormones (e.g., dexamethasone), growth factors (e.g., epidermal growth factor), and physiological concentrations of insulin is recommended to maintain hepatocyte-specific gene expression and metabolic function. Culture media and dietary supplementsThe choice of culture medium significantly influences IGF-1 production. Williams’ E medium and Dulbecco’s Modified Eagle Medium supplemented with fetal bovine serum, insulin, transferrin, selenium, and glucocorticoids (e.g., hydrocortisone or dexamethasone) are commonly used. Glucocorticoids enhance hepatocyte differentiation and stimulate GH receptor expression, which indirectly promotes IGF-1 synthesis (Kisiday et al., 2005). Serum-free systems are increasingly favored for controlled IGF-1 quantification and downstream applications. In such systems, defined supplements must be optimized to balance cell survival with functional activity (Miescher et al., 2023). Dynamics of IGF-1 secretionIn vitro IGF-1 secretion by hepatocytes is time- and density-dependent. Peak levels are usually observed within the first 24–48 hours post-seeding and gradually decline due to cell stress and dedifferentiation (Yakar et al., 2002). Therefore, IGF-1 production is often transient unless culture conditions are optimized to extend the hepatic phenotype. Enzyme-linked immunosorbent assay and qPCR targeting IGF1 mRNA are routinely employed to assess IGF-1 production (Wacharasindhu et al., 2002). These methods also help monitor the effect of experimental treatments (e.g., GH supplementation) on IGF-1 expression. Comparative analysis of IGF-1 production methodsSeveral methods have been explored for IGF-1 production to support its application in biomedical and reproductive biotechnology, including bovine IVF. Recombinant protein expression and liver-derived IGF-1 via hepatocyte culture are the two primary approaches. Recombinant IGF-1 is commonly produced using Escherichia coli or mammalian expression systems, offering high yield, scalability, and consistency (Venkatesan et al., 2022). Its advantages include ease of production, well-defined purification protocols, and standardized bioactivity, making it suitable for use in commercial embryo culture media. However, recombinant IGF-1, particularly from prokaryotic systems, often lacks post-translational modifications such as glycosylation and may require complex protein refolding procedures to restore biological activity (Iranpoor et al., 2015). In contrast, IGF-1 derived from primary hepatocyte cultures more closely mimics endogenous production, complete with physiologically relevant modifications and co-secretion of IGFBPs, which regulate IGF-1 bioavailability and stability (Sandhu et al., 2002). However, this method suffers from low yield, batch variability, and rapid dedifferentiation of hepatocytes in monolayer culture, limiting its utility for large-scale or long-term applications. Hepatocyte culture requires specialized techniques and maintenance conditions, making it less accessible for routine use (de Hoyos-Vega et al., 2021). While recombinant IGF-1 is widely used and validated in bovine embryo culture, liver-derived IGF-1 offers a model for studying hormonal regulation and metabolic interactions in a more physiological context (Carrillo-Gonzalez et al., 2024). Future research may benefit from integrating these approaches, such as using recombinant IGF-1, while leveraging liver-derived systems to explore regulatory mechanisms and species-specific responses in reproductive protocols. IGF-1 applications and trendsIGF-1 is a multifunctional polypeptide hormone with pivotal roles in cellular proliferation, differentiation, survival, and metabolism across diverse tissues. Although its role in reproductive biology—particularly in enhancing oocyte maturation and embryo development—has been extensively studied, IGF-1 has far broader physiological and clinical significance (Toori et al., 2014). IGF-1 is essential for normal postnatal growth in somatic tissues, acting as a mediator of GH effects and influencing bone elongation, skeletal muscle hypertrophy, and cartilage development (Racine and Serrat, 2020). In metabolic regulation, IGF-1 contributes to glucose homeostasis and lipid metabolism, showing insulin-like effects on peripheral tissues, which has led to growing interest in its role in insulin resistance and T2DM (Rajpathak et al., 2009). In regenerative medicine, IGF-1 has emerged as a potent factor in promoting tissue repair and regeneration, especially in muscle injury, neurogenesis, and cardiac remodeling after Meiosis I (Macvanin et al., 2023). IGF-1 enhances stem cell proliferation and survival and facilitates tissue integration, making it a valuable adjunct in cell-based therapies (Huat et al., 2014). Clinically, recombinant human IGF-1 has been approved for the treatment of growth failure in children with severe primary IGF-1 deficiency, and its therapeutic potential in age-related muscle wasting (sarcopenia), neurodegenerative diseases, and wound healing is being explored (Puche and Castilla-Cortázar, 2012). Recent trends in IGF-1 research have also focused on its interactions with IGFBPs, the development of long-acting IGF-1 analogs, and the use of targeted delivery systems, such as nanoparticles, to improve tissue specificity and reduce systemic side effects (Kotsifaki et al., 2024). The epigenetic regulation of IGF-1 gene expression and its crosstalk with other signaling pathways (e.g., mTOR, PI3K-AKT, and MAPK) are receiving increasing attention as researchers seek to harness the pleiotropic effects of IGF-1 in both physiological and pathological contexts (Yoshida and Delafontaine, 2020). Thus, while IGF-1 remains a molecule of interest in assisted reproductive technologies, its expanding role in therapeutic interventions highlights its growing importance in translational biomedical research. Cumulus oophorus culture as an estrogen and progesterone sourceCumulus oophorus cells play a crucial role in both oocyte formation and ovulation (Turathum et al., 2021). These cells play a particular function in the synthesis of IGF-1, a vital growth factor for effective fertilization in cattle (Tanghe et al., 2003). The cumulus oophorus cells of the ovarian follicle encircle the developing oocyte and nourish and sustain it as it matures (Turathum et al., 2021). They are essential for ovulation and aid in the oocyte’s communication with its surroundings, including the availability of growth hormones like IGF-1 (Del Bianco et al., 2024). IGF-1 is produced by Cumulus oophorus cells and released into the culture medium (Sirotkin et al., 1998). This implies that the cumulus cells left in culture after oocytes are removed for IVF can still aid in IGF-1 synthesis. IGF-1 production by cumulus cells has a major impact on the development of embryos and the quality of oocytes (Sato et al., 2017). Cumulus oophorus cells produce insulin-like growth factor-1, which is involved in the development of oocytes (Turathum et al., 2021). The presence of IGF-1 enhances the quality and maturation rate of oocytes recovered from IVF culture. Mature oocytes of superior quality are necessary for both effective fertilization and embryo growth (Yu et al., 2015). Communication between the oocyte and cumulus oophorus cells is crucial for promoting healthy oocyte development (Martinez et al., 2023). This process can help in vitro fertilization succeed while also increasing IGF-1 production by cumulus cells. The cumulus oophorus cells of the ovarian follicles, which encircle the oocytes, are crucial to the IVF process in cattle because they produce the hormones progesterone and estrogen (Ledwaba et al., 2025). Cumulus oophorus cells may help the follicle produce estrogen by converting androstenedione into estrogens, such as estradiol (Emori et al., 2013). Together with the granulosa cells that also envelop the oocyte, these cells contribute to the manufacturing of estrogen and aid in the formation of follicles and the maturation of oocytes (Turathum et al., 2021). The interaction between cumulus and granulosus cells regulates reproductive hormone synthesis. The release of estrogen by cumulus cells aids in inducing the corpus luteum to produce progesterone following fertilization (Stocco et al., 2007). The cumulus oophorus, which is the cells that encircle the oocyte in the ovarian follicle, is essential for the growth of the follicle and ovulation (Emori and Sugiura, 2014). The female reproductive cycle is heavily dependent on the sex hormone estrogen, particularly estradiol (Farkas et al., 2022). The steroid hormone estrogen is derived from cholesterol (Holst et al., 2004). Estradiol (E2) is the most physiologically active type of estrogen, which is crucial for controlling reproductive processes (Yoh et al., 2023). The hormone estrogen helps females develop secondary sexual traits, prepares the endometrium for implantation, and controls the menstrual cycle (Yu et al., 2022). Granulosa cells affixed to the oocyte make up the cumulus oophorus. During follicle development, these cells serve to support and provide the oocyte with the best possible conditions (Khamsi and Roberge, 2001). The cumulus oophorus also contributes to the synthesis of estrogen and other hormones (Sugiura et al., 2010). The cumulus oophorus undergoes multiple estrogen production steps that involve hormones, enzymes, and cell-to-cell interactions. The anterior pituitary gland secretes follicle-stimulating hormone, which promotes the growth and function of granulosa cells during the follicular phase of the menstrual cycle (Park et al., 2022). Estradiol and other estrogen concentrations increase in response to FSH stimulation (Laven and Fauser, 2006). The mechanism of estrogen synthesis is explained in Figure 3. A signaling cascade involving Rho GTPase and N-cadherin protein is activated following FSH stimulation. This leads to a rise in the expression of genes that show the manufacture of important enzymes in the estrogen biosynthesis pathway, including aromatase (CYP19A1) (Lundqvist et al., 2013).
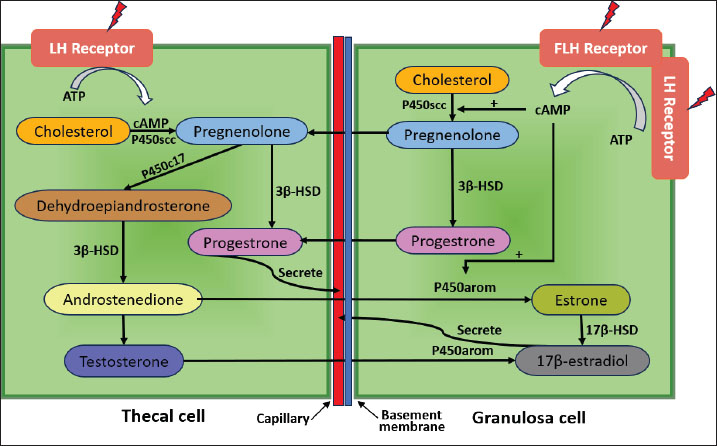
Fig. 3. E2 and ER signaling pathway mediated. The cholesterol-binding protein (StAR; Steroidogenic Acute Regulatory protein) transports cholesterol, the primary precursor for the synthesis of steroid hormones, into the mitochondria (Christenson and Strauss, 2000). Low-density lipoprotein (LDL) and very low-density lipoprotein attached to the cell membrane provide cholesterol for granulosa cells to absorb (Zhang et al., 2023). The cytochrome P450scc (CYP11A1) enzyme transforms cholesterol into pregnenolone in the mitochondria (McCarty et al., 2024). Pregnenolone, a steroid, serves as the starting point for estrogen production (Manna et al., 2016). Enzymes such as 17α-hydroxylase (CYP17A1) and 3β-hydroxysteroid dehydrogenase (3β-HSD) aid in the conversion of pregnenolone to progesterone and then to androstenedione (Miller and Auchus, 2011). The enzyme aromatase is essential for estrogen production because it transforms androgens, such as testosterone and androstenedione, into estrogen (Chan et al., 2016). Granulosa cells in the cumulus oophorus use aromatase to convert androstenedione into estradiol (Liu et al., 2021). Many variables strictly control the estrogen production in cumulus oophorus. FSH promotes GC proliferation and estrogen synthesis (Wu et al., 2022). Luteinizing hormone (LH) contributes to the transition of granulosa cells into the corpus luteum, which generates estrogen, following ovulation (Przygrodzka et al., 2021). Hepatocytes produce insulin-like growth factor-1, which promotes granulosa cell development and activity and increases estrogen production (Kineman et al., 2018). The interplay of ovarian stromal cells, granulosa cells, and oocytes influences the regulation of estrogen synthesis (Papageorgiou et al., 2021). Local follicle factors such as cytokines can also occasionally influence hormone synthesis (Guzeloglu-Kayisli et al., 2009). Successful fertilization requires proper oocyte maturation, which is facilitated by estrogen released by cumulus cells (Turathum et al., 2021). Increases the likelihood that mature oocytes will successfully fertilize and grow into healthy embryos. Estrogen and other hormones generated by cumulus cells aid in the growth of embryos during culture, improving the quality and chance of survival of the embryos that will be placed in the uterus of the surrogate heifer (Fatehi et al., 2005). The capacity of cumulus cells to generate estrogen and aid in oocyte maturation can be influenced by ideal growing conditions (Lucidi et al., 2003). Essential hormone production can be increased by making changes to the culture medium, such as adding growth factors or keeping an eye on the nutritional conditions. Although cumulus cells have been shown to produce small amounts of progesterone in vitro, their contribution is minimal compared with that of the corpus luteum (Salehnia and Zavareh, 2013). Therefore, their potential as an alternative progesterone source remains theoretical and would likely require substantial cell mass to achieve physiologically relevant levels. Granulosa cells that make up the cumulus oophorus encircle the oocyte in the ovarian follicle (Kulus et al., 2020). In addition to providing oocyte nourishment, these cells aid in the manufacture of several hormones, such as progesterone and estrogen (Cavalcanti et al., 2023). Granulosa cells in the cumulus oophorus are stimulated to produce progesterone by the release of LH by the anterior pituitary gland (Fig. 4). The corpus luteum, the main location of progesterone synthesis, develops from the cellular structure inside the follicle because of the increase in LH levels (Christenson and Devoto, 2003).
Fig. 4. Interaction of theca and granulosa cells in estrogen synthesis. Cholesterol is the primary building block for the production of progesterone and all other steroid hormones (Hu et al., 2010). The cholesterol transporter protein (StAR; Steroidogenic Acute Regulatory protein) helps move cholesterol into the mitochondria in GCs (Miller, 2007). This procedure is a crucial regulatory stage in steroid hormone production. Cholesterol is metabolized to pregnenolone in the mitochondria by the enzyme cytochrome P450scc (CYP11A1) (Mast et al., 2011). The crucial chemical pregnenolone serves as the starting point for progesterone synthesis (Liang and Rasmusson, 2018). Subsequently, pregnenolone undergoes a number of transformations, including conversion via the enzyme 3β-HSD (Slominski et al., 2013). Hormones and growth factors, such as LH and IGF-1, can control the activity of enzymes like 3β-HSD that are part of the progesterone production pathway (Hayes et al., 2024). Environmental factors such as growth factors, hormone concentrations, and culture media can influence progesterone synthesis in cumulus oophorus cultures (Lucidi et al., 2003). Generally, progesterone synthesis is significantly increased in cumulus oophorus cultures treated with LH and IGF-1 (Turathum et al., 2021). To establish conditions that promote hormone synthesis activity, culture media must be properly chosen. Serum IGF-1 and LH can boost progesterone production (Fig. 5).
Fig. 5. Progesterone synthesis requires cholesterol from LDL and high-density lipoprotein. Although cumulus oophorus cells possess steroidogenic capacity and can produce progesterone in vitro, their practical application as an alternative progesterone source is restricted by several biological and technical limitations. First, the number of cumulus cells retrieved from each cumulus–oocyte complex is inherently limited, which limits the total hormone yield. Since these cells are primary somatic cells, their lifespan in culture is short, and their steroidogenic activity declines rapidly after isolation, resulting in a brief window for optimal hormone production (Turathum et al., 2021). Second, progesterone synthesis in cumulus cells exhibits substantial variability and is influenced by donor-specific factors such as age, reproductive status, follicular stage, and prior hormonal stimulation (Pan et al., 2024). This biological variability can compromise reproducibility across experiments or ART cycles. Moreover, in vitro luteinization—while enhancing progesterone secretion—often alters the estrogen-to-progesterone ratio, potentially creating a hormonal profile that is not ideal for certain stages of oocyte maturation or embryo development (Mesen and Young, 2015). Third, progesterone output from cumulus oophorus cells is highly sensitive to culture parameters, including medium composition, gonadotropin or growth factor supplementation, oxygen tension, and temperature stability (Simon et al., 2020). Even minor deviations from optimal conditions can reduce steroidogenic activity. Additionally, because their natural role is to support the oocyte rather than act as a primary endocrine source, the quantity of progesterone they produce in vitro is considerably lower than that of the corpus luteum, making it challenging to achieve physiologically relevant concentrations without substantial cell mass (da Silva Rosa et al., 2024). In vitro embryonic developmentIn culture media such as Tissue Culture Medium-199, Potassium Simplex Optimized Medium, Ham’s F-10, human tubal fluid medium, and others that contain protein, energy sources, and buffers, zygotes generated from in vitro fertilization can grow and divide into 2, 4, 8, and 16 cells to form morula and blastocysts (Gardner et al., 2000). Furthermore, in order for cells to grow and divide adequately, embryo culture must be conducted in an environment with 5% CO2. The in vitro embryo culture technique significantly impacts the success of additional embryo development and the implantation procedure in the recipient’s body (Hemkemeyer et al., 2014). Amino acids can influence the rate of development, boost the number of blastocyst cells, and raise maternal mRNA levels in oocyte maturation medium (Xiong et al., 2024). The addition of glutamate and non-essential amino acids to culture media can greatly accelerate development to the 8–16 cell and blastocyst stages (Itami et al., 2024). However, it cannot promote embryonic development if glutamine alone is administered. This further demonstrates that glutamine functions as an energy source and a medium osmolarity regulator (Huang et al., 2020). The addition of non-essential amino acids along with glutamine has a more stimulating effect than the mere presence of essential amino acids. This demonstrates unequivocally that sheep, cows, mice, and hamsters have different transport systems for essential and non-essential amino acids in their cells (Watford, 2015). Nevertheless, the medium’s amino acids may also raise ammonium levels, which may prevent blastocyst growth and cell division (Van Winkle, 2021). Furthermore, the presence of amino acids in the media may exacerbate the occurrence of cytoplasmic fragmentation in sheep embryos (Da Broi et al., 2018). A fresh culture medium needs to be used every 48–72 hours to get around this. Early embryonic development frequently encounters challenges. Inhibition typically occurs at the two-cell stage in mouse and rat embryos and at the eight-cell stage in cow and sheep embryos (Latham, 2023). This situation can be solved by replacing glucose as an energy source with pyruvate and lactate in culture media. According to Hong et al. (2023), blastocyst development is correlated with glucose use. The embryo uses pyruvate throughout the preimplantation phase before reaching the blastocyst, but glucose is more necessary during blastocyst development (Mu et al., 2022). Throughout the early stages of cow embryo development, glucose metabolism is very low, although glutamine and pyruvate metabolism increase (Krisher and Prather, 2012). Only during the 8–16 cell stage, which is linked to the activation of the embryonic genome, does glucose metabolism start to rise (Wang et al., 2023). The sheep embryo uses glucose to transform it into lactate, which is then extracted from the cells (Darby et al., 2023). Cattle developmental delay in the 8-cell stage is linked to oocyte cytoplasmic quality. Embryos that are unable to transcribe their own genome will not develop, but oocytes require all proteins and mRNAs to reach the 4 or 5 cell stage (Mu et al., 2024). The oocyte cytoplasm, which is critical for healthy embryo development, has a significant impact on the active demethylation of cytosine methylation in the spermatozoa genome to produce the zygote nucleus in mammals (Beaujean et al., 2004). Culturing oocytes and embryos in high quantities can increase the quality of low-quality embryos. Group culture of 20 embryos in 500 ml of media improves the growth ability of the embryos (Smith et al, 2012). The male and female sexes impact the blastocyst formation and embryo cell division rates. Cell division and blastocyst formation occur more quickly in cattle embryos derived from parents of various breeds than in those derived from parents of the same breed (Shorten et al., 2018). Embryos that develop to the 8-cell stage within 48 hours of fertilization produce blastocysts more often than not (Zhu et al., 2014). IGF-1 plays a major role in cattle FIV. IGF-1 can improve oocytes’ capacity for fertilization because it plays a role in the maturation process (Barrera et al., 2023). IGF-1-exposed oocytes are often of superior quality and have a greater chance of developing into healthy embryos following fertilization (Bonavina and Taylor, 2022). An embryo is created after fertilization, which needs a nurturing environment to grow. IGF-1 may help with this process by promoting early-stage embryo survival and facilitating embryo development (Kasprzak and Szaflarski, 2020). This could lead to higher rates of embryo implantation in replacement heifers. In addition, IGF-1 regulates metabolism, which is critical for reproductive health. IGF-1 facilitates the energy metabolism required for fertilization and embryonic development by regulating the availability of nutrients to cells (Feng and Levine, 2010). A healthy energy balance can improve the likelihood of successful in vitro fertilization. Consequently, IGF-1 improves the culture conditions for embryo development (Fig. 6).
Fig. 6. Role of IGF-1 in preventing the apoptosis cascade through the PI3K/Akt pathway. Estrogen, particularly through its function in controlling oocyte development and the reproductive cycle, significantly influences cattle’s FIV process. The maturation process of oocytes is influenced by estrogen. Estrogen promotes the growth of healthy, mature oocytes by inducing papillary follicle growth (Guo et al., 2004). The likelihood of successful fertilization and high-quality embryo production is higher for mature oocytes (Yu et al., 2015). Estrogen is frequently added to the embryo culture medium during IVF procedures to promote cell development and embryo growth. Estrogen can enhance the quality and survival rate of embryos in culture by fostering an environment that is abundant in nutrients and development factors (Salek et al., 2025). Additionally, estrogen increases cellular metabolism, which is crucial for embryo growth. Estrogen helps guarantee that the embryo has enough energy and nutrients to develop normally by increasing the metabolic rate (Mahboobifard et al., 2022). The FIV process in cows is significantly influenced by progesterone. Progesterone may have an impact on the quality of embryos (Yovel et al., 1995; Salehnia and Zavareh, 2013; Kalakota et al., 2022). Progesterone can help create a healthy, high-quality embryo by creating a stable and encouraging environment. Literature integrationA comprehensive and critical literature review reveals the potential and current limitations of using growth factors—particularly IGF-1—and sex steroid hormones derived from cultured hepatic and cumulus cells in supporting bovine IVF. IGF-1 enhances oocyte maturation, promotes embryonic development, and improves blastocyst quality in various species, including cattle, swine, and mice (Pereira et al., 2019; Sato et al., 2017; Barrera et al., 2023; Lin et al., 2003). However, a closer examination of the available data indicates that these outcomes are often context-dependent, varying with IGF-1 concentration, timing, and specific developmental stage. While some studies support the use of exogenous IGF-1 in oocyte maturation and embryo culture media, others report limited or inconsistent improvements in developmental competence, suggesting the need for more standardized protocols and comparative studies (Fernandez-Gonzalez et al., 2021; Carrillo-Gonzalez et al., 2024). Cumulus cells have been shown to synthesize small amounts of estradiol and progesterone in vitro, contributing to the oocyte microenvironment; however, the endocrine significance of these secretions remains unclear without in vivo validation or dose-response analyses (Harl et al., 2025). The literature also reflects a gap in experimental studies evaluating the direct use of liver- or cumulus-derived IGF-1 and steroids in practical IVF systems (Ohlsson et al., 2009). Most current findings are extrapolated from indirect observations or model organisms, underscoring the need for species-specific, mechanistic investigations in bovine models. Few studies have addressed the technical aspects of IGF-1 purification from primary cell cultures or its stability in culture media—critical factors for successful translation to applied reproductive biotechnology (Park et al., 2020; Venkatesan et al., 2022). Taken together, the literature not only offers promising insights but also highlights the fragmented and preliminary nature of current knowledge. Therefore, the use of liver and cumulus cell culture products as bioactive supplements for IVF remains largely theoretical and warrants further research through well-controlled, reproducible, and comparative studies to establish biological relevance, efficacy, and scalability (Cairo Consensus Group C, 2020). Limitations and future perspectivesThe scarcity of recent in vivo data on the practical use of liver- and cumulus cell-derived factors in bovine IVF limits this review. Most current findings originate from in vitro studies or non-bovine models, which may limit the direct applicability of the results to cattle reproduction. Furthermore, significant challenges remain in the purification, standardization, and stability of bioactive molecules, potentially affecting their reproducibility and scalability. Future research should focus on validating these approaches through well-controlled animal studies, optimizing protocols for large-scale application, and exploring biotechnological innovations that can ensure consistent quality and cost-effective production of these factors. ConclusionCattle liver, a slaughterhouse by-product, can serve as an IGF-1 source, while the ovaries produce estrogen and progesterone. Supplementation of these bioactive compounds obtained through tissue culture may enhance the success of IVF in cattle. This strategy offers the potential to produce genetically superior males for frozen semen production, thereby improving the productivity of both dairy and beef cattle. AcknowledgmentsThe authors thank Universitas Airlangga and Badan Riset dan Inovasi Nasional. Author’s contributionsSM, ARK, IM, and FAR drafted the manuscript. RZA, SW, DAAK, and AOA revised and edited the manuscript. UFH, ATK, and BWKW prepared and critically checked this manuscript. LA, LL, and BPP edited the references. All authors have read and approved the final version of the manuscript. Conflict of interestThe authors declare no conflict of interest. FundingThe authors funded this study. Data availabilityAll references are open access, so data can be obtained from the internet. ReferencesAhmad, S.S., Ahmad, K., Lee, E.J., Lee, Y.H. and Choi, I. 2020. Implications of insulin-like growth factor-1 in skeletal muscle and various diseases. Cells 9(8), 1773. Al-Samerria, S. and Radovick, S. 2021. The role of insulin-like growth factor-1 (IGF-1) in the control of neuroendocrine regulation of growth. Cells 10(10), 2664. Al-Samerria, S. and Radovick, S. 2023. Exploring the therapeutic potential of targeting GH and IGF-1 in the management of obesity: insights from the interplay between these hormones and metabolism. Int. J. Mol. Sci. 24(11), 9556. Anifandis, G., Messini, C., Dafopoulos, K., Sotiriou, S. and Messinis, I. 2014. Molecular and cellular mechanisms of sperm-oocyte interactions opinions relative to in vitro fertilization (IVF). Int. J. Mol. Sci. 15(7), 12972–12997. Annes, K., De Lima, C.B., Ispada, J., Dos Santos, E.C., Fontes, P.K., Nichi, M., Nogueira, M.F.G., Sudano, M.J. and Milazzotto, M.P. 2023. Insulin-like growth factor-1 (IGF-1) selectively modulates the metabolic and lipid profile of bovine embryos according to their kinetics of development. Theriogenology 204(1), 1–7. Arias, M.E., Vargas, T., Gallardo, V., Aguila, L. and Felmer, R. 2022. Simple and efficient chemically defined in vitro maturation and embryo culture system for bovine embryos. Animals 12(21), 3057. Bailes, J. and Soloviev, M. 2021. Insulin-like growth factor-1 (IGF-1) and Its monitoring in medical diagnostic and in sports. Biomolecules 11(2), 217. Barrera, S.S., Naranjo-Gomez, J.S. and Rondón-Barragán, I.S. 2023. Thermoprotective molecules: effect of insulin-like growth factor type I (IGF-1) in cattle oocytes exposed to high temperatures. Heliyon 9(3), e14375. Bastide, A. and David, A. 2018. Interaction of rRNA with mRNA and tRNA in translating mammalian ribosome: functional implications in health and disease. Biomolecules 8(4), 100. Baumgarten, S.C., Armouti, M., Ko, C. and Stocco, C. 2017. IGF1R expression in ovarian granulosa cells is essential for steroidogenesis, follicle survival, and fertility in female mice. Endocrinology 158(7), 2309–2318. Beaujean, N., Taylor, J., Gardner, J., Wilmut, I., Meehan, R. and Young, L. 2004. Effect of limited DNA methylation reprogramming in the normal sheep embryo on somatic cell nuclear transfer. Biol. Reprod. 71(1), 185–193. Bikle, D.D., Tahimic, C., Chang, W., Wang, Y., Philippou, A. and Barton, E.R. 2015. Role of IGF-I signaling in muscle bone interactions. Bone 80(1), 79–88. Blum, W.F., Alherbish, A., Alsagheir, A., El Awwa, A., Kaplan, W., Koledova, E. and Savage, M.O. 2018. The growth hormone-insulin-like growth factor-I axis in the diagnosis and treatment of growth disorders. Endocr. Connect. 7(6), R212–R222. Bonavina, G., Taylor, H. and S. 2022. Endometriosis-associated infertility: from pathophysiology to tailored treatment. Front. Endocrinol. (Lausanne). 13(1), 1020827. Britt, J.H., Cushman, R.A., Dechow, C.D., Dobson, H., Humblot, P., Hutjens, M.F., Jones, G.A., Ruegg, P.S., Sheldon, I.M. and Stevenson, J.S. 2018. Invited review: learning from the future-A vision for dairy farms and cows in 2067. J. Dairy Sci. 101(5), 3722–3741. Cairo Consensus Group. 2020. ‘There is only one thing that is truly important in an IVF laboratory: everything’. Reprod. Biomed. Online. 40(1), 33–60. Caputo, M., Pigni, S., Agosti, E., Daffara, T., Ferrero, A., Filigheddu, N. and Prodam, F. 2021. Regulation of GH and GH signaling by nutrients. Cells 10(6), 1376. Carrillo-Gonzalez, D.F., Hernández-Herrera, D.Y., Medina-Montes, A.F. and Otero-Arroyo, R. 2024. Effect of the addition of IGF-1 during in vitro culture on the embryonic development speed from different crossbreed bovine embryos. Trop. Anim. Health Prod. 56(8), 368. Cavalcanti, G.S., Carvalho, K.C., Ferreira, C.D.S., Alvarez, P.A.C., Monteleone, P.A.A., Baracat, E.C. and Júnior, J.M.S. 2023. Granulosa cells and follicular development: a brief review. Rev. Assoc. Med. Bras. (1992). 69(6), e20230175.69. Chan, H.J., Petrossian, K. and Chen, S. 2016. Structural and functional characterization of aromatase, estrogen receptor, and their genes in endocrine-responsive and -resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 161(1), 73–83. Christenson, L.K. and Devoto, L. 2003. Cholesterol transport and steroidogenesis by the corpus luteum. Reproductive Biol. Endocrinol. 1(1), 90. Christenson, L.K. and Strauss, J.F. 2000. Steroidogenic acute regulatory protein (StAR) and the intramitochondrial translocation of cholesterol. Biochim. Biophys. Acta 1529(1–3), 175–187. Da Broi, M.G., Giorgi, V.S.I., Wang, F., Keefe, D.L., Albertini, D. and Navarro, P.A. 2018. Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications. J. Assist. Reprod. Genet. 35(5), 735–751. Da Silva Rosa, P.M., Bridi, A., De Ávila Ferronato, G., Prado, C.M., Bastos, N.M., Sangalli, J.R., Meirelles, F.V., Perecin, F. and Da Silveira, J.C. 2024. Corpus luteum presence in the bovine ovary increase intrafollicular progesterone concentration: consequences in follicular cells gene expression and follicular fluid small extracellular vesicles miRNA contents. J. Ovarian Res. 17(1), 65. Darby, J.R.T., Zhang, S., Holman, S.L., Muhlhausler, B.S., Mcmillen, I.C. and Morrison, J.L. 2023. Cardiac growth and metabolism of the fetal sheep are not vulnerable to a 10 day increase in fetal glucose and insulin concentrations during late gestation. Heliyon 9(7), e18292. De Hoyos-vega, J.M., Hong, H.J., Stybayeva, G. and Revzin, A. 2021. Hepatocyte cultures: from collagen gel sandwiches to microfluidic devices with integrated biosensors. APL. Bioeng. 5(4), 041504. Dehkhoda, F., Lee, C.M.M., Medina, J. and Brooks, A.J. 2018. The growth hormone receptor: mechanism of receptor activation, cell signaling, and physiological aspects. Front. Endocrinol. (Lausanne). 9(1), 35. Del Bianco, D., Gentile, R., Sallicandro, L., Biagini, A., Quellari, P.T., Gliozheni, E., Sabbatini, P., Ragonese, F., Malvasi, A., D’Amato, A., Baldini, G.M., Trojano, G., Tinelli, A. and Fioretti, B. 2024. Electro-metabolic coupling of cumulus-oocyte complex. Int. J. Mol. Sci. 25(10), 5349. Drummond, A.E. 2006. The role of steroids in follicular growth. Reproductive Biol. Endocrinol. 4(1), 16. Echternkamp, S.E., Aad, P.Y., Eborn, D.R. and Spicer, L.J. 2012. Increased abundance of aromatase and follicle stimulating hormone receptor mRNA and decreased insulin-like growth factor-2 receptor mRNA in small ovarian follicles of cattle selected for twin births. J. Anim. Sci. 90(7), 2193–2200. Emori, C. and Sugiura, K. 2014. Role of oocyte‐derived paracrine factors in follicular development. Anim. Sci. J. 85(6), 627–633. Emori, C., Wigglesworth, K., Fujii, W., Naito, K., Eppig, J.J. and Sugiura, K. 2013. Cooperative effects of 17β-estradiol and oocyte-derived paracrine factors on the transcriptome of mouse cumulus cells. Endocrinology 154(12), 4859–4872. Erdem, H., Karasahin, T., Alkan, H., Dursun, S., Satilmis, F. and Guler, M. 2020. Effect of embryo quality and developmental stages on pregnancy rate during fresh embryo transfer in beef heifers. Trop. Anim. Health Prod. 52(5), 2541–2547. Fanton, M., Cho, J.H., Baker, V.L. and Loewke, K. 2023. A higher number of oocytes retrieved is associated with an increase in fertilized oocytes, blastocysts, and cumulative live birth rates. Fertil. Steril. 119(5), 762–769. Farkas, S., Szabó, A., Hegyi, A.E., Török, B., Fazekas, C.L., Ernszt, D., Kovács, T. and Zelena, D. 2022. Estradiol and Estrogen-like Alternative Therapies in Use: the Importance of the Selective and Non-Classical Actions. Biomedicines 10(4), 861. Fatehi, A.N., Roelen, B.A.J., Colenbrander, B., Schoevers, E.J., Gadella, B.M., Bevers, M.M. and Van Den Hurk, R. 2005. Presence of cumulus cells during in vitro fertilization protects the bovine oocyte against oxidative stress and improves first cleavage but does not affect further development. Zygote 13(2), 177–185. Feng, Z. and Levine, A.J. 2010. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol. 20(7), 427–434. Fernandez-Gonzalez, L., Kozhevnikova, V., Brusentsev, E., Jänsch, S., Amstislavsky, S. and Jewgenow, K. 2021. IGF-I medium supplementation improves singly cultured cat oocyte maturation and embryo development In Vitro. Animals 11(7), 1909. Fortune, J.E., Rivera, G.M., Evans, A.C. and Turzillo, A.M. 2001. Differentiation of dominant versus subordinate follicles in cattle. Biol. Reprod. 65(3), 648–654. Furth, P.A., Nakles, R.E., Millman, S., Diaz-Cruz, E.S. and Cabrera, M.C. 2011. Signal transducer and activator of transcription 5 as a key signaling pathway in normal mammary gland developmental biology and breast cancer. Breast Cancer Res. 13(5), 220. Gallelli, M.F., Bianchi, C., Zampini, E., Aba, M., Gambarotta, M. and Miragaya, M. 2020. Plasma IGF1 and 17β-estradiol concentrations during the follicular wave in llamas. Front. Vet. Sci. 7(1), 555261. Gao-Ping, Z., Pei-Xin, S., Qing, C., Zi-Xin, W., Yun-Xia, L., Li-Xia, Z., Wei, S., Meng, W., Si-Qin, B., Gui-Fang, C. and Xi-He, L. 2023. Effects of insulin-like growth factor 1, leukemia inhibitory factor, and basic fibroblast growth factor on goat oocyte maturation and early embryonic development in vitro. Small Ruminant Res. 226(1), 107038. Gardner, D.K., Lane, M., Stevens, J., Schlenker, T. and Schoolcraft, W.B. 2000. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil. Steril. 73(6), 1155–1158. Green, C.J., Charlton, C.A., Wang, L.M., Silva, M., Morten, K.J. and Hodson, L. 2017. The isolation of primary hepatocytes from human tissue: optimising the use of small non-encapsulated liver resection surplus. Cell Tissue Bank 18(4), 597–604. Gualtieri, R., De Gregorio, V., Candela, A., Travaglione, A., Genovese, V., Barbato, V. and Talevi, R. 2024. In vitro culture of mammalian embryos: is there room for improvement?. Cells 13(12), 996. Guo, Y., Guo, K.J., Huang, L., Tong, X.G. and Li, X. 2004. Effect of estrogen deprivation on follicle/oocyte maturation and embryo development in mice. Chin. Med. J. (Engl). 117(4), 498–502. Gupta, S. and Savaliya, C. 2012. Application of biotechnology to improve livestock products. Vet. World 5(10), 634–638. Guzeloglu-Kayisli, O., Kayisli, U.A. and Taylor, H.S. 2009. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin. Reprod. Med. 27(1), 62–79. Harl, A.W., Negrón-Pérez, V.M., Stewart, J.W., Perry, G.A., Ealy, A.D. and Rhoads, M.L. 2025. Maturation of bovine cumulus oocyte complexes in follicular fluid with or without estradiol, progesterone or the combination affects cumulus cell expansion and blastocyst development. PLoS One 20(6), e0321266. Hayes, E., Winston, N. and Stocco, C. 2024. Molecular crosstalk between insulin-like growth factors and follicle-stimulating hormone in the regulation of granulosa cell function. Reprod. Med. Biol. 23(1), e12575. Hellström, A., Ley, D., Hansen‐Pupp, I., Hallberg, B., Löfqvist, C., Van Marter, L., Van Weissenbruch, M., Ramenghi, L.A., Beardsall, K., Dunger, D., Hård, A.L. and Smith, L.E.H. 2016. Insulin‐like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 105(6), 576–586. Hemkemeyer, S.A., Schwarzer, C., Boiani, M., Ehmcke, J., Le Gac, S., Schlatt, S. and Nordhoff, V. 2014. Effects of embryo culture media do not persist after implantation: a histological study in mice. Hum. Reprod. 29(2), 220–233. Holst, J.P., Soldin, O.P., Guo, T. and Soldin, S.J. 2004. Steroid hormones: relevance and measurement in the clinical laboratory. Clin. Lab. Med. 24(1), 105–118. Hong, J., Tong, H., Wang, X., Lv, X., He, L., Yang, X., Wang, Y., Xu, K., Liang, Q., Feng, Q., Niu, T., Niu, X. and Lu, Y. 2023. Embryonic diapause due to high glucose is related to changes in glycolysis and oxidative phosphorylation, as well as abnormalities in the TCA cycle and amino acid metabolism. Front. Endocrinol. (Lausanne). 14(1), 1135837. Hu, C. and Li, L. 2015. In vitro culture of isolated primary hepatocytes and stem cell-derived hepatocyte-like cells for liver regeneration. Protein Cell 6(8), 562–574. Hu, J., Zhang, Z., Shen, W.J. and Azhar, S. 2010. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. (Lond). 7(1), 47. Huang, L., Li, G., Du, C., Jia, Y., Yang, J., Fan, W., Xu, Y.Z., Cheng, H. and Zhou, Y. 2023. The polyA tail facilitates splicing of last introns with weak 3′ splice sites via PABPN1. EMBO Rep. 24(10), e57128. Huang, P.C., Liu, T.Y., Hu, M.Y., Casties, I. and Tseng, Y.C. 2020. Energy and nitrogenous waste from glutamate/glutamine catabolism facilitates acute osmotic adjustment in non-neuroectodermal branchial cells. Sci. Rep. 10(1), 9460. Huat, T.J., Khan, A.A., Pati, S., Mustafa, Z., Abdullah, J.M. and Jaafar, H. 2014. IGF-1 enhances cell proliferation and survival during early differentiation of mesenchymal stem cells to neural progenitor-like cells. BMC. Neurosci. 15(1), 91. Hull, K.L. and Harvey, S. 2014. Growth hormone and reproduction: a review of endocrine and autocrine/paracrine interactions. Int. J. Endocrinol. 2014(1), 234014. Ipsa, E., Cruzat, V.F., Kagize, J.N., Yovich, J.L. and Keane, K.N. 2019. Growth hormone and insulin-like growth factor action in reproductive tissues. Front. Endocrinol. (Lausanne). 10(1), 777. Iranpoor, H., Omidinia, E., Vatankhah, V., Gharanjik, V. and Shahbazi, M. 2015. Expression of recombinant human insulin-like growth factor type 1 (rhIGF-1) in Escherichia coli. Avicenna J. Med. Biotechnol. 7(3), 101–105. Iresjö, B.M., Diep, L. and Lundholm, K. 2022. Initiation of muscle protein synthesis was unrelated to simultaneously upregulated local production of IGF-1 by amino acids in non-proliferating L6 muscle cells. PLoS One 17(7), e0270927. Itami, N., Akagi, S. and Hirao, Y. 2024. Excluding alanine from minimum essential medium (MEM) nonessential amino acid supplementation of the culture medium facilitates post-fertilization events and early cleavages of bovine oocytes fertilized in vitro. J. Reprod. Dev. 70(4), 223–228. Kalakota, N.R., George, L.C., Morelli, S.S., Douglas, N.C. and Babwah, A.V. 2022. Towards an improved understanding of the effects of elevated progesterone levels on human endometrial receptivity and oocyte/embryo quality during assisted reproductive technologies. Cells 11(9), 1405. Kalous, J., Aleshkina, D. and Anger, M. 2023. A role of PI3K/Akt signaling in oocyte maturation and early embryo development. Cells 12(14), 1830. Kang, L.I., Mars, W.M. and Michalopoulos, G.K. 2012. Signals and cells involved in regulating liver regeneration. Cells 1(4), 1261–1292. Kasprzak, A. and Szaflarski, W. 2020. Role of alternatively spliced messenger RNA (mRNA) isoforms of the insulin-like growth factor 1 (IGF1) in selected human tumors. Int. J. Mol. Sci. 21(19), 6995. Khamsi, F. and Roberge, S. 2001. Granulosa cells of the cumulus oophorus are different from mural granulosa cells in their response to gonadotrophins and IGF-I. J. Endocrinol. 170(3), 565–573. Khan, M.Z., Zugaza, J.L. and Torres Aleman, I. 2025. The signaling landscape of insulin-like growth factor 1. J. Biol. Chem. 301(1), 108047. Kholodenko, I.V. and Yarygin, K.N. 2017. Cellular mechanisms of liver regeneration and cell-based therapies of liver diseases. Biomed. Res. Int. 2017(1), 8910821. Kim, H., Fu, Y., Hong, H.J., Lee, S.G., Lee, D.S. and Kim, H.M. 2022. Structural basis for assembly and disassembly of the IGF/IGFBP/ALS ternary complex. Nature Commun. 13(1), 4434. Kim, M., Park, J.E., Lee, Y., Lee, S.T., Lee, G.S., Hyun, S.H., Lee, E. and Lee, J. 2023. Effect of growth factors and hormones during in vitro growth culture of cumulus-oocyte-complexes derived from small antral follicles in pigs. Animals (Basel). 13(7), 1206. Kineman, R.D., Del Rio-moreno, M. and Sarmento-Cabral, A. 2018. 40 YEARS of IGF1: understanding the tissue-specific roles of IGF1/IGF1R in regulating metabolism using the Cre/loxP system. J. Mol. Endocrinol. 61(1), T187–T198. Kisiday, J.D., Kurz, B., Dimicco, M.A. and Grodzinsky, A.J. 2005. Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng. 11(1–2), 141–151. Kotsifaki, A., Maroulaki, S., Karalexis, E., Stathaki, M. and Armakolas, A. 2024. Decoding the role of insulin-like growth factor 1 and its isoforms in breast cancer. Int. J. Mol. Sci. 25(17), 9302. Krisher, R.L. and Prather, R.S. 2012. A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation. Mol. Reprod. Dev. 79(5), 311–320. Kulus, M., Kranc, W., Sujka-Kordowska, P., Mozdziak, P., Jankowski, M., Konwerska, A., Kulus, J., Bukowska, D., Skowroński, M., Piotrowska-Kempisty, H., Nowicki, M., Kempisty, B. and Antosik, P. 2020. The processes of cellular growth, aging, and programmed cell death are involved in lifespan of ovarian granulosa cells during short-term IVC - Study based on animal model. Theriogenology 148(1), 76–88. Laron, Z. 2001. Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol. Pathol. 54(5), 311–316. Latham, K.E. 2023. Preimplantation embryo gene expression: 56 years of discovery, and counting. Mol. Reprod. Dev. 90(4), 169–200. Laven, J.S. and Fauser, B.C. 2006. What role of estrogens in ovarian stimulation. Maturitas 54(4), 356–362. Lecluyse, E.L., Witek, R.P., Andersen, M.E. and Powers, M.J. 2012. Organotypic liver culture models: meeting current challenges in toxicity testing. Crit. Rev. Toxicol. 42(6), 501–548. Ledwaba, M.R., O’Neill, H.A., Thema, M.A., Maqhashu, A. and Mphaphathi, M.L. 2025. Techniques for in vitro fertilisation of vitrified cattle oocytes: challenges and new developments. Agriculture 15(4), 363. Leroith, D., Holly, J.M.P. and Forbes, B.E. 2021. Insulin-like growth factors: ligands, binding proteins, and receptors. Mol. Metab. 52(1), 101245. Lewitt, M.S. and Boyd, G.W. 2019. The role of insulin-like growth factors and insulin-like growth factor-binding proteins in the nervous system. Biochem. Insights 12(1), 1178626419842176. Li, Y., Li, R.Q., Ou, S.B., Zhang, N.F., Ren, L., Wei, L.N., Zhang, Q.X. and Yang, D.Z. 2014. Increased GDF9 and BMP15 mRNA levels in cumulus granulosa cells correlate with oocyte maturation, fertilization, and embryo quality in humans. Reproductive Biol. Endocrinol. 12(1), 81. Liang, J.J. and Rasmusson, A.M. 2018. Overview of the molecular steps in steroidogenesis of the GABAergic neurosteroids allopregnanolone and pregnanolone. Chronic Stress (Thousand. Oaks). 2(1), 2470547018818555. Lin, T.C., Yen, J.M., Gong, K.B., Hsu, T.T. and Chen, L.R. 2003. IGF-1/IGFBP-1 increases blastocyst formation and total blastocyst cell number in mouse embryo culture and facilitates the establishment of a stem-cell line. BMC. Cell Biol. 4(1), 14. Liu, T., Huang, Y. and Lin, H. 2021. Estrogen disorders: interpreting the abnormal regulation of aromatase in granulosa cells (Review). Int. J. Mol. Med. 47(5), 73. Liu, X., Bushnell, D.A. and Kornberg, R.D. 2013. RNA polymerase II transcription: structure and mechanism. Biochim Biophys. Acta 1829(1), 2–8. Lucidi, P., Bernabò, N., Turriani, M., Barboni, B. and Mattioli, M. 2003. Cumulus cells steroidogenesis is influenced by the degree of oocyte maturation. Reprod. Biol. Endocrinol. 1(1), 45. Lundqvist, J., Hansen, S.K. and Lykkesfeldt, A.E. 2013. Vitamin D analog EB1089 inhibits aromatase expression by dissociation of comodulator WSTF from the CYP19A1 promoter-a new regulatory pathway for aromatase. Biochim. Biophys. 1833(1), 40–47. Ma, I., L., Stanley, T. and L. 2023. Growth hormone and nonalcoholic fatty liver disease. Immunometabolism (Cobham). 5(3), 30. Macháčková, K., Collinsová, M., Chrudinová, M., Selicharová, I., Pícha, J., Buděšínský, M., Vaněk, V., Žáková, L., Brzozowski, A.M. and Jiráček, J. 2017. Insulin-like growth factor 1 analogs clicked in the C domain: chemical synthesis and biological activities. J. MedChem. 60(24), 10105–10117. Macvanin, M., Gluvic, Z., Radovanovic, J., Essack, M., Gao, X. and Isenovic, E.R. 2023. New insights on the cardiovascular effects of IGF-1. Front. Endocrinol. (Lausanne). 14(1), 1142644. Mahboobifard, F., Pourgholami, M.H., Jorjani, M., Dargahi, L., Amiri, M., Sadeghi, S. and Tehrani, F.R. 2022. Estrogen as a key regulator of energy homeostasis and metabolic health. Biomed. Pharmacother. 156(1), 113808. Makarevich, A., V. and Markkula, M. 2002. Apoptosis and cell proliferation potential of bovine embryos stimulated with insulin-like growth factor i during in vitro maturation and culture. Biol. Reprod. 66(2), 386–392. Mani, A.M., Fenwick, M.A., Cheng, Z., Sharma, M.K., Singh, D. and Wathes, D.C. 2010. IGF1 induces up-regulation of steroidogenic and apoptotic regulatory genes via activation of phosphatidylinositol-dependent kinase/AKT in bovine granulosa cells. Reproduction 139(1), 139–151. Manna, P.R., Stetson, C.L., Slominski, A.T. and Pruitt, K. 2016. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 51(1), 7–21. Marantha, S.D.I., Purnomo, S.H. and Sari, A.I. 2024. Study of the financial feasibility of a dairy farming business after the FMD outbreak in kampung susu lawu, Plaosan district, magetan regency. Livestock Anim. Res. 22(2), 139–149. Martín, A.I., Priego, T., Moreno-Ruperez, A., González-Hedström, D., Granado, M. and López-Calderón, A. 2021. IGF-1 and IGFBP-3 in inflammatory cachexia. Int. J. Mol. Sci. 22(17), 9469. Martinez, C.A., Rizos, D., Rodriguez-Martinez, H. and Funahashi, H. 2023. Oocyte-cumulus cells crosstalk: new comparative insights. Theriogenology 205(1), 87–93. Martínez-Moreno, C.G., Calderón-Vallejo, D., Harvey, S., Arámburo, C. and Quintanar, J.L. 2018. Growth hormone (GH) and gonadotropin-releasing hormone (GnRH) in the central nervous system: a potential neurological combinatory therapy?. Int. J. Mol. Sci. 19(2), 375. Mast, N., Annalora, A.J., Lodowski, D.T., Palczewski, K., Stout, C.D. and Pikuleva, I.A. 2011. Structural basis for three-step sequential catalysis by the cholesterol side chain cleavage enzyme CYP11A1. J. Biol. Chem. 286(7), 5607–5613. Mccarty, K.D., Liu, L., Tateishi, Y., Wapshott-Stehli, H.L. and Guengerich, F.P. 2024. The multistep oxidation of cholesterol to pregnenolone by human cytochrome P450 11A1 is highly processive. J. Biol. Chem. 300(1), 105495. Mcdermott, N., O’Shea, S., Rieger, L., Cox, O.T. and O’Connor, R. 2025. Β1-integrin controls IGF-1R internalization and intracellular signaling. J. Biol. Chem. 301(1), 108021. Mehta, B.N., Chimote, N.M., Chimote, M.N., Chimote, N.N. and Nath, N.M. 2013. Follicular fluid insulin like growth factor-1 (FF IGF-1) is a biochemical marker of embryo quality and implantation rates in in vitro fertilization cycles. J. Hum. Reprod. Sci. 6(2), 140–146. Mesen, T., B., Young, S. and L. 2015. Progesterone and the luteal phase: a requisite to reproduction. Obstet. Gynecol. Clin. North Am. 42(1), 135–151. Michalopoulos, G.K. 2010. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am. J. Pathol. 176(1), 2–13. Miescher, I., Rieber, J., Calcagni, M. and Buschmann, J. 2023. In vitro and in vivo effects of IGF-1 delivery strategies on tendon healing: a review. Int. J. Mol. Sci. 24(3), 2370. Miller, W.L. 2007. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim. Biophys. Acta. 1771(6), 663–676. Miller, W.L. and Auchus, R.J. 2011. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32(1), 81–151. Monniaux, D., Pisselet, C. and Fontaine, J. 1994. Uncoupling between proliferation and differentiation of ovine granulosa cells in vitro. J. Endocrinol. 142(3), 497–510. Mu, J., Zhou, Z., Sang, Q. and Wang, L. 2022. The physiological and pathological mechanisms of early embryonic development. Fundam. Res. 2(6), 859–872. Ogo, Y., Taniuchi, S., Ojima, F., Hayashi, S., Murakami, I., Saito, Y., Takeuchi, S., Kudo, T. and Takahashi, S. 2014. IGF-1 gene expression is differentially regulated by estrogen receptors α and β in mouse endometrial stromal cells and ovarian granulosa cells. J. Reprod. Dev. 60(3), 216–223. Ohlsson, C., Mohan, S., Sjögren, K., Tivesten, A., Isgaard, J., Isaksson, O., Jansson, J.O. and Svensson, J. 2009. The role of liver-derived insulin-like growth factor-I. Endocr. Rev. 30(5), 494–535. Pan, Y., Pan, C. and Zhang, C. 2024. Unraveling the complexity of follicular fluid: insights into its composition, function, and clinical implications. J. Ovarian Res. 17(1), 237. Papageorgiou, K., Mastora, E., Zikopoulos, A., Grigoriou, M.E., Georgiou, I. and Michaelidis, T.M. 2021. Interplay between mTOR and hippo signaling in the ovary: clinical choice guidance between different gonadotropin preparations for better IVF. Front. Endocrinol. (Lausanne). 12(1), 702446. Park, J., Yan, G., Kwon, K.C., Liu, M., Gonnella, P.A., Yang, S. and Daniell, H. 2020. Oral delivery of novel human IGF-1 bioencapsulated in lettuce cells promotes musculoskeletal cell proliferation, differentiation and diabetic fracture healing. Biomaterials 233(1), 119591. Park, S.R., Kim, S.R., Kim, S.K., Park, J.R. and Hong, I.S. 2022. A novel role of follicle-stimulating hormone (FSH) in various regeneration-related functions of endometrial stem cells. Exp. Mol. Med. 54(9), 1524–1535. Pech-Pool, S., Berumen, L.C., Martínez-Moreno, C.G., García-Alcocer, G., Carranza, M., Luna, M. and Arámburo, C. 2020. Thyrotropin-releasing hormone (TRH) and somatostatin (SST), but not growth hormone-releasing hormone (GHRH) nor Ghrelin (GHRL), regulate expression and release of immune growth hormone (GH) from chicken bursal B-lymphocyte cultures. Int. J. Mol. Sci. 21(4), 1436. Pereira, B.A., Zangeronimo, M.G., Castillo-Martín, M., Gadani, B., Chaves, B.R., Rodríguez-Gil, J.E., Bonet, S. and Yeste, M. 2019. Supplementing maturation medium with insulin growth factor i and vitrification-warming solutions with reduced glutathione enhances survival rates and development ability of in vitro matured vitrified-warmed pig oocytes. Front. Physiol. 9(1), 1894. Poreba, E. and Durzynska, J. 2020. Nuclear localization and actions of the insulin-like growth factor 1 (IGF-1) system components: transcriptional regulation and DNA damage response. Mutat. Res. Rev. Mutat. Res. 784(1), 108307. Przygrodzka, E., Plewes, M.R. and Davis, J.S. 2021. Luteinizing hormone regulation of inter-organelle communication and fate of the corpus luteum. Int. J. Mol. Sci. 22(18), 9972. Puche, J.E. and Castilla-Cortázar, I. 2012. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. J. Transl. Med. 10(1), 224. Racine, H.L. and Serrat, M.A. 2020. The actions of IGF-1 in the growth plate and its role in postnatal bone elongation. Curr. Osteoporos. Rep. 18(3), 210–227. Rajpathak, S.N., Gunter, M.J., Wylie-Rosett, J., Ho, G.Y., Kaplan, R.C., Muzumdar, R., Rohan, T.E. and Strickler, H.D. 2009. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab. Res. Rev. 25(1), 3–12. Salehnia, M. and Zavareh, S. 2013. The effects of progesterone on oocyte maturation and embryo development. Int. J. Fertil. Steril. 7(2), 74–81. Salek, F., Guest, A., Johnson, C., Kastelic, J.P. and Thundathil, J. 2025. Factors affecting the success of ovum pick-up, in vitro production and cryopreservation of embryos in cattle. Animals (Basel). 15(3), 344. Sandhu, M.S., Dunger, D.B. and Giovannucci, E.L. 2002. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J. Natl. Cancer Inst. 94(13), 972–980. Sarjito, A. 2024. Free nutritious meal program as a human resource development strategy to support national defence. Int. J. Admin. Bus. &. Org. 5(5), 129–141. Sato, A., Sarentonglaga, B., Ogata, K., Yamaguchi, M., Hara, A., Atchalalt, K., Sugane, N., Fukumori, R. and Nagao, Y. 2018. Effects of insulin-like growth factor-1 on the in vitro maturation of canine oocytes. J. Reprod. Dev. 64(1), 83–88. Shorten, P.R., Ledgard, A.M., Donnison, M., Pfeffer, P.L., Mcdonald, R.M. and Berg, D.K. 2018. A mathematical model of the interaction between bovine blastocyst developmental stage and progesterone-stimulated uterine factors on differential embryonic development observed on Day 15 of gestation. J. Dairy Sci. 101(1), 736–751. Silva, J.R., Figueiredo, J.R. and Van Den Hurk, R. 2009. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 71(8), 1193–1208. Simon, L.E., Kumar, T.R. and Duncan, F.E. 2020. In vitro ovarian follicle growth: a comprehensive analysis of key protocol variables. Biol. Reprod. 103(3), 455–470. Sirotkin, A.V., Taradajnik, T.E., Makarevich, A.V. and Bulla, J. 1998. Effect of follicular cells, IGF-I and tyrosine kinase blockers on oocyte maturation. Anim. Reprod. Sci. 51(4), 333–344. Skinner, M.K., Schmidt, M., Savenkova, M.I., Sadler-Riggleman, I. and Nilsson, E.E. 2008. Regulation of granulosa and theca cell transcriptomes during ovarian antral follicle development. Mol. Reprod. Dev. 75(9), 1457–1472. Slominski, A., Zbytek, B., Nikolakis, G., Manna, P.R., Skobowiat, C., Zmijewski, M., Li, W., Janjetovic, Z., Postlethwaite, A., Zouboulis, C.C. and Tuckey, R.C. 2013. Steroidogenesis in the skin: implications for local immune functions. J. Steroid Biochem. Mol. Biol. 137(1), 107–123. Smith, G.D., Takayama, S. and Swain, J.E. 2012. Rethinking in vitroembryo culture: new developments in culture platforms and potential to improve assisted reproductive technologies. Biol. Reprod. 86(3), 62. Stocco, C., Telleria, C. and Gibori, G. 2007. The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 28(1), 117–149. Sugiura, K., Su, Y.Q., Li, Q., Wigglesworth, K., Matzuk, M.M. and Eppig, J.J. 2010. Estrogen promotes the development of mouse cumulus cells in coordination with oocyte-derived GDF9 and BMP15. Mol. Endocrinol. 24(12), 2303–2314. Talia, C., Connolly, L. and Fowler, P.A. 2021. The insulin-like growth factor system: a target for endocrine disruptors?. Environ. Int. 147(1), 106311. Tang, Y., Li, X., Hu, C., Guan, R., Wang, Z., Zhang, S., Tao, G., Qu, J. and Gong, F. 2025. Exploring the potential benefits of growth hormone co-treatment on embryo quality in IVF: a randomized controlled open-label trial. Reprod. Biol. Endocrinol. 23(1), 42. Tanghe, S., Van Soom, A., Mehrzad, J., Maes, D., Duchateau, L. and De Kruif, A. 2003. Cumulus contributions during bovine fertilization in vitro. Theriogenology 60(1), 135–149. Toori, M.A., Mosavi, E., Nikseresht, M., Barmak, M.J. and Mahmoudi, R. 2014. Influence of insulin-like growth factor-i on maturation and fertilization rate of immature oocyte and embryo development in NMRI mouse with TCM199 and α-MEM medium. J. Clin. Diagn. Res. 8(12), AC05–AC08. Turathum, B., Gao, E.M. and Chian, R.C. 2021. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells 10(9), 2292. Van Winkle, L.J. 2021. Amino acid transport and metabolism regulate early embryo development: species differences, clinical significance, and evolutionary implications. Cells 10(11), 3154. Velazquez, M.A., Spicer, L.J. and Wathes, D.C. 2008. The role of endocrine insulin-like growth factor-I (IGF-I) in female bovine reproduction. Domest. Anim. Endocrinol. 35(4), 325–342. Velazquez, M.A., Zaraza, J., Oropeza, A., Webb, R. and Niemann, H. 2009. The role of IGF1 in the in vivo production of bovine embryos from superovulated donors. REPRODUCTION 137(2), 161–180. Venkatesan, M., Semper, C., Skrivergaard, S., Di Leo, R., Mesa, N., Rasmussen, M.K., Young, J.F., Therkildsen, M., Stogios, P.J. and Savchenko, A. 2022. Recombinant production of growth factors for application in cell culture. iScience 25(10), 105054. Voudouri, K., Berdiaki, A., Tzardi, M., Tzanakakis, G.N. and Nikitovic, D. 2015. Insulin-like growth factor and epidermal growth factor signaling in breast cancer cell growth: focus on endocrine resistant disease. Anal. Cell Pathol.2015(1), 975495. Wacharasindhu, S., Aroonparkmongkol, S. and Srivuthana, S. 2002. Measurement of IGF-1, IGFBP-3 and free IGF-1 levels by ELISA in growth hormone (GH) deficient children before and after GH replacement. Asian Pac. J. Allergy Immunol. 20(3), 155–160. Wang, M., Xue, J., Li, C., Qi, L., Nie, L. and Xue, Z. 2023. Glucose promoting the early embryonic development by increasing the lipid synthesis at 2-cell stage. Front. Cell Develop. Biol. 11(1), 1208501. Watford, M. 2015. Glutamine and glutamate: nonessential or essential amino acids?. Anim. Nutr. 1(3), 119–122. Werner, H. 2023. The IGF1 signaling pathway: from basic concepts to therapeutic opportunities. Int. J. Mol. Sci. 24(19), 14882. Wu, G., Li, C., Tao, J., Liu, Z., Li, X., Zang, Z., Fu, C., Wei, J., Yang, Y., Zhu, Q., Zhang, J.Q., Shen, M. and Liu, H. 2022. FSH mediates estradiol synthesis in hypoxic granulosa cells by activating glycolytic metabolism through the HIF-1α–AMPK–GLUT1 signaling pathway. J. Biol. Chem. 298(5), 101830. Xiong, Y.Y., Zhu, H.Y., Shi, R.J., Wu, Y.F., Fan, Y. and Jin, L. 2024. Regulation of glucose metabolism: effects on oocyte, preimplantation embryo, assisted reproductive technology and embryonic stem cell. Heliyon 10(19), e38551. Xu, X.L., Huang, Z.Y., Yu, K., Li, J., Fu, X.W. and Deng, S.L. 2022. Estrogen biosynthesis and signal transduction in ovarian disease. Front. Endocrinol. (Lausanne). 13(1), 827032. Yakar, S., Rosen, C.J., Beamer, W.G., Ackert-Bicknell, C.L., Wu, Y., Liu, J.L., Ooi, G.T., Setser, J., Frystyk, J., Boisclair, Y.R. and Leroith, D. 2002. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Invest. 110(6), 771–781. Yang, R.F., Xiong, X.R. and Zi, X.D. 2019. Effect of cysteine, insulin-like growth factor-1 and epidermis growth factor during in vitro oocyte maturation and in vitro culture of yak-cattle crossbred embryos. J. Appl. Anim. Res. 47(1), 463–466. Yang, S., Yang, Y., Hao, H., Du, W., Pang, Y., Zhao, S., Zou, H., Zhu, H., Zhang, P. and Zhao, X. 2022. Supplementation of EGF, IGF-1, and Connexin 37 in IVM Medium Significantly Improved the Maturation of Bovine Oocytes and Vitrification of Their IVF Blastocysts. Genes (Basel). 13(5), 805. Yoh, K., Ikeda, K., Horie, K. and Inoue, S. 2023. Roles of estrogen, estrogen receptors, and estrogen-related receptors in skeletal muscle: regulation of mitochondrial function. Int. J. Mol. Sci. 24(3), 1853. Yoshida, T. and Delafontaine, P. 2020. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 9(9), 1970. Yovel, I., Yaron, Y., Amit, A., Peyser, M.R., David, M.P., Kogosowski, A. and Lessing, J.B. 1995. High progesterone levels adversely affect embryo quality and pregnancy rates in in vitro fertilization and oocyte donation programs. Fertil. Steril. 64(1), 128–131. Yu, E.J., Ahn, H., Lee, J.M., Jee, B.C. and Kim, S.H. 2015. Fertilization and embryo quality of mature oocytes with specific morphological abnormalities. Clin. Exp. Reproductive Med. 42(4), 156–162. Yu, K., Huang, Z.Y., Xu, X.L., Li, J., Fu, X.W. and Deng, S.L. 2022. Estrogen Receptor Function: impact on the Human Endometrium. Front. Endocrinol. (Lausanne). 13(1), 827724. Zhang, C.H., Liu, X.Y. and Wang, J. 2023. Essential role of granulosa cell glucose and lipid metabolism on oocytes and the potential metabolic imbalance in polycystic ovary syndrome. Int. J. Mol. Sci. 24(22), 16247. Zhao, Z., Zhang, N., Li, A., Zhou, B., Chen, Y., Chen, S., Huang, M., Wu, F. and Zhang, L. 2020. Insulin-like growth factor-1 receptor induces immunosuppression in lung cancer by upregulating B7–H4 expression through the MEK/ERK signaling pathway. Cancer Lett. 485(1), 14–26. Zhou, C., Lv, M., Wang, P., Guo, C., Ni, Z., Bao, H., Tang, Y., Cai, H., Lu, J., Deng, W., Yang, X., Xia, G., Wang, H., Wang, C. and Kong, S. 2021. Sequential activation of uterine epithelial IGF1R by stromal IGF1 and embryonic IGF2 directs normal uterine preparation for embryo implantation. J. Mol. Cell. Biol. 13(9), 646–661. Zhu, H.B., Zhang, Z.H., Fadlalla, E., Wang, R.X., Geng, D.F. and Liu, R.Z. 2014. Culturing surplus poor-quality embryos to blastocyst stage have positive predictive value of clinical pregnancy rate. Iran. J. Reprod. Med. 12(9), 609–616. Zhu, M., Wang, D., Zou, K., Wang, F., Zhang, Z., Song, X., Jia, C. and Wei, Z. 2022. Insulin-like growth factor-1 regulates follicle selection of hens by promoting proliferation and inhibiting apoptosis of granulosa cells in prehierarchical follicles in vitro. Anim. Reprod. Sci. 247(1), 107091. | ||
| How to Cite this Article |
| Pubmed Style Mulyati S, Rantam FA, Khairullah AR, Mustofa I, Akintunde AO, Ahmad RZ, Handayani UF, Khalisa AT, Anggraini L, Pratama BP, Latifah L, Wardhani BWK, Kurniasih DAA, Wibowo S. Potential of growth factors and steroid hormones from cell cultures to improve in vitro fertilization in bovine. Open Vet. J.. 2025; 15(9): 3961-3979. doi:10.5455/OVJ.2025.v15.i9.4 Web Style Mulyati S, Rantam FA, Khairullah AR, Mustofa I, Akintunde AO, Ahmad RZ, Handayani UF, Khalisa AT, Anggraini L, Pratama BP, Latifah L, Wardhani BWK, Kurniasih DAA, Wibowo S. Potential of growth factors and steroid hormones from cell cultures to improve in vitro fertilization in bovine. https://www.openveterinaryjournal.com/?mno=250013 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i9.4 AMA (American Medical Association) Style Mulyati S, Rantam FA, Khairullah AR, Mustofa I, Akintunde AO, Ahmad RZ, Handayani UF, Khalisa AT, Anggraini L, Pratama BP, Latifah L, Wardhani BWK, Kurniasih DAA, Wibowo S. Potential of growth factors and steroid hormones from cell cultures to improve in vitro fertilization in bovine. Open Vet. J.. 2025; 15(9): 3961-3979. doi:10.5455/OVJ.2025.v15.i9.4 Vancouver/ICMJE Style Mulyati S, Rantam FA, Khairullah AR, Mustofa I, Akintunde AO, Ahmad RZ, Handayani UF, Khalisa AT, Anggraini L, Pratama BP, Latifah L, Wardhani BWK, Kurniasih DAA, Wibowo S. Potential of growth factors and steroid hormones from cell cultures to improve in vitro fertilization in bovine. Open Vet. J.. (2025), [cited January 12, 2026]; 15(9): 3961-3979. doi:10.5455/OVJ.2025.v15.i9.4 Harvard Style Mulyati, S., Rantam, . F. A., Khairullah, . A. R., Mustofa, . I., Akintunde, . A. O., Ahmad, . R. Z., Handayani, . U. F., Khalisa, . A. T., Anggraini, . L., Pratama, . B. P., Latifah, . L., Wardhani, . B. W. K., Kurniasih, . D. A. A. & Wibowo, . S. (2025) Potential of growth factors and steroid hormones from cell cultures to improve in vitro fertilization in bovine. Open Vet. J., 15 (9), 3961-3979. doi:10.5455/OVJ.2025.v15.i9.4 Turabian Style Mulyati, Sri, Fedik Abdul Rantam, Aswin Rafif Khairullah, Imam Mustofa, Adeyinka Oye Akintunde, Riza Zainuddin Ahmad, Ulvi Fitri Handayani, Andi Thafida Khalisa, Lili Anggraini, Bima Putra Pratama, Latifah Latifah, Bantari Wisynu Kusuma Wardhani, Dea Anita Ariani Kurniasih, and Syahputra Wibowo. 2025. Potential of growth factors and steroid hormones from cell cultures to improve in vitro fertilization in bovine. Open Veterinary Journal, 15 (9), 3961-3979. doi:10.5455/OVJ.2025.v15.i9.4 Chicago Style Mulyati, Sri, Fedik Abdul Rantam, Aswin Rafif Khairullah, Imam Mustofa, Adeyinka Oye Akintunde, Riza Zainuddin Ahmad, Ulvi Fitri Handayani, Andi Thafida Khalisa, Lili Anggraini, Bima Putra Pratama, Latifah Latifah, Bantari Wisynu Kusuma Wardhani, Dea Anita Ariani Kurniasih, and Syahputra Wibowo. "Potential of growth factors and steroid hormones from cell cultures to improve in vitro fertilization in bovine." Open Veterinary Journal 15 (2025), 3961-3979. doi:10.5455/OVJ.2025.v15.i9.4 MLA (The Modern Language Association) Style Mulyati, Sri, Fedik Abdul Rantam, Aswin Rafif Khairullah, Imam Mustofa, Adeyinka Oye Akintunde, Riza Zainuddin Ahmad, Ulvi Fitri Handayani, Andi Thafida Khalisa, Lili Anggraini, Bima Putra Pratama, Latifah Latifah, Bantari Wisynu Kusuma Wardhani, Dea Anita Ariani Kurniasih, and Syahputra Wibowo. "Potential of growth factors and steroid hormones from cell cultures to improve in vitro fertilization in bovine." Open Veterinary Journal 15.9 (2025), 3961-3979. Print. doi:10.5455/OVJ.2025.v15.i9.4 APA (American Psychological Association) Style Mulyati, S., Rantam, . F. A., Khairullah, . A. R., Mustofa, . I., Akintunde, . A. O., Ahmad, . R. Z., Handayani, . U. F., Khalisa, . A. T., Anggraini, . L., Pratama, . B. P., Latifah, . L., Wardhani, . B. W. K., Kurniasih, . D. A. A. & Wibowo, . S. (2025) Potential of growth factors and steroid hormones from cell cultures to improve in vitro fertilization in bovine. Open Veterinary Journal, 15 (9), 3961-3979. doi:10.5455/OVJ.2025.v15.i9.4 |