| Review Article | ||
Open Vet. J.. 2025; 15(9): 3980-4006 Open Veterinary Journal, (2025), Vol. 15(9): 3980-4006 Review Article Antibiotic resistance in mastitis-causing bacteria: Exploring antibiotic-resistance genes, underlying mechanisms, and their implications for dairy animal and public healthShima Kazemzadeh1,2*, Olga Korneeva1, Sergey Shabunin3 and Mikhail Syromyatnikov1,41Laboratory of Metagenomics and Food Biotechnology, Voronezh State University of Engineering Technologies, Voronezh, Russia 2Department of Biochemistry and Biotechnology, Voronezh State University of Engineering Technologies, Voronezh, Russia 3FSBSI All-Russian Veterinary Research Institute of Pathology, Pharmacology and Therapy, Voronezh, Russia 4Department of Genetics, Cytology and Bioengineering, Voronezh State University, Voronezh, Russia *Corresponding Author: Shima Kazemzadeh. Laboratory of Metagenomics and Food Biotechnology, Voronezh State University of Engineering Technologies, 394036 Voronezh, Russia. Email: shimakazemzadeh [at] yahoo.com Submitted: 01/04/2025 Revised: 29/07/2025 Accepted: 12/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
ABSTRACTThe development and spread of superbugs, which are bacterial strains resistant to several types of antibiotics, threatening the lives of myriad people and animals worldwide, is one of the most concerning issues facing both global and animal health. Dairy animals are considered to be key reservoirs of antibiotic-resistant bacteria, which are closely correlated with the widespread and inappropriate application of antibiotics in agriculture and veterinary medicine, particularly for mastitis treatment. Although antimicrobial agents are administered in dairy farming for various conditions beyond mastitis, such as respiratory infections and digestive disorders, as well as prophylaxis and growth promotion, the most common reason for antimicrobial use in this industry is mastitis treatment. Since raw milk can be contaminated with opportunistic pathogens carrying antimicrobial resistance genes, these pathogens increase the gene pool from which pathogenic bacteria can acquire resistance traits. Indeed, these resistance genes may be horizontally transferred from livestock to human pathogens through mobile genetic elements through the consumption of raw milk. This phenomenon poses a global health threat, emphasizing the necessity of applying the “One Health” approach in global health and medicine to safeguard animal health and public health. Given the high prevalence and economic impact of mastitis and the evidence supporting mastitis as a major driver of antimicrobial use in dairy farming, this review summarizes recent genomic and metagenomic studies on major mastitis-causing pathogens (Staphylococcus aureus, Escherichia coli, Streptococcus spp., and Pseudomonas spp.) in dairy animals, detailing their primary resistance mechanisms. We highlight advanced surveillance tools, such as metagenomics, whole-genome sequencing, and quantitative polymerase chain reaction, for the rapid detection of resistance genes and mobile elements in the dairy chain. Keywords: Antimicrobial resistance, Mastitis, Opportunistic pathogens, Resistance genes, Veterinary. IntroductionOver the last few decades, the diagnosis and control of human foodborne diseases have become one of the main public health challenges worldwide. Dairy foods have been reported as the confirmed source of several foodborne illnesses; thus, this category of products, especially raw milk, should be consumed with caution and health supervision (Sapp et al., 2023). Potential health risks in raw milk arise from the presence of opportunistic pathogens that can cause foodborne illnesses. Contrary to old beliefs, milk is not sterile; however, it is a diverse ecosystem inhabited by myriad of beneficial or pathogenic microorganisms. Despite the nutritional and probiotic properties of beneficial microbes, which confer health benefits on the gastrointestinal tract and host immune system, the development of antibiotic resistance in potentially harmful bacterial populations is a growing concern (Rubiola et al., 2020; Hassani et al., 2022). The microbial communities of raw milk are shaped by various sources, including the udder, the exterior of the teats, milking equipment, and the farm environment (Ouamba et al., 2022). Some of these communities may carry antibiotic resistance genes (ARGs), and in certain instances, these resistance genes may be horizontally transferred to human pathogens by mobile genetic elements, namely plasmids or transposons (Manimaran et al., 2025). A recent shotgun-metagenomic analysis performed on raw bovine milk revealed over 150 different ARGs, including β-lactamases, aminoglycoside-modifying enzymes (AMEs), and tetracycline efflux pumps, carried on diverse plasmids and transposons (da Silva Duarte et al., 2025). In 2025, a whole-genome metagenomic study of raw milk identified a link between ARGs and mobile genetic elements. This research demonstrated that ARGs found on contigs carrying plasmid replicons or phage-associated recombinase genes, providing direct evidence of active horizontal gene transfer (HGT) within the milk microbiome (Manimaran et al., 2025). Notably, antibiotic-resistant bacteria can infiltrate milk as a consequence of prolonged and inappropriate administration of antibiotics to dairy cattle as part of veterinary therapies (Kovačević et al., 2022; Morales-Ubaldo et al., 2023). However, in 2024, a 15-month surveillance study from European pasture-based dairy farms used culture-independent qPCR of raw milk filters and detected low but detectable levels of ARGs, including aadA, sul1, and tetM, which shows the “silent background” ARG load in milk even without recent antibiotic usage (Collis et al., 2024). Although antibiotics are used for various purposes in the dairy and farming industry, their major application is in the treatment of mastitis, from treating different bacterial infections in the respiratory and digestive systems to serving as growth promoters or prophylactic agents. According to the 2014 report on U.S. dairy operations by the USDA’s National Animal Health Monitoring System, with some supporting evidence from recent studies by Farrell et al. (2023) and Ruegg et al. (2021), treatment of clinical mastitis in adult dairy cows is the most common reason for antimicrobial use. Given the high prevalence and economic impact of mastitis, the evolution of antibiotic-resistant bacteria, developed through various mutations and selective pressure, poses a global threat to both human and livestock health. Therefore, the implementation of genomic surveillance to achieve a deeper understanding of bacterial dynamics, resistance mechanisms adopted by different bacterial strains, and resistance distribution through dairy consumption has been subject to much debate over time. This review article focuses on antibiotic-resistant bacteria in raw milk associated with mastitis, examining their prevalence, resistance mechanisms, and health risks. Moreover, it addresses the potential distribution of resistance genes in the dairy chain, highlighting their role in the broader challenge of antibiotic resistance. Consequently, the growing global concern about antimicrobial resistance (AMR) emphasizes the need to identify the etiology and effective countermeasures. Milk microbiotaSeveral studies have examined the microbiome of milk, particularly in humans and cattle, using non-culture methods. Although milk was traditionally believed to be sterile, investigations have identified a diverse range of microorganisms in milk, including bacteria, fungi, and viruses (Lima et al., 2018). Staphylococcus, Streptococcus, Escherichia coli, Pseudomonas, and Bifidobacterium are the most frequently detected bacterial species in both human and bovine milk (Nguyen et al., 2020). These microorganisms have a significant impact on human and livestock health, contributing to the development of the immune system and gastrointestinal tract of infants, although the composition of milk microbiota could vary between individuals and species (Oikonomou et al., 2020). Nonetheless, some studies propose a shared composition of milk microbiota across different host species, necessitating further research to comprehend its composition, functions, and potential implications for host health and development (Li et al., 2017). The composition of the milk microbiota depends on several factors, including the milk source, sanitation, and environmental factors (Yuan et al., 2022). Reviewing microbiological articles reveals milk-inhabiting microorganisms. Some bacterial species with probiotic properties, such as Lactobacillus, Streptococcus, and Bifidobacterium, inhabit raw milk and play a major role in fermenting milk into products such as yogurt and cheese. Moreover, other species, such as Staphylococci, coliform bacteria, yeasts, and even molds, are abundant in raw milk. However, pathogenic bacteria, such as Salmonella, Listeria, and Campylobacter, have also been identified in raw milk. In addition to the beneficial health effects of milk microbiota, it may cause serious health risks, especially in cases of contamination or poor hygiene (Quigley et al., 2013; Dubey et al., 2022). MastitisMastitis, one of the most prevalent diseases in the dairy industry, is defined broadly as inflammation of the mammary gland and udder tissues due to trauma or microbial infections. Bacterial infections are considered the primary cause of mastitis; therefore, several studies have investigated the main bacterial species. According to these studies, Staphylococcus, particularly S. aureus, coagulase-negative staphylococci (CNS), E. coli, and Streptococcus species, namely S. agalactiae, S. uberi, and S. dysgalactiae, are commonly implicated in bovine mastitis (Srithanasuwan et al., 2023). Mastitis-causing microorganisms can be classified as either infectious or environmental. Infectious pathogens, such as Staphylococcus aureus and S. agalactiae, are adapted to survive within the mammary gland. Their transmission primarily occurred from cow to cow, especially during the milking process (Crespi et al., 2022). Conversely, environmental pathogens, namely, Streptococcus uberis and E. coli, which are opportunistic invaders that originate from the contaminated environment, cause infections typically between the milking process and during the dry period (Schneider et al., 2023). Bovine mastitis poses a significant economic burden on the global dairy industry and human health. The epidemiology of mastitis has undergone changes over the past 70 years, attributed to the implementation of regulations on the dairy industry as well as mastitis control measures (Campos et al., 2022). Mastitis control is complicated due to various factors, including the diversity of pathogens, antibiotic resistance, and management practices (Stanek et al., 2024). Furthermore, following the correct withdrawal periods post treatment, with the aim of preventing antimicrobial residues in milk, more challenges arise (de Oliveira et al., 2022; Pascu et al., 2022). Staphylococcus aureusStaphylococcus aureus, a member of the Bacillotais family, is a highly opportunistic pathogen that affects both humans and animals worldwide. Studies have shown that a significant portion of bovine mastitis cases, approximately 40%, is caused by this gram-positive bacterium (Sharifi et al., 2023). This condition leads to udder-related health issues in livestock, impacting milk quality and production. Improper milk processing is one of the most significant elements in the contamination of dairy products with S. aureus (Abebe et al., 2016). The pathogenesis of S. aureus is mediated by the release of toxins, such as Panton–Valentine leukocidin and exfoliative toxins, or other virulence factors located on the bacterial surface. It has been demonstrated that the contribution of these cell-surface proteins to bacterial functions is quite important, namely, in the formation of biofilms, bacterial adhesion to target cells, invading host cells by proteolytic enzymes or inducing endocytosis, and bacterial evasion of innate immune defenses (Mbindyo et al., 2021). Staphylococcal infections can lead to potentially life-threatening conditions by suppressing the immune system and worsening inflammation (Abril et al., 2020; Naranjo-Lucena and Slowey, 2023). Antibiotic resistance of S. aureusAntibiotic prescription, particularly udder infusion, is considered the most predominant therapeutic strategy for treating and preventing bovine mastitis caused by S. aureus (Yang et al., 2023). Nonetheless, these therapeutic approaches promote the possibility of antibiotic resistance in S. aureus through previously noted mechanisms (Majumder et al., 2023; Silva et al., 2023). Khanal et al. (2022) conducted a recent systematic review and meta-analysis and estimated the pooled prevalence of methicillin-resistant S. aureus (MRSA) in dairy farms to be 4.12%, highlighting the ongoing concern of MRSA in dairy production (Khanal et al., 2022). Notably, the presence of resistance genes on transferable genetic elements, such as plasmids and transposons, facilitates the horizontal transfer of resistance between bacterial strains (Liu et al., 2022). The emergence of antibiotic-resistant S. aureus, with prolonged hospitalization, elevated morbidity and mortality rates, and reduced dairy-industry production and quality, poses a global threat to both human and livestock health, emphasizing the necessity of “One Health Approach” in this regard (Zhang et al., 2022; Silva et al., 2023). Regarding intrinsic resistance, S. aureus exhibits reduced fitness when exposed to certain antibiotics, activated by intrinsic factors such as mprF, ndh, fmtA, graR, or dltA. Chromosomally encoded multidrug-efflux pumps such as NorA or LmrS contribute to resistance or reduced susceptibility to specific antibiotics. Various resistance genes have been identified in Staphylococci from bovine mastitis, including those associated with resistance to trimethoprim (dfrA, dfrD, dfrG, and dfrK), fluoroquinolones (gyrA mutation, grlA mutation, and mepA), phenicols (fexA), and sulfonamides (AMR-associated residues in the folP gene) (Kadlec et al., 2012; Xu et al., 2014; Shoaib et al., 2023). Additionally, some Staphylococci, such as S. aureus, that are not inherently resistant to novobiocin can acquire resistance through the accumulation of point mutations in the genes parE and gyrB (Lee et al., 2022). CNS intrinsically resists novobiocin due to the expression of a novobiocin-resistant GyrB protein (Jensen and Lyon, 2009; Silva et al., 2023). Intrinsic resistance factors may lead to reduced susceptibility to specific antimicrobials, and acquired resistance mechanisms may coexist in some isolates (Pérez et al., 2020; Naranjo-Lucena and Slowey, 2023). Staphylococcus species isolated from bovine mastitis cases and milk samples demonstrate acquired resistance to various antibiotic classes, which will be discussed further in the future. Mechanisms of S. aureus resistance to β-lactamsThe extensive and occasionally irresponsible use of β-lactam antibiotics, including penicillins, cephalosporins, and related compounds, in clinical and agricultural settings has contributed to the emergence and widespread distribution of resistant isolates. In the 1940s and 1950s, methicillin was introduced as a therapeutic solution by medical societies, although in the 1970s, the initial identification of MRSA made it a serious global concern in livestock infections and healthcare. (Naranjo-Lucena and Slowey, 2023). MRSA has become one of the most concerning universal health issues due to its resistance not only to new semisynthetic β-lactams, but also to a broad spectrum of antimicrobial agents, particularly aminoglycosides, macrolides, chloramphenicol, tetracycline, and fluoroquinolones (Liang et al., 2022). In the livestock industry, MRSA was originally isolated from dairy cows with mastitis, which were considered the natural host of MRSA. Indeed, MRSA has a significant clinical impact on the treatment and management of clinical mastitis, as these coagulase-positive Staphylococcus along with methicillin-resistant coagulase-negative Staphylococcus (MRCoNS), particularly Staphylococcus epidermidis, were found in cow’s milk with mastitis (Nelli et al., 2022). Therefore, MRS transmission puts dairy cows and individuals in contact with them at high risk. Furthermore, the emergence of these resistant isolates poses a potential health threat for those consuming or handling raw milk products (Khanal et al., 2022; Nandhini et al., 2022). Despite the existence of different genetic bases for resistance to β-lactams, some regulatory mechanisms are similar (Mlynarczyk-Bonikowska et al., 2022). It is worth mentioning that β-lactamase enzymes have generated significant interest due to their ability to confer resistance to penicillin and methicillin, the most commonly used family of antibiotics. It has been extensively proven that the blaZ gene, encoding PC1 β-lactamase, is responsible for penicillin resistance in S. aureus and CNS species isolated from mastitis cases. This enzyme hydrolyzes the β-lactam ring of penicillin, making it inactive. Its expression is induced by β-lactam antibiotics through a regulatory system involving the sensor protein BlaR1 and repressor BlaI, where BlaR1 detects the antibiotic and inactivates BlaI, allowing blaZ transcription (Lade and Kim, 2023). According to cytogenetic mapping and sequence inspection, this gene is situated in the mobile genetic element transposon Tn552, either in the bacterial chromosome or a plasmid, and is regulated by blaR1 and blaI genes (Rocha et al., 2022; Alexander et al., 2023). In the molecular basis, in terms of resistance to methicillin and oxacillin, the gene encoding PBP2a with low β-lactam affinity, known as mecA, has been identified. PBP2a replaces penicillin-binding proteins during cell wall synthesis and enables peptidoglycan cross-linking even in the presence of β-lactams. β-lactam exposure normally induces the expression of mecA gene via the mecR1/mecI system. However, many MRSA strains have deletions in these regulators and rely on the blaR1/blaI system for mecA activation (Lade and Kim, 2023). This gene is localized within a unique segment of DNA known as the Staphylococcal chromosome cassette (SCC), which is a mobile genetic element integrated into the bacterial chromosome at a specific site. Gene expression analysis revealed that mecA expression is regulated by the inducer-repressor genes mecR1 and mecI. Although the mechanism of genetic transfer is not fully understood, it involves conjugation, transduction, and transformation (Uehara, 2022). Thirteen types of SCCmec have been identified, and a mecA homolog, mecC, has been shown to confer resistance to penicillinase-resistant penicillins. The mecC gene encodes PBP2c, which has a low affinity for β-lactams and is mainly detected in strains associated with livestock. Similar to mecA, its expression is induced under β-lactam stress (Dierikx et al., 2023). Moreover, the CNS, which carries mecA genes in SCCmec elements, plays a crucial role in the spread of resistance. Methicillin resistance attributed to mecA is frequently detected in Staphylococcus species, which can lead to bovine mastitis (de Moura et al., 2023). Nonetheless, methicillin-susceptible isolates carrying mecA have been reported. These bacterial isolates may appear phenotypically susceptible due to the suppression of mecA expression or mutations, but they can rapidly develop resistance under selective pressure, which shows the importance of genotypic and phenotypic testing to ensure precise and reliable results (Lade and Kim, 2023). Furthermore, investigations have reported another isolate, characterized as oxacillin-sensitive mecA-positive S. aureus, in clinical settings and animal products, which is frequently misidentified as methicillin-sensitive S. aureus despite carrying mecA. This may lead to the development of high levels of resistance under β-lactam antibiotic selection pressure (Martins et al., 2015; Ning et al., 2023). Mechanisms of S. aureus resistance to tetracyclinesHaving a broad antimicrobial spectrum, tetracyclines are widely prescribed in human and veterinary medicine to treat an extensive variety of infections, especially Staphylococcal infections (Scaria et al., 2021). Tetracyclines interfere with protein synthesis in bacterial cells by binding to 30s ribosomal subunits and blocking the attachment of tRNA to the acceptor site in the ribosomal complex, thus inhibiting protein formation (de Freitas et al., 2023). In S. aureus, resistance develops either through ribosomal protection, encoded by the tetM gene, or an efflux pump system, encoded by tetK (Bitrus et al., 2018). tetM encodes a ribosomal protection protein that binds to the 30S subunit and displaces tetracycline, thereby restoring protein synthesis; its expression is triggered by sub-inhibitory levels of tetracycline that activate the promoter of the conjugated transposon (Shi et al., 2025). tetK and the related tetL encode efflux pumps of the major facilitator superfamily located in the bacterial membrane that export tetracycline out of the cell. These pumps are often plasmid-encoded and induced under antibiotic stress, with a prevalence of up to 45% in mastitis-associated isolates (Kakooza et al., 2024). Both resistance mechanisms are induced by tetracycline subinhibitory concentrations. Analyzing mastitis sampleshas proven that Staphylococcal resistance in mastitis isolates involves tetK and tetL genes, coding for membrane-associated efflux proteins, which are transferred by plasmids, while tetM gene is located in conjugative transposons. Moreover, chromosomally encoded tet38 can be overexpressed from a plasmid. These resistance genes have been identified in S. aureus and CNS from dairy farms globally (Alian et al., 2012). In a survey of dairy herds in China in 2023, tetK was detected in 12.2% of S. aureus and 17.5% of CNS isolates (with tetM in 9.9% and 12.5%, respectively), and all tetK-positive isolates were phenotypically tetracycline-resistant (Yang et al., 2023). Investigations revealed that tetK, tetL, and tetM are more prevalent in non-aureus isolates, and isolates may contain combinations of tetK and tetM (Karzis et al., 2021). Notably, tetracycline-resistant S. aureus strains are more frequently isolated from farmers and veterinarians in contact with livestock, especially pigs (Pérez et al., 2020). Mechanisms of S. aureus resistance to aminoglycosides and aminocyclitolsAminoglycosides are bactericidal antimicrobial agents that are widely used worldwide against mastitis infections and disrupt protein synthesis by targeting ribosomal RNA. Resistance to aminoglycosides can develop through the acquisition of AMEs, which add specific chemical molecules to the compound, or through 16S rRNA ribosomal protein-targeting mutation (Zhang et al., 2023). Key AMEs include Aph(3′)-IIIa, an ATP and Mg2+ dependent phosphotransferase that catalyzes the phosphorylation of the 3′ hydroxyl of the aminoglycoside ring; Aac(6′)-Ie–Aph(2″)-Ia, a bifunctional enzyme with GCN5-related N-acetyltransferaseacetyltransferase and phosphotransferase domains targeting the 6′ amino and 2″ hydroxyl positions; and AadD, an adenylyltransferase that transfers adenosine monophosphate to the 4′ hydroxyl. These modifications sterically block drug binding to the site of the 16S rRNA and eliminate its ability to induce misreading (Naderi et al., 2025). According to studies on Staphylococcal mastitis, the development of resistance to aminoglycosides is primarily associated with aphA3, aacA-aphD, and aadD genes, which encode inactivating enzymes. Genome analyses have demonstrated that these AME genes are primarily carried on Tn4001- or Tn5405-related transposons, which facilitates the rapid horizontal spread of staphylococci in antibiotic-treated livestock (Aguirre-Sánchez et al., 2024). The aphA3 gene encodes a phosphotransferase, which is responsible for conferring resistance to kanamycin, neomycin, and amikacin (Mushtaq et al., 2019). Conversely, acetyltransferase and phosphotransferase, which are encoded by the gene aacA-aphD, develop resistance to gentamicin, kanamycin, tobramycin, and amikacin when overexpressed. Resistance genes may transpose between plasmids or be present within transposons in the bacterial chromosome (Wang et al., 2022). In a Chinese investigation on Staphylococcal species associated with bovine mastitis, the aphA3 gene was reported to be more prevalent in non-aureus isolates, while the aacA-aphD gene was commonly identified in S. aureus (Qu et al., 2019). Furthermore, other resistance genes have been detected in mastitis S. aureus and CNS isolates, namely aadE, ant(6)-Ia, and str, which mediate streptomycin resistance. Moreover, class 1 integrons containing aadA1/dfrA1 cassettes occur together with AMEs, which link aminoglycoside and trimethoprim resistance in approximately 30% of bovine isolates (Haq et al., 2024). Remarkably, these genes, along with lsaE and lnuB genes, are part of a multi-resistant gene cluster in Staphylococcal mastitis (Naranjo-Lucena and Slowey, 2023). In addition to S. aureus mastitis isolates, CNS from bovine mastitis milk was genetically examined, which led to the detection of other aminoglycoside resistance genes, including aph(3′)-IIIa, ant(4′)-Ia3, and aac(6′)/aph(2ʺ)-3, in these samples (Schwarz et al., 2018). Moreover, in the case of aminocyclitols, genes spc and spw (spectinomycin resistance genes) were identified in methicillin-resistant S. aureus and CNS from bovine mastitis (Omwenga et al., 2021). Spectinomycin resistance results from Spc acetyltransferases that use acetyl-CoA to modify the 9-amino groups of spectinomycin, blocking its binding to the head domain of the 30S subunit. Similar to pT181, spc/spw are often carried on rolling circle plasmids induced by sub-Minimum Inhibitory Concentration spectinomycin (Haq et al., 2024). Recent studies have characterized class 1 integrons and gene cassettes in S. aureus isolated from cows with mastitis. Gene cassettes, specifically dfrA1-aadA1, aadA2, dfrA12-orfX2-aadA2, and aadA1, were prevalent in mastitis isolates, leading to phenotypic resistance to aminoglycosides and, in some cases, resistance to trimethoprim-sulfamethoxazole (Naranjo-Lucena and Slowey, 2023). Additionally, a new finding involved the identification of a class 1 integron in S. aureus isolated from Ruminants, which contains a dfrA15 gene cassette (Li and Zhao, 2018). This is significant because integrons in gram-positive bacteria are not well characterized, whereas increasing evidence indicates similar findings in human-derived isolates. This could highlight the development of multidrug resistance in S. aureus associated with the presence of class 1 or 2 integrons (El-Baz et al., 2021). Mechanisms of S. aureus resistance to macrolides, lincosamides, and streptograminsEvolution of resistance to the macrolide-lincosamide-streptogramin B class (MLS) of antibiotics in S. aureus involves methylation of ribosomal receptor binding sites. Erythromycin ribosomal methyltransferase (ErmA/C) dimethylate the N6 position of A2058 in domain V of 23S rRNA using S adenosyl L methionine. This prevents hydrogen bonding with the macrolactone ring, which blocks drug binding and confers MLS B cross-resistance (Shi et al., 2025). As the MLS binding sites on 23S rRNA overlap, adenine methylation causes cross-resistance toward these three antibiotic classes, although they are structurally unrelated. Erythromycin ribosomal methyltransferase (Erm), encoded by ermA, ermB, and ermC genes, is responsible for the catalysis and post-transcriptional modification of the 23S rRNA structure (Wendlandt et al., 2015; Bitrus et al., 2018). Erm gene-mediated methylase expression in S. aureus is either inducible or constitutive, with macrolides inducing erm gene expression facilitated by an efflux pump system, which is encoded by the msrA gene (Mahdavi et al., 2019). MsrA is an ATP-binding ABC efflux transporter specific for macrolides and streptogramin B that hydrolyzes ATP to export these antibiotics out of the cytoplasm. The global regulator MgrA upregulates its expression under antibiotic pressure (Monistero et al., 2025). This mechanism does not lead to resistance to streptogramins or lincosamide (Tsitou et al., 2021). Generally, in the context of MLSB antibiotics used in Staphylococcal infections, the major associated genes include erm, msr, mph, vat, and lnu (Andreadis, 2020). ErmA is highly prevalent in MRSA, whereas ermB is highly prevalent in Livestock-associated MRSA (Rahi et al., 2020). Moreover, the msrA and msrB genes encode efflux pump proteins, providing MLSB resistance in Staphylococcal mastitis, whereas the others, such as mphC, ereA, lnuA, and lnuB, encode modifying enzymes. More accurately, mphC encodes a macrolide 2′-phosphotransferase that transfers the γ-phosphate of guanosine triphosphate to the 2′-hydroxyl of the desosamine sugar, inactivating erythromycin; ereA codes for esterase, along with lnuA and lnuB genes that encode nucleotidyltransferase, leading to antibiotic resistance by enzymatic-target-modification. lnuB is a uridine triphosphate-dependent lincosamide nucleotidyltransferase that adds uridine monophosphate to lincomycin, both enzymes preventing drug ribosome interactions (Jamali et al., 2015; Antók et al., 2019; Yang et al., 2024). The detection of these resistance genes in both S. aureus and CNS isolates from mastitis cases indicates their widespread presence, except for salA, which has only been identified in S. sciuri from bovine mastitis. The presence of isolates with multiple resistance genes raises concerns for treating staphylococcal infections in both humans and animals (Wendlandt et al., 2013). Mechanisms of S. aureus resistance to vancomycinPharmacologically, vancomycin is prescribed as the last-line defense against gram-positive bacteria resistant to other antibiotics. Vancomycin interferes with the synthesis of bacterial cell walls by inhibiting peptidoglycan layer transpeptidation. In other words, it prevents the formation of proper cross-linking of peptidoglycan in the bacterial cell wall. Despite the widespread use of this antimicrobial agent to treat S. aureus infections, vancomycin-resistant S. aureus (VRSA) has been increasingly reported in various regions of the world (Muzammil et al., 2023). Two distinct mechanisms have been determined by which resistance develops in S. aureus. The first is VanA-mediated resistance, which is associated with the vanA operon located on a transposable element, encoding a dehydrogenase, VanH. The vanA operon (vanR/S–vanH–vanA–vanX–vanY) is regulated by the VanR/VanS two-component system: VanS autophosphorylate upon glycopeptide binding, transferring the phosphate to VanR, which then activates PvanA (Gökdağ and Çiftci, 2021). It is noteworthy that acquisition of VanA-mediated resistance in S. aureus is likely acquired through in vivo transfer of the vanA operon from vancomycin-resistant Enterococci (Mohamadi et al., 2023; Zahid et al., 2023). Investigations provide additional insight into the molecular evolution of VanA-mediated resistance involving transposon Tn1546. VanA ligase synthesizes d-Ala-d-Lac, leading to the formation of depsipeptides. In contrast, VanX hydrolyzes residual d-Ala-d-Ala, and VanY removes the C-terminal d-Ala, resulting in target modification and inducing vancomycin resistance (Dong, 2023; Weigel et al., 2003). The second mechanism is resistance due to a thickened cell wall, which is associated with S. aureus showing intermediate resistance to vancomycin (VISA) (Srinivasan et al., 2002). VISA results from point mutations in the two-component regulators WalKR and VraSR, leading to upregulation of cell wall biosynthesis genes (e.g., murF, pbp2) and increased peptidoglycan synthesis but decreased cross-linking, which creates a deceptive “false target” layer that separates vancomycin molecules before they reach the cytoplasmic membrane (Tartor et al., 2024). Based on these findings, VISA strains exhibit reduced susceptibility to vancomycin by cell wall thickening and decreased peptidoglycan cross-linking, resulting in decreased vancomycin uptake (Bitrus et al., 2018). Multidrug-resistant S. aureusThe emergence of multidrug-resistant (MDR) and extensively drug-resistant strains of S. aureus has raised global concerns about their potential impact on human and animal health, as well as livestock-derived products. Milk from mastitis cases is considered one of the most dominant reservoirs of these MDR strains (Mbindyo et al., 2021). MDR phenotypes often combine β-lactamases, AMEs, Tet efflux pumps, Erm methylases, and Msr/Mph enzymes, with colocalization on large mosaic plasmids (e.g., pSK1 family) containing multiple resistance operons and toxin/adhesion sites. Intensive antibiotic use selects for the maintenance of these plasmids through toxin–antitoxin addiction modules (Aguirre-Sánchez et al., 2024; Na et al., 2025). The decreased effectiveness of antimicrobial agents against mastitis pathogens, including Staphylococcus species, is partially attributed to the development of multidrug resistance, emphasizing the establishment of effective veterinary surveillance methods as well as antimicrobial susceptibility testing (Mbindyo et al., 2021; Ghali-Mohammed et al., 2023). Monitoring and evaluating the AMR rates in S. aureus isolates, along with the prevalence of MRSA and VRSA in raw milk, is of paramount importance for investigations and the development of novel antibiotics and resistance-modifying agents. Moreover, this can facilitate the identification of appropriate antimicrobial treatments for animal diseases from a food safety perspective (Ning et al., 2023). Escherichia coliEscherichia coli is one of the most frequent bacterial etiologies of acute clinical mastitis. Although this Gram-negative bacterium is a normal intestinal microbiota in endothermic organisms, it causes several infections in humans and animals(Mwasinga et al., 2023). The emergence of MDR E. coli strains, frequently discovered in dairy cattle feces, unpasteurized cow’s milk, and culled dairy cow beef, poses a serious challenge for livestock and public health (Massé et al., 2023). Nevertheless, studies have revealed that some of these MDR E. coli strains can contaminate various sources even without the administration of antimicrobial therapies. Furthermore, it has been shown that young calves carry higher resistance levels than older cows, regardless of drug exposure (Hang et al., 2019). Indeed, these calves could acquire ARBs from their dams or the surrounding environments; however, several genetic elements contribute to the enrichment and persistence of these bacterial species in their gut (Haley et al., 2023). In addition to the fecal-oral route, the transmission of resistant E. coli is constantly occurring in dairy cattle, particularly in livestock milk, which is rich in genetically diverse E. coli strains carrying ARGs (Widodo et al., 2023a). Indeed, this close interaction between humans and livestock provides a pathway for AMR transmission, leading to the integration of ARGs into the human intestinal microbiota through HGT (Dowidar and Khalifa, 2023). Furthermore, the increased incidence of extended-spectrum β-lactamase (ESBL)-producing E. coli in livestock and dairies is the most concerning aspect (Nahar et al., 2023). It is noteworthy that AMR monitoring has traditionally relied on phenotypic tests; however, nowadays, whole genome sequencing enhances phenotypic techniques, offering a more comprehensive approach (Haley et al., 2023; Massé et al., 2023). Mechanisms of E. coli resistance to β-LactamsThe production of β-lactamases is the major defense mechanism of gram-negative bacteria against β-lactam antibiotics, contributing to global β-lactam resistance challenges in mastitis pathogens. In bovine mastitis caused by E. coli, enzymes, including Temoniera β-lactamase-1, SHV-1, and SHV-2, which are plasmid-encoded β-lactamases, catalyze the hydrolysis of the amide bond in the β-lactam ring (Murinda et al., 2019). Mastitis isolates in China have been shown to carry blaTEM and blaCTX-M simultaneously, both of which encode class A β-lactamases that hydrolyze extended-spectrum cephalosporins (Xu et al., 2022b). According to the in vitro experiments conducted by Speksnijder et al. (2022), intramammary administration of first-generation cephalosporins has been demonstrated to select for ESBL-producing E. coli in bovine feces, which highlights the risk of resistance development from antibiotic use in mastitis treatment (Speksnijder et al., 2022). TEM-type enzymes are variants of the original plasmid-mediated β-lactamases, which are responsible for 90% of ampicillin resistance in E. coli, with over 170 variants. Bacteriophage-associated AMR has been demonstrated in mastitis-causing E. coli, revealing the significance of bacteriophages as reservoirs for resistant blaTEM genes, which contribute to β-lactam resistance distribution (Widodo et al., 2023b). Moreover, another globally prevalent plasmid-encoded β-lactamase is sulfhydryl-variable (SHV), encoded by the blaSHV gene, exhibiting diverse resistance patterns (Ramasamy et al., 2021). In Bangladesh, blaSHV coexisted with AmpC-type DHA β-lactamase, which confers resistance by hydrolyzing the β-lactam ring of penicillins and 3rd-generation cephalosporins (e.g., ceftazidime) (Afroz et al., 2024). Along with HGT-mediated β-lactamase expression, the porin impenetrability of the external membrane of E. coli and efflux pumps collectively affect resistance to β-lactams, particularly carbapenem (El-Halem et al., 2021; Hussain et al., 2021). Metallo-β-lactamases (MBLs), including Imipenemase, Seoul Imipenemase, German Imipenemase, Sao Paulo Metallo-β-lactamase, New Delhi Metallo-β-lactamase, and Verona Integron-encoded Metallo-β-lactamase, are another group of β-lactamases that hydrolyze most β-lactam agents, especially carbapenems, contributing to the development of broad-spectrum β-lactam resistance (Tewari et al., 2019). The first report of an E. coli isolate carrying MBL (NDM-5) was reported from a milk sample of mastitis cattle in eastern India in 2012 (Ghatak et al., 2013). Furthermore, the co-occurrence of MBL genes, including blaIMP-1, blaKPC-3, blaNDM-1, and blaVIM-2, has been reported in E. coli isolates globally (Mollenkopf, 2017; Hussain et al., 2021). Moreover, studies have provided insights into other groups of ESBLs detected in mastitis E. coli isolates, namely CTX-M-15, CTX-M-1, CTX-M-2, and SHV-12, as well as carbapenemase-producing Enterobacteriaceae. In addition to posing a threat to livestock health by intensifying bacterial resistance, these ESBL-producing E. coli increase nosocomial infections in humans. Although ESBLs initially developed resistance to first-generation β-lactams, they gradually evolved to be resistant to oxyimino-cephalosporins (Skočková et al., 2015; Oliver et al., 2020). Thus, the evolution of carbapenemases and ESBLs, namely Klebsiella pneumoniae carbapenemase, IMP, VIM, Oxacillinase, and NDM, necessitates the development of more effective therapeutic approaches to E. coli mastitis (Uyanik et al., 2022). Mechanisms of E. coli resistance to tetracyclinesThe expression of resistance genes, including tetA, tetB, tetC, tetD, and tetG, coding for an energy-dependent efflux pump system that exports tetracycline out of the cell, lowering the intracellular drug concentration and preventing it from binding the 30S ribosome, was investigated in E. coli isolates from mastitis (Ramasamy et al., 2021). For instance, the TetA protein encoded by the tetA gene in mastitis isolates functions as an antiporter, exchanging tetracycline for protons across the inner membrane. tetA is the dominant tet gene in mastitis isolates (Emon et al., 2024). For instance, a recent investigation revealed that more than 75% of isolates from buffalo subclinical mastitis carried the tetA gene (Chowdhury et al., 2025). Investigations have revealed that the integration of non-conjugative transposons (Tn1721 and Tn10) into plasmids accelerates the dissemination of the prevalent resistance genes tetA and tetB (Poirel et al., 2018). Moreover, these genes have been identified in E. coli isolates from various countries, including Ireland, Switzerland, the United States, and Germany, despite the variations in their prevalence, as recent research revealed specific patterns in Jordan, China, the United States, and Iran. For instance, according to a recent Jordanian study, a high prevalence of tetB, tetE, and tetG genes has been detected in E. coli isolates from bovine mastitis (Naranjo-Lucena and Slowey, 2023). The distribution of tetracycline resistance genes varies in China, with tetC being the only gene detected in one study. Furthermore, in the United States, tetC was more prevalent than tetA and tetB (Lan et al., 2020; Yu et al., 2020). Monitoring these trends and the distribution patterns of resistance genes are essential tools for devising effective strategies to deal with antibiotic resistance globally (Ahmed et al., 2021). Mechanisms of E. coli resistance to aminoglycosidesInvestigations on E. coli from bovine mastitis revealed that theaminoglycoside-resistant isolates acquire resistance through two main mechanisms, namely, target modification and enzymatic inactivation (Majumder et al., 2021). Enzymatic inactivation in Enterobacteriaceae is generally mediated by three of the best-known types of AMEs, including acetyltransferases, phosphotransferases, and nucleotidyltransferases. Nonetheless, 16S RNA methylase, encoded by the rmtB gene, has also been identified and reported in E. coli isolates from raw milk in Ningxia Province, China (Yu et al., 2020). Resistance is mainly due to AMEs and 16S methyltransferases. Specifically, the aac(3)-IV gene has been detected in approximately 70% of mastitis isolates and encodes an enzyme that acetylates the 3-amino group of aminoglycosides, which inactivates the drug (Chowdhury et al., 2025). Regarding acetyltransferases, classified based on the acetylation position in the chemical structure of the drug, the aminoglycoside N-acetyltransferases AAC(3)-II/IV and AAC(6)-Ib are considered the most prevalent ones in E. coli, which are frequently found in gene cassettes in integrons. Based on previous studies, the gene variant aac(6’)-Ib-cr can simultaneously develop resistance to aminoglycosides and fluoroquinolones (Poirel et al., 2018). Moreover, gene cassettes in class 1 integrons contain codes for nucleotidyltransferase ANT(2”) and ANT(3”), commonly found in E. coli from food-producing animals. These adenyltransferases chemically modify aminoglycosides, preventing the binding of the 30S ribosomal subunit (Emon et al., 2024). Interestingly, these enzymes, encoded by the aadB and aadA (aadA1 and aadA5) genes, coexist with resistance genes targeting various antimicrobial classes within the integron. (Poirel et al., 2018; Urban-Chmiel et al., 2022). Furthermore, the strA and strB genes encode phosphotransferase APH(6)-Ia and APH(6)-Id, which contribute to streptomycin resistance. However, some evidence suggests that these genes are occasionally associated with the kanamycin resistance genes, aph(3″)-I/II genes (Poirel et al., 2018). Overall, resistance mechanisms to aminoglycosides may vary according to the diverse combinations of genes contributing to AMR in different bacterial isolates (Findlay et al., 2020). Mechanisms of E. coli resistance to quinolonesNumerous investigations have been conducted to discover the mechanism of resistance to quinolone in bacteria, as it became clear that mutations in chromosomal genes that encode recombinant enzymes are mainly responsible for the development of quinolone resistance in E. coli isolates. Indeed, the gyrA, gyrB, parC, and parE genes encoding DNA gyrase and topoisomerase IV are the key players in the antibiotic resistance scenario because they are essential bacterial enzymes for modulating chromosomal supercoiling, which is required for vital nucleic acid processes (Massella et al., 2021). Fluoroquinolone-resistant mastitis isolates show the classical double mutations in gyrA (Ser83→Leu and Asp87→Asn), often combined with diverse parC mutations, which reduce quinolone binding affinity. Mutations in gyrB and parE (e.g., GyrB-Pro385→Ala, ParE-Ser458→Ala) have been reported in these isolates, which contribute to higher-level fluoroquinolone resistance (Emon et al., 2024). Moreover, a Chinese study has identified a mutation rate of over 95% in the gyrA and gyrB genes in E. coli isolated from mastitis samples (Lan et al., 2020). Nonetheless, upregulation of some plasmid-borne quinolone resistance determinants, such as QepA and OqxAB, that contribute to the efflux pump system in bacteria, is considered a non-specific mechanism of antibiotic resistance in E. coli mastitis. The oqxAB gene not only contributes to quinolone resistance but also reduces susceptibility to other antimicrobial agents. Furthermore, plasmid-mediated resistance developed by either the qnr genes or aac(6′)-Ib-cr gene is more frequently observed in mastitis-causing E. coli. More precisely, qnrA, qnrB, and qnrS genes encode topoisomerase protective proteins, and aac(6′)-Ib-cr gene encodes aminoglycoside acetyltransferase AAC(6′)-Ib, which is crucial for quinolone resistance in E. coli (Li et al., 2019). More interestingly, resistance to ciprofloxacin is often associated with the development of resistance to nalidixic acid in E. coli, as a result of mutations in gyrA or parC genes (Mahdavi et al., 2022). Overall, further investigation into quinolone-resistant E. coli in mastitis is needed, since the interplay between chromosomal resistance and plasmid-mediated resistance contributes to the complexity of antibiotic resistance in this case (Kang et al., 2014). Mechanisms of E. coli resistance to sulfonamides and trimethoprimInvestigations into bovine mastitis have shown that resistance to sulfonamides and trimethoprim is mediated by drug-insensitive target enzymes encoded by sul and dfr genes (Emon et al., 2024). Several genes, namely sul1, sul2, and sul3, are mainly involved in developing plasmid-borne resistance to sulfonamide antibiotics in E. coli (Fazel et al., 2019). In updated surveys of mastitis E. coli, the sul1 gene, which encodes a resistant dihydropteroate synthase, was detected in over 50% of isolates (Chowdhury et al., 2025). Nonetheless, other AMR genes conferring resistance to sulfonamides have also been identified. A recent study in the United States detected mutations in the folP gene, which contribute to sulfonamide resistance in E. coli (Adnan et al., 2017). Furthermore, regarding trimethoprim resistance, studies proposed a large number of dfr genes identified in Enterobacteriaceae from bovine mastitis, which are typically located in the gene cassettes within integrons class 1 or 2 (Todorović et al., 2018). Nevertheless, only the following genes are frequently detected in E. coli mastitis: dfrA1, dfrA5, dfrA7, dfrA12, dfrA15, dfrA16, and dfrA17 (Ahmed et al., 2021). Approximately 30% of clinical mastitis isolates carried class-1 integrons with aadA and dfrA variants. These genes allow folate synthesis enzymes to function despite the presence of sulfonamides or trimethoprim, which may explain the high resistance rates (Emon et al., 2024). This highlights the diverse genetic mechanisms underlying trimethoprim resistance in mastitis-causing bacterial populations (Behera et al., 2023). Streptococcus SpeciesOver the years, more than 130 pathogens, including various Streptococcus species, have been identified as the main cause of bovine mastitis. Although many strains of Streptococcus are classified as commensal microbiota, some specific strains, such as S. agalactiae, S. dysgalactiae, and S. uberis, have been identified as the cause of severe diseases, such as mastitis (Kabelitz et al., 2021). Epidemiological studies have revealed the diverse prevalence of Streptococcus species in bovine mastitis. According to the Department of Agriculture, Food and the Marine, the most commonly isolated species reported in Ireland, France, Sweden, and Finland in 2021 were S. uberis and S. dysgalactiae. However, in Portugal and Germany, the prevalence of S. agalactiae has been reported to be equal to or greater than that of S. uberis and S. dysgalactiae (Naranjo-Lucena and Slowey, 2023). Thus, the high prevalence and emergence of antibiotic resistance in Streptococcus species due to the overuse of antimicrobial agents for mastitis treatment have made this opportunistic pathogen a global concern (Saed and Ibrahim, 2020). Mechanisms of Streptococcal resistance to β-LactamsRegarding the use of β-lactams, the prevalence of resistance to these antibiotics in Streptococcus isolates from bovine mastitis is low, although some studies have reported resistant isolates with decreased susceptibility to β-Lactams. Indeed, according to investigations, Streptococci face various challenges in acquiring exogenous β-lactam resistance genes by HGT (Lopardo et al., 2022; Ma et al., 2023). Nevertheless, it has been demonstrated that in S. uberis, resistance can be developed through substitution mutations in penicillin-binding proteins (PBPs), which lead to acquire resistance to penicillin and ampicillin (McDougall et al., 2020). Type II PBP (PBP2X) is a transpeptidase, which is essential for cell-wall crosslinking; mutations in PBP2X, such as E381K and Q554E, alter the conformation of the transpeptidase active-site loop, reducing the acylation rate of β-lactam antibiotics. More precisely, these mutations can decrease β-lactam binding or inhibit the enzyme (McDougall et al., 2020). The presence of the bl2b gene in both S. uberis and S. dysgalactiae has also been reported. Further investigations provided insights into the genetic basis of resistance development in these streptococcal strains, resulting in the identification of a large number of TEM genes in Strep. uberis (TEM-1, TEM-127, TEM-136, TEM-157, TEM-163, TEM-47, TEM-89, and TEM-95) and Strep. dysgalactiae (TEM-71, TEM-1, TEM-136, TEM-157, and TEM-47). Notably, the presence of these genes does not always correspond to phenotypic resistance (Reyes et al., 2019). According to the findings of a study in Poland, despite the presence of blaZ gene in Strep. uberis and Strep. dysgalactiae, most of these bacterial isolates exhibited phenotypic susceptibility to penicillin (Kaczorek et al., 2017). Furthermore, Chinese studies have reported a low correlation between phenotypic resistance and the presence of β-lactam resistance genes (Tian et al., 2019; Zhao et al., 2022). Mechanisms of Streptococci resistance to tetracyclinesAs reported, the prevalence of tetracycline resistance among different Streptococcal species may vary, whereas S. dysgalactiae frequently exhibits a higher prevalence of resistance. In this regard, various genes have been implicated, including tetK and tetL, which encode membrane efflux systems, and tetM, tetO, and tetS, which encode ribosomal protection enzymes (Donkin, 2016; Shen et al., 2021). tetM is the most frequently detected gene, although all five genes have been identified in S. uberis, S. agalactiae, or S. dysgalactiae across different countries (Zhang et al., 2021). tetM is a 70 kDa ribosomal protection protein that uses GTP to induce conformational changes in the ribosome; it physically dislodges tetracycline from its binding site on the 30S subunit and restores translation (HajiAhmadi et al., 2025). Moreover, S. agalactiae has been detected in buffalo milk with mastitis, expressing the resistance genes (tetO, tetM, and tetK) in approximately half of the strains (Tuzcu et al., 2023). Mechanisms of Streptococci resistance to macrolides, lincosamides, and streptograminsA myriad of investigations have been conducted on streptococci causing bovine mastitis that exhibit resistance to MLS antibiotics. According to the findings, erm genes, which mediate the resistance mechanism, including ribosomal methylation, are the most prevalent resistance genes in this regard; so ermB in Strep. uberis, ermA, or ermB in S. agalactiae, and both of them are detected in S. dysgalactiae (Zeng et al., 2022; Bustos et al., 2023; Singh et al., 2023). ermB encodes a methyltransferase that dimethylated adenine A2058 in the 23S rRNA peptidyl transferase center, preventing hydrogen bonding with macrolides, lincosamide, and streptogramin B. This steric barrier blocks antibiotic binding (León Saldaña, 2025). Although ermB is a prevalent gene for MLS resistance, other genes encoding efflux pumps, including the mef, msr, and mre gene families, also contribute significantly to the development of MLS resistance in Streptococci (Huang et al., 2023). Studies from various regions of the world have indicated the diversity of the distribution of resistance genes in Streptococci. Based on previous studies, in the United States, S. uberis predominantly carries ermB, whereas in Germany, in addition to S. uberis, genes ermB and ermC are present in S. agalactiae and S. dysgalactiae. The combinations of resistance genes within the same isolate have been frequently reported (Duarte et al., 2005; Entorf et al., 2016). Additionally, new genes, such as mel or mefA, have been discovered in S. uberis from Australia (Vezina et al., 2021). Resistance to macrolides involves mph genes, with mphB emerging in French S. uberis isolates. Furthermore, investigations revealed that resistance to lincosamide relies on lnu genes, and lnuB has been frequently detected in various streptococci strains globally (Naranjo-Lucena and Slowey, 2023). Mechanisms of Streptococcal resistance to other antibioticsAlong with resistance to β-lactams, tetracyclines, and MLS, there is evidence of resistance to other antibiotic families in bovine mastitis Streptococcus isolates. Studies have demonstrated that fluoroquinolone resistance is mainly attributed to point mutations in the gyrA and parC genes in these bacteria (Bustos et al., 2023). Mutations in the quinolone-resistance-determining region (e.g., gyrA Ser81Phe and parC Ser79Tyr) within DNA gyrase/topoisomerase IV reduce the binding affinity of the drugs to the DNA–enzyme complex, enabling DNA replication despite quinolone presence (Stefańska et al., 2025). Nonetheless, some investigations revealed that quinolone resistance could be found in Streptococcus isolates, even when they are not carrying resistance genes (Zhang et al., 2018; Tian et al., 2019). Moreover, studies observed a higher phenotypic prevalence than genetic prevalence for resistance to chloramphenicol and sulfonamides, which refers to the expression of cat1/2 and sul1/2/3 genes, respectively (Tian et al., 2019). When exploring aminoglycoside resistance in Streptococci, aphA-3 and aad-6 genes have been detected in S. uberis and S. dysgalactiae, albeit sporadically (Kaczorek et al., 2017). AphA-3 is a phosphotransferase that phosphorylates the 3′-hydroxyl group of aminoglycosides (e.g., kanamycin), blocking ribosomal binding. Aad-6 (nucleotidyltransferase) adenylates the 6′-hydroxyl of streptomycin, both preventing interaction with the 16S A-site (Ozavci et al., 2023). However, the phenotypic resistance levels were higher because these species inherently have lower susceptibility to this group of antibiotics. Resistance to streptogramin A in S. uberis is associated with the vatD gene (Vezina et al., 2021). VatD is an acetyltransferase that acetylates streptogramin A on the hydroxyl group of the lactone ring, preventing its binding to the peptidyl transferase center on the 50S subunit (Ozavci et al., 2023). Pseudomonas SpeciesPseudomonas species are Gram-negative, aerobic bacilli that play a significant role in the deterioration of dairy and food products(Du et al., 2022). Primarily known as psychrotrophic bacteria, these opportunistic pathogens, especially Pseudomonas aeruginosa, are widely distributed in water, soil, and sediment. They exhibit a wide metabolic adaptability that allows them to thrive in temperatures between 4°C and 42°C. Pseudomonas spp. are capable of multiplying at cold temperatures and comprise over half of the bacteria in milk (Meng et al., 2017). Moreover, heat-stable extracellular peptidases and lipases are produced in these bacterial species, contributing to the spoilage of raw milk (Savaşan and Sezener, 2022). Apart from the development of antibiotic-resistant Pseudomonas spp. through the widespread administration of antibiotics, the long-term survival of Pseudomonas in aquatic environments, including raw milk, raises the risk of spreading ARGs between pathogenic and non-pathogenic bacteria through mobile genetic elements (Sekhri et al., 2021). Furthermore, the development of inherent resistance mechanisms, namely efflux pumps, in Pseudomonas spp. could raise concerns about the prolonged viability of these bacteria in bulk-tank milk and the potential risk of spreading ARGs, emphasizing the importance of Pseudomonas detection for animal and human health (Quintieri et al., 2019). Moreover, a large number of investigations have been conducted on the antimicrobial susceptibility of Pseudomonas spp. isolated from cases of mastitis to various antibiotics. Based on these findings, Pseudomonas spp. are particularly sensitive to gentamicin, ciprofloxacin, tetracycline, and levofloxacin, although they are significantly resistant to imipenem, trimethoprim-sulfamethoxazole, aztreonam, chloramphenicol, and meropenem, as well as MDR has been reported (Sekhri et al., 2021; Ahamd et al., 2022; de Souza et al., 2023). Mechanisms of antibiotic resistance in Pseudomonas spp.The genetic basis of antibiotic resistance and related mechanisms in Pseudomonas isolates from raw milk has been identified in various investigations. Regarding aminoglycoside resistance, the aph(6)-Ic gene has been reported as the most prevalent AMR gene, found in a composite transposon Tn5. aph(6)-Ic encodes an aminoglycoside phosphotransferase that specifically phosphorylates the 6′-hydroxyl group on aminoglycosides (e.g., streptomycin), blocking binding to the 16S rRNA A-site, and consequently making the drug inactive (Meng et al., 2020). Moreover, studies revealed that genes ant(3”)-Ia, aph(6)-Id, and aph(3’)-IIc, along with rpsL mutations associated with streptomycin resistance, have been identified in Pseudomonas isolates as well. ant(3″)-Ia encodes an aminoglycoside nucleotidyltransferase that adenylates aminoglycosides at the 3″-hydroxyl, preventing ribosomal binding, while rpsL mutations alter ribosomal protein S12, reducing streptomycin binding to the decoding center (Ramirez and Tolmasky, 2010; Meng et al., 2020). Furthermore, research on β-lactam resistance provides insight into the wide variety of genes mediating resistance in Pseudomonas spp. isolated from raw milk and mastitis, with fourteen different β-lactam resistance genes identified in these isolates over the years. Nonetheless, despite the high total number of identified genes, the individual prevalence of each gene was quite low. In this regard, OCH-8 was reported as a mediator of resistance to third-generation cephalosporins. OCH-8 is a class C β-lactamase that hydrolyzes the β-lactam ring of cephalosporins via serine-mediated acyl-enzyme intermediate formation, which inactivates the antibiotic (Sultana et al., 2025). Moreover, LRA-13 confers resistance to amoxicillin, ampicillin, cephalexin, and carbenicillin (Alonso et al., 2017). Moreover, mutations in PBPs, including PBP1a, PBP1b, and PBP2, have been detected in the isolates, contributing to resistance to β-lactams (Kang, 2022). A number of studies have been conducted to discover the lipopeptide resistance gene cluster in Pseudomonas spp., including the phoP and phoQ genes, providing resistance to polymyxin. phoPQ is a two-component regulatory system that, when activated, upregulates the arnBCADTEF operon, leading to the addition of 4-amino-4-deoxy-L-arabinose to lipid A; this reduces its net negative charge, decreasing polymyxin binding (Castanheira et al., 2022). Additionally, the bacA and floR genes have been reported to confer resistance to bacitracin and florfenicol, respectively (Masschalck et al., 2003; Pitta et al., 2016). Mutations in the liaR, liaS, cls, pgsA, and rpoC genes are responsible for the development of resistance to daptomycin, although it has been observed in a few Pseudomonas strains. Mutations in liaFSR and cls genes alter membrane phospholipid composition and fluidity, which prevents daptomycin from inserting and forming lethal calcium-dependent oligomeric pores (Meng et al., 2020). Interestingly, some investigations speculated the possibility of transfer of resistance genes to rifampin and isoniazid from other bacterial species, namely E. coli, Mycobacterium tuberculosis, and S. aureus. In this regard, the genes gyrA, gyrB, rpsL, PBP1a, PBP1b, PBP2, liaR, liaS, cls, pgsA, rpoC, rpoB, katG, and kasA have been detected in different studies. These genes play a significant role in the development of resistance to various groups of antibiotics, including aminocoumarin, aminoglycoside, β-lactam, fluoroquinolones, fosfomycin, lipopeptides, rifampin, and isoniazid. Bacterial efflux pumps, along with the relevant genes, have been elucidated as a resistance mechanism in Pseudomonas spp. Several studies have identified genes associated with resistance-nodulation-cell division and ATP-binding cassette antibiotic efflux pumps. These include AcrAB-TolC, MexAB-OprM, MexCD-OprJ, MexEF-OprN, MexJK-OpmH, MexMN-OprM, MexPQ-OpmE, MexVW-OprM, and EfrAB (Chen and Duan, 2016). The aforementioned efflux pumps, excluding MexAB-OprM and MexXY-OprM, can be generated by various genetic mutations, mainly local repressor genes, global regulatory genes, or other mutations. Overexpression of RND efflux pumps is frequently due to mutations in repressor genes (mexR, nalC, nalD); these pumps can export aminoglycosides, β-lactams, and fluoroquinolones out of the cell, and increase MICs (Meng et al., 2020). Moreover, these pumps contribute to intrinsic multidrug resistance in P. aeruginosa PAO1 (Piddock, 2006). DiscussionThe challenge of surviving in the modern era of drug-resistant opportunistic bacteria is considered the greatest concern for human health, livestock health, and the environment. Emergence of new antimicrobial-resistant microorganisms threatening health is an inevitable process reflecting the general course of biological evolution. Conversely, the main factors leading to the emergence and increase of antibiotic resistance in the microbiota of raw milk are the universal availability of antibiotics, the extensive and irresponsible administration of antibiotics for treating infections, particularly mastitis in dairy animals, and the misuse of these agents not only to prevent infections but also in animal feed as growth stimulants. Thus, the development of antibiotic-resistant strains poses a significant challenge in the management of bovine mastitis worldwide due to the high global prevalence of mastitis and its economic impacts on dairy cattle. Recent studies have demonstrated that mastitis-causing bacteria, such as S. aureus, E. coli, Streptococcus spp., and Pseudomonas spp., are significant carriers of ARGs, which can make antibiotics less effective and pose risks to both animal and human health. For instance, a 2023 study, “Is There a Relationship between Antimicrobial Use and Antibiotic Resistance of the Most Common Mastitis Pathogens in Dairy Cows?”, found that there is a clear association between antibiotic use and resistance patterns in dairy cows. The pathogens (S. aureus and E. coli) isolated from mastitis cases frequently exhibited multidrug resistance to penicillin and tetracycline, highlighting the importance of these pathogens in driving antibiotic resistance and AMR (Kovačević et al., 2022). Another 2022 study titled “Etiology of Mastitis and Antimicrobial Resistance in Dairy Cattle Farms in the Western Part of Romania,” reported the high prevalence of MDR bacteria in bovine mastitis isolates. Moreover, the investigation revealed that these resistant pathogens can spread to humans via milk consumption or direct contact, posing a public health threat (Pascu et al., 2022). Tóth, et al. (2020) suggested that the ARG content of unprocessed dairy products and raw milk plays a role in the development of AMR in human pathogens, increasing AMR risks for humans (Tóth et al., 2020). Notably, while pasteurization reduces AMR risks to public health, the consumption of raw milk, which is still practiced in some regions of the world due to local traditions or perceptions about nutritional benefits, reinforces the transmission of resistant bacteria from mastitic cows to humans (Xu et al., 2022a). The presence of MRSA and ESBL-producing E. coli in dairy farms poses significant challenges for treatment efficacy and public health, as these resistant strains can be transmitted through raw milk consumption (Kovačević et al., 2022). Bacteria can develop resistance to antibiotics through several mechanisms, namely by mutating the target sites of antibiotics, reducing the permeability of the cell membrane, or acquiring corresponding resistance genes (Figs. 1 and 2). Therefore, to prevent the spread of antibiotic resistance, it is necessary to fundamentally change the use of existing antibiotics, intensify the search for new antimicrobial drugs, and develop new diagnostic techniques for quickly monitoring the formation and spread of drug resistance.
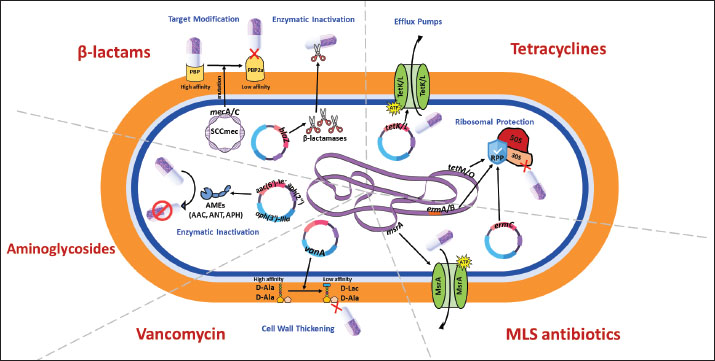
Fig. 1. Molecular mechanisms of antibiotic resistance in Gram-positive mastitis pathogens (Staphylococcus aureus and Streptococcus spp.) Schematic cross-section of a Gram-positive bacterial cell highlighting the predominant resistance strategies against the following key antibiotic classes: β-Lactams, tetracyclines, aminoglycosides, MLS (macrolides, lincosamides, streptogramins), and vancomycin.
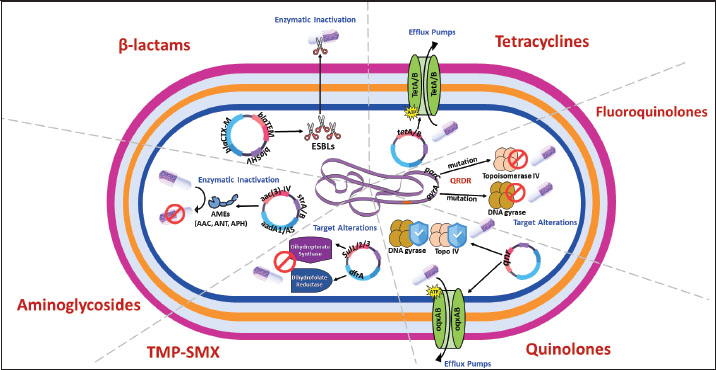
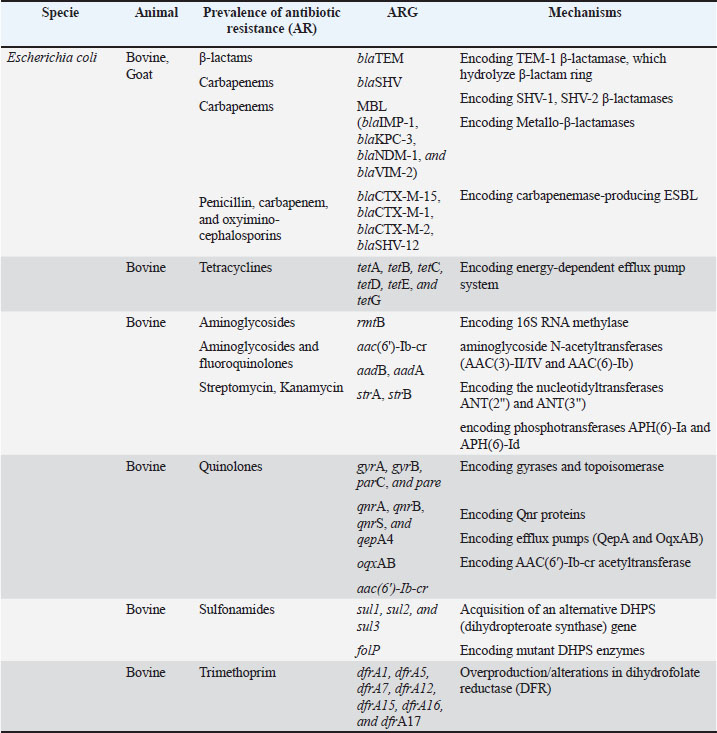
Fig. 2. Molecular mechanisms of antibiotic resistance in Gram-negative mastitis pathogens (Escherichia coli and Pseudomonas spp.). Schematic cross-section of a Gram-negative bacterial cell highlighting the predominant resistance strategies against the following key antibiotic classes: β-Lactams, tetracyclines, aminoglycosides, fluoroquinolones, quinolones, and trimethoprim-sulfonamides (TMP-SMX). Tables 1 and 2 provide a comprehensive overview of the antibiotic resistance gene profiles found in gram-positive and gram-negative bacteria associated with the occurrence of mastitis in bovine, goat, and sheep populations. Understanding the intricate dynamics of resistance mechanisms in bacterial species is crucial for developing more effective strategies to mitigate adverse effects on both animal and public health. In this regard, many techniques and methods have been developed for the more effective application of existing antimicrobial drugs, including combination therapy, immunotherapy, targeted delivery of antimicrobial agents using bacteriophage vectors, recording the antibiotic prescriptions following genotyping and metagenomic analysis of the bacterial source of infection, and more significantly, applying pathogen genomic surveillance for characterizing the development and progression of ARGs within bacterial populations in dairy livestock (Neculai-Valeanu and Ariton, 2022; Elbayoumy et al., 2024; Nesterova et al., 2024; Venkataraman et al., 2024). Furthermore, the necessity of evolving veterinary practices, as well as applying alternative treatment approaches for mastitis, has been highlighted in several articles (Nale and McEwan, 2023). Given the rising concerns of AMR, there is a growing interest in using probiotics, bacteriophages, phage endolysins, bacteriocins (bacteria-derived antimicrobials), animal-derived antimicrobials (such as lactoferrin and propolis), silver nanoparticles, and plant-derived compounds (especially essential oils) (Angelopoulou et al., 2019; Tomanić et al., 2023). These alternatives may help reduce the reliance on antibiotics and the spread of resistance. Table 1. Profiles of ARGs of Gram-positive bacteria isolated from raw milk.
Table 2. Antibiotic resistance gene profiles of Gram-negative bacteria isolated from raw milk.
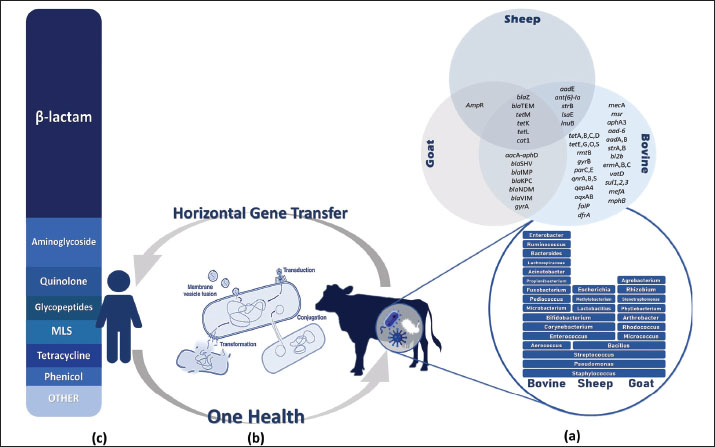
AMR genes in raw milk are characterized by complex genetic diversity as a selective pressure, which poses complicated challenges in predicting and managing the evolution of resistance over time. Moreover, the distribution of this genetic diversity through HGT among bacteria enhances the resistance of mastitis (Nery Garcia et al., 2024). Since HGT serves as a bridge between mastitis-causing bacteria in livestock and human pathogens, numerous studies have explored the dynamics of antibiotic resistance, focusing on the dissemination mechanisms of ARG from raw milk to the human microbiome. Antibiotic-resistant bacteria and their ARGs are transmitted to humans through the consumption of raw milk or undercooked dairy products, as well as direct contact with infected animals (Sharma et al., 2018). As reported in a 2020 metagenomic analysis of raw milk samples, the samples contained several ARGs associated with mobile genetic elements that accelerate their transmission to other bacteria, including those in the human gut (Tóth et al., 2020). Furthermore, this issue is particularly concerning in the context of bovine mastitis, since there is compelling evidence that mastitis-causing pathogens, including S. aureus and E. coli, carry and disseminate ARGs. These MDR bacteria are not only extremely hazardous for public health but also present significant challenges for human medicine (Kovačević et al., 2022; Pascu et al., 2022). Identification of ARGs and the underlying molecular resistance mechanisms in key mastitis-causing pathogens are critical early step in controlling the veterinary antibiotic resistance crisis. This information could help scientists implement targeted diagnostics in the livestock industry by developing rapid, on-farm multiplex assays (qPCR or LAMP) to detect key ARGs, including mecA, blaZ, tetM, and aac(6’)-Ie-aph(2″)-Ia, enabling veterinarians to choose effective antibiotics rather than broad-spectrum drugs (Fragas Quintero, 2025). Furthermore, the use of enzyme inhibitors and efflux-pump modulators is another strategy for managing AMR. Studies suggest co-administration of β-lactamase inhibitors (e.g., clavulanate) in cases where plasmid-encoded β-lactamases are predominant (Prescott and Hardefeldt, 2024) and the exploration of compounds that inhibit RND or ABC efflux pumps (e.g., MexAB-OprM) to restore intracellular levels of tetracycline or macrolide in strains overexpressing Tet and Msr pumps (Mech, 2024). However, the application of bacteriophages or phage-derived lysins targeting specific resistance mechanisms, such as altered PBPs or ribosomal protection, has shown efficacy in clearing resistance strains in murine models (Vander Elst, 2024; Cho et al., 2025). There are several areas that scientists and veterinarians need to address regarding the challenges of combating antibiotic resistance in the dairy industry. First and foremost is the presence of low-level silent reservoirs of ARG (e.g., aadA, sul1, and tetM) in the raw milk microbiome even without recent antibiotic use, making contamination difficult to detect without genomic surveillance (Collis et al., 2024). The second challenge is HGT via mobile elements, including plasmids, transposons, and integrons, which shuttle ARGs across species and facilitate the spread of resistance among bacteria (Da Silva Duarte et al., 2025). Moreover, some diagnostic gaps exist in current methods. For instance, conventional culture and susceptibility tests may miss many resistance mechanisms (e.g., low-level mecA expression and non-expressed blaZ carriers), which delays targeted therapy (Abdelhameed et al., 2025). On the other hand, farmers may lack access to surveillance practices, leading to the overuse of antibiotics for prophylactic or growth-promotion purposes that sustain selection pressure. Therefore, the implementation of new diagnostic methods or phage therapies requires training to assess feasibility in real-world dairy settings as well as farmer adoption and compliance (Rodriguez et al., 2025). Figure 3 highlights the need for a One Health approach to tackle the issue of AMR in bacteria associated with mastitis (World Health Organization, 2017). This approach supports the collaborative efforts among veterinary medicine, environmental science, and public health to implement surveillance programs and regulatory frameworks based on the use of biomedical knowledge, the strictly regulated use of antimicrobial drugs, and compliance with the basics of hygiene and epidemiology. By adopting a One Health perspective, scientists can develop comprehensive strategies to encompass the entire ecosystem, which serves as a reservoir of antibiotic resistance (Algammal et al., 2020).
Fig. 3. Diagram of the antibiotic resistance cycle from the perspective of (a) resistant bacteria and resistance genes subdivided by the host (bovine, sheep, and goat), (b) the mechanism of resistance distribution through HGT, and (c) the rate of resistance to different antibiotic groups in humans. In conclusion, it is our collective responsibility to foster our understanding of antibiotic resistance, a globally complex, multifaceted phenomenon, and leverage this knowledge to increase sustainability in veterinary practice, improve food safety in the dairy supply chain, and protect public health in the face of evolving microbial challenges. Although improving milking hygiene and cow comfort (dry-cow therapy and bedding management) are critical to reducing the incidence of mastitis and antibiotic demand, some specific strategies can be adopted to slow the rate of antibiotic resistance in the dairy industry. First, it is recommended to use rapid gene-based diagnostics, such as on-farm ARG testing, before administering antibiotics to guide targeted therapy (De Jong, 2024). Second, implementing alternative therapeutics, namely phage cocktails (e.g., SW21/SW25 for MRSA; StaphLyse™), endolysins, and vaccines against common mastitis pathogens (e.g., S. aureus and E. coli) to reduce antibiotics consumption while combating in vivo dynamics that are difficult to treat (Brouillette et al., 2023). Additionally, routine genomic surveillance, particularly bulk-milk shotgun metagenomics and targeted qPCR screening, could effectively track ARG prevalence and emerging threats in the dairy industry. Finally, a One Health approach was adopted to track ARG flows across farm, food, and clinical settings to facilitate early warnings and policy responses. AcknowledgmentsNone. Conflict of interestThe authors declare no conflict of interest. FundingThe research was conducted within the state assignment of the Russian Federation’s Ministry of Science and Higher Education (project FZGW-2024-0003). Authors’ contributionsShima Kazemzadeh and Mikhail Syromyatnikov substantially contributed to the design of this review article, as well as the interpretation of the scientific material, drafting the manuscript, and critically reviewing it for important intellectual content. Olga Korneeva and Sergey Shabunin contributed to the conception of the study. The final version of the review article was approved by Olga Korneeva and Sergey Shabunin. All authors are accountable for all aspects of the work, ensuring that any questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Data availabilityAll data were provided in the review article. ReferencesAbdelhameed, M.F.M., Almansoori, A.E., Zaid, R. and Ibrahim, M.T. 2025. Comparison bBetween the cConventional bacterial culture and the real time PCR in identifying the dairy cow mastitis causative agents. Sultan Qaboos Univ. J. For Sci. 30, 44–50. Abebe, R., Hatiya, H., Abera, M., Megersa, B. and Asmare, K. 2016. Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC. Vet. Res. 12, 270. Abril, G.A., Villa, G.T., Barros-Velázquez, J., Cañas, B., Sánchez-Pérez, A., Calo-Mata, P. and Carrera, M. 2020. Staphylococcus aureus exotoxins and their detection in the dairy industry and mastitis. Toxins 12, 537. Adnan, M., Khan, H., Kashif, J., Ahmad, S., Gohar, A., Ali, A., Khan, M.A., Shah, S.S.A., Hassan, M.F. and Irshad, M. 2017. Clonal expansion of sulfonamide resistant Escherichia coli isolates recovered from diarrheic calves. Pak. Vet. J. 37, 230–232. Afroz, F., Shifat, H., Begum, M., Akter, M. and Islam, M. 2024. Molecular characterization of extended spectrum β-lactamase producing Escherichia coli isolated from bovine mastitic milk in Northern Bangladesh. Eur. J. Microbiol. Infect. Dis.,1. Aguirre-Sánchez, J.R., Castro-del Campo, N., Medrano-Félix, J.A., Martínez-Torres, A.O., Chaidez, C., Querol-Audi, J. and Castro-del Campo, N. 2024. Genomic insights of S. aureus associated with bovine mastitis in a high livestock activity region of Mexico. J. Vet. Sci. 25, 42. Ahamd, J., Khan, W., Khan, M.K., Shah, K., Yasir, M., Ullah, I., Ullah, H., Samee, A., Zaman, A. and Khan, R.U. 2022. Antimicrobial resistant and sensitivity profile of bacteria isolated from raw milk in Peshawar, KPK, Pakistan. Ann. Romanian. Soc. for Cell Biol. 26, 364–374. Ahmed, W., Neubauer, H., Tomaso, H., El Hofy, F.I., Monecke, S., Abd El-tawab, A.A. and Hotzel, H. 2021. Characterization of Enterococci- and ESBL-Producing Escherichia coli Isolated from Milk of Bovides with Mastitis in Egypt. Pathogens 10, 97. Alexander, J.A.N., Worrall, L.J., Hu, J., Vuckovic, M., Satishkumar, N., Poon, R., Sobhanifar, S., Rosell, F.I., Jenkins, J., Chiang, D., Mosimann, W.A., Chambers, H.F., Paetzel, M., Chatterjee, S.S. and Strynadka, N.C.J. 2023. Structural basis of broad-spectrum β-lactam resistance in Staphylococcus aureus. Nature 613, 375–382. Algammal, A.M., Hetta, H.F., Elkelish, A., Alkhalifah, D.H.H., Hozzein, W.N., Batiha, G.S., El Nahhas, N. and Mabrok, M.A. 2020. Methicillin-Resistant Staphylococcus aureus (MRSA): one health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infectdrug. Resist. 3255, 3255–3265. Alian, F., Rahimi, E., Shakerian, A., Momtaz, H., Riahi, M. and Momeni, M. 2012. Antimicrobial resistance of Staphylococcus aureus isolated from bovine, sheep and goat raw milk. Global Veterinaria. 8, 111–114. Alonso, C.A., Kwabugge, Y.A., Anyanwu, M.U., Torres, C. and Chah, K.F. 2017. Diversity of Ochrobactrum species in food animals, antibiotic resistance phenotypes and polymorphisms in the blaOCH gene. FEMS Microbiol. Lett. 364, 178. Andreadis, T., 2020. Characterisationof phenotypic and genotypic antibiotic resistance with special consideration of macrolide, lincosamide and streptogramin B (MLS) antibiotics in methicillin-resistant Staphylococcus aureus (MRSA) isolated from European hares (Lepus europaeus) and companion animals originating from the German North Frisian Island Pellworm (European hares) as well as Austria (companion animals, European hares), University of Veterinary Medicine Vienna, Vienna, Austria. Angelopoulou, A., Warda, A.K., Hill, C. and Ross, R.P. 2019. Non-antibiotic microbial solutions for bovine mastitis - live biotherapeutics, bacteriophage, and phage lysins. Crit. Rev. Microbiol. 45, 564–580. Antók, F.I., Mayrhofer, R., Marbach, H., Masengesho, J.C., Keinprecht, H., Nyirimbuga, V., Fischer, O., Lepuschitz, S., Ruppitsch, W., Ehling-Schulz, M., Feßler, A.T., Schwarz, S., Monecke, S., Ehricht, R., Grunert, T., Spergser, J. and Loncaric, I. 2019. Characterization of antibiotic and biocide resistance genes and virulence factors of Staphylococcus species associated with Bovine mastitis in Rwanda. Antibiotics 9, 1. Behera, M., Parmanand., Roshan, M., Rajput, S., Gautam, D., Vats, A., Ghorai, S.M. and De, S. 2023. Novel aadA5 and dfrA17 variants of class 1 integron in multidrug-resistant Escherichia colicausing bovine mastitis. Appl. Microbiol. Biotechnol. 107, 433–446. Bitrus, A.A., Peter, O.M., Abbas, M.A. and Goni, M.D. 2018. Staphylococcus aureus: a review of antimicrobial resistance mechanisms. Vet. Sci. Res. Rev. 4, 43–54. Brouillette, E., Millette, G., Chamberland, S., Roy, J.P., Ster, C., Kiros, T., Hickey, S., Hittle, L., Woolston, J. and Malouin, F. 2023. Effective treatment of Staphylococcus aureus intramammary infection in a murine model using the bacteriophage cocktail StaphLyse™. Viruses 15, 887. Bustos, C.P., Retamar, G., Leiva, R., Frosth, S., Ivanissevich, A., Demarchi, M.E., Walsh, S., Frykberg, L., Guss, B., Mesplet, M. and Waller, A. 2023. Novel genotype of Streptococcus dysgalactiae subsp. equisimilis associated with mastitis in an Arabian filly: genomic approaches and phenotypic properties. J. Equine Vet. Sci. 130, 104913. Campos, B., Pickering, A.C., Rocha, L.S., Aguilar, A.P., Fabres-Klein, M.H., De Oliveira Mendes, T.A., Fitzgerald, J.R. and De Oliveira Barros Ribon, A. 2022. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: current understanding and future perspectives. BMC Vet. Res. 18, 115. Castanheira, M., Doyle, T.B., Hubler, C.M., Collingsworth, T.D., Devries, S. and Mendes, R.E. 2022. The plethora of resistance mechanisms in Pseudomonas aeruginosa: transcriptome analysis reveals a potential role of lipopolysaccharide pathway proteins to novel β-lactam/β-lactamase inhibitor combinations. J. Glob. Antimicrob. Resist. 31, 72–79. Chen, L. and Duan, K. 2016. A PhoPQ-regulated ABC transporter system exports tetracycline in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 60, 3016–3024. Cho, J.H., Lee, G.M., Ko, S., Kim, Y. and Kim, D. 2025. Characterization and therapeutic potential of newly isolated bacteriophages against Staphylococcus species in bovine mastitis. J. Virol. 99, 1901. Chowdhury, M.S.R., Hossain, H., Rahman, M.N., Rahman, A., Ghosh, P.K., Uddin, M.B., Nazmul Hoque, M., Hossain, M.M. and Rahman, M.M. 2025. Emergence of highly virulent multidrug and extensively drug resistant Escherichia coli and Klebsiella pneumoniae in buffalo subclinical mastitis cases. Sci. Rep. 15, 11704. Collis, R.M., Biggs, P.J., Burgess, S.A., Midwinter, A.C., Liu, J., Brightwell, G. and Cookson, A.L. 2024. Assessing antimicrobial resistance in pasture-based dairy farms: a 15-month surveillance study in New Zealand. Appl. Environ. Microbiol. 90, e0139024. Crespi, E., Pereyra, A.M., Puigdevall, T., Rumi, M.V., Testorelli, M.F., Caggiano, N., Gulone, L., Mollerach, M., Gentilini, E.R. and Srednik, M.E. 2022. Antimicrobial resistance studies in staphylococci and streptococci isolated from cows with mastitis in Argentina. J. Vet. Sci. 23, e12. da Silva Duarte, V., Franklin, F.V., Krysmann, A.M. and Porcellato, D. 2025. Longitudinal study of the udder microbiome of Norwegian Red dairy cows using metataxonomic and shotgun metagenomic approaches: insights into pathogen-driven microbial adaptation and succession. bioRxiv 2004, 647183. de Freitas, J., Ferreira, A.P.G. and Cavalheiro, E.T.G. 2023. Thermal analysis of tetracyclines: a review. J. Thermal Anal. Calorimetry 149, 11375–11387. De Jong, E. 2024. Enhancing antimicrobial stewardship on dairy farms with a focus on selective treatment of clinical mastitis. de Moura, G.S., De Carvalho, E., Ramos Sanchez, E.M., Sellera, F.P., Marques, M.F.S., Heinemann, M.B., De Vliegher, S., Souza, F.N. and Mota, R.A. 2023. Emergence of livestock-associated Mammaliicoccus sciuri ST71 co-harbouring mecA and mecC genes in Brazil. Vet. Microbiol. 283, 109792. de Oliveira, R.P., Aragão, B.B., De Melo, R.P.B., Da Silva, D.M.S., De Carvalho, R.G., Juliano, M.A., Farias, M.P.O., De Lira, N.S.C. and Mota, R.A. 2022. Bovine mastitis in northeastern Brazil: occurrence of emergent bacteria and their phenotypic and genotypic profile of antimicrobial resistance. Comp. Immunol. Microbiol. Infect. Dis. 85, 101802. de Souza, Z.N., De Moura, D.F., De Almeida Campos, L.A., Córdula, C.R. and Cavalcanti, I.M.F. 2023. Antibiotic resistance profiles on pathogenic bacteria in the Brazilian environments. Arch. Microbiol. 205, 185. Dierikx, C., Hengeveld, P., Witteveen, S., Van Hoek, A., Van Santen-verheuvel, M., Montizaan, M., Kik, M., Maas, M., Schouls, L., Hendrickx, A. and Van Duijkeren, E. 2023. Genomic comparison of mecC-carrying methicillin-resistant Staphylococcus aureus from hedgehogs and humans in the Netherlands. J. Antimicrob. Chemother. 78, 1168–1174. Dong, L. 2023. Effects of cephalosporin treatment on the microbiota and resistance genes in milk and feces, and the presence of antibiotic heteroresistant strains. Donkin, R.W. 2016. Assessment of antimicrobial resistance in pathogens responsible for causing bovine mastitis in Kentucky dairy farms. Dowidar, H.A. and Khalifa, M.I. 2023. Molecular isolation and identification of multidrug-resistant Escherichia coli from Milk, meat, and product samples. J. Adv. Vet. Res. 13, 643–646. Du, B., Meng, L., Liu, H., Zheng, N., Zhang, Y., Zhao, S., Li, M. and Wang, J. 2022. Diversity and proteolytic activity of Pseudomonas species isolated from raw cow milk samples across China. Sci. Total Environ. 838, 156382. Duarte, R.S., Bellei, B.C., Miranda, O.P., Brito, M.A.V.P. and Teixeira, L.M. 2005. Distribution of antimicrobial resistance and virulence-related genes among Brazilian group B Streptococci recovered from Bovine and human sources. Antimicrob. Agents Chemother. 49, 97–103. Dubey, K.K., Raj, T. and Kumar, P. 2022. Pathogenic microorganisms in milk: their source, hazardous role and identification. In Advances in dairy microbial products. Eds., Singh, J. and Vyas, A. Amsterdam, The Netherlands: Elsevier, pp: 145–61. Elbayoumy, M., Allam, A., Ghazy, A. and Nasr, S. 2024. Advances in controlling bacterial mastitis in dairy cows. Egypt. J. Vet. Sci. 55, 1–21. El-Baz, A.M., Yahya, G., Mansour, B., El-Sokkary, M.M.A., Alshaman, R., Alattar, A. and El-Ganiny, A.M. 2021. The link between occurrence of class I integron and acquired aminoglycoside resistance in Clinical MRSA Isolates. Antibiotics 10, 488. El-Halem, A., Sahar, G., Attia, I. and El-Derea, H. 2021. Antibiotic resistance and extended-spectrum b-lactamases producing Escherichia coli in raw milk and dairy products collected from Alexandria, Egypt. Egypt. J. Dairy Sci. 49. Emon, A.A., Hossain, H., Chowdhury, M.S.R., Rahman, M.A., Tanni, F.Y., Asha, M.N., Akter, H., Hossain, M.M., Islam, M.R. and Rahman, M.M. 2024. Prevalence, antimicrobial susceptibility profiles and resistant gene identification of bovine subclinical mastitis pathogens in Bangladesh. Heliyon 10, e34567. Entorf, M., Feßler, A.T., Kaspar, H., Kadlec, K., Peters, T. and Schwarz, S. 2016. Comparative erythromycin and tylosin susceptibility testing of streptococci from bovine mastitis. Vet. Microbiol. 194, 36–42. Farrell, S., Benson, T., Mckernan, C., Regan, A., Burrell, A.M.G. and Dean, M. 2023. Factors influencing dairy farmers’ antibiotic use: an application of the COM-B model. J. Dairy Sci. 106, 4059–4071. Fazel, F., Jamshidi, A. and Khoramian, B. 2019. Phenotypic and genotypic study on antimicrobial resistance patterns of E. coli isolates from bovine mastitis. Microb. Pathog. 132, 355–361. Findlay, J., Mounsey, O., Lee, W.W.Y., Newbold, N., Morley, K., Schubert, H., Gould, V.C., Cogan, T.A., Reyher, K.K. and Avison, M.B. 2020. Molecular epidemiology of Escherichia coli producing CTX-M and pAmpC β-lactamases from dairy farms identifies a dominant Plasmid Encoding CTX-M-32 but No Evidence for Transmission to Humans in the Same Geographical Region. Appl. Environ. Microbiol. 87, e01842-01820. Fragas Quintero, A. 2025. Rapid detection of problematic Staphylococcus aureus strains in milk production. Ghali-Mohammed, I., Ade-Yusuf, R., Adewoye, A., Adetona, M., Ahmed, O., Alhaji, N. and Odetokun, I. 2023. Prevalence and antimicrobial susceptibility profile of Staphylococcus aureus isolated from marketed milk and cheese in Ilorin, Nigeria. Ghatak, S., Singha, A., Sen, A., Guha, C., Ahuja, A., Bhattacharjee, U., Das, S., Pradhan, N.R., Puro, K., Jana, C., Dey, T.K., Prashantkumar, K.L., Das, A., Shakuntala, I., Biswas, U. and Jana, P.S. 2013. Detection of New Delhi metallo-beta-lactamase and extended-spectrum beta-lactamase genes in Escherichia coli isolated from mastitic milk samples. Transbound. Emerg. Dis. 60, 385–389. Gökdağ, M.O. and Çiftci, A. 2021. Antibiotic resistance and virulence gene profiles in Staphylococci isolated from cattle with mastitis. J. Anatolian Environ. Anim. Sci. 6, 395–402. HajiAhmadi, P., Momtaz, H. and Tajbakhsh, E. 2025. Capsular typing and molecular characterization of Streptococcus agalactiae strains isolated from Bovine mastitis in Iran. Vet. Med. Sci. 11, e70275. Haley, B.J., Kim, S.W., Salaheen, S., Hovingh, E. and Van Kessel, J.A.S. 2023. Genome-wide analysis of Escherichia coli isolated from dairy animals identifies virulence factors and genes enriched in multidrug-resistant strains. Antibiotics 12, 1559. Hang, B.P.T., Wredle, E., Börjesson, S., Sjaunja, K.S., Dicksved, J. and Duse, A. 2019. High level of multidrug-resistant Escherichia coli in young dairy calves in southern Vietnam. Trop. Anim. Health Prod. 51, 1405–1411. Haq, I.U., Kamal, M., Swelum, A.A., Khan, S., Ríos-Escalante, P.R.L. and Usman, T. 2024. Alarming multidrug resistance in Staphylococcus aureus isolated from raw milk of cows with subclinical mastitis: antibiotic resistance patterns and occurrence of selected resistance genes. PLoS One 19, e0301200. Hassani, S., Moosavy, M.H., Gharajalar, S.N., Khatibi, S.A., Hajibemani, A. and Barabadi, Z. 2022. High prevalence of antibiotic resistance in pathogenic foodborne bacteria isolated from bovine milk. Sci. Rep. 12, 3878. Huang, J., Dai, X., Wu, Z., Hu, X., Sun, J., Tang, Y., Zhang, W., Han, P., Zhao, J., Liu, G., Wang, X., Mao, S., Wang, Y., Call, D.R., Liu, J. and Wang, L. 2023. Conjugative transfer of Streptococcal prophages harboring antibiotic resistance and virulence genes. ISME J. 17, 1467–1481. Hussain, H.I., Aqib, A.I., Seleem, M.N., Shabbir, M.A., Hao, H., Iqbal, Z., Kulyar, M.F., Zaheer, T. and Li, K. 2021. Genetic basis of molecular mechanisms in β-lactam resistant gram-negative bacteria. Microb. Pathog. 158, 105040. Jamali, H., Paydar, M., Radmehr, B., Ismail, S. and Dadrasnia, A. 2015. Prevalence and antimicrobial resistance of Staphylococcus aureus isolated from raw milk and dairy products. Food Control 54, 383–388. Jensen, S.O. and Lyon, B.R. 2009. Genetics of antimicrobial resistance in Staphylococcus aureus. Future Microbiol. 4, 565–582. Kabelitz, T., Aubry, E., Van Vorst, K., Amon, T. and Fulde, M. 2021. The role of Streptococcus spp. in Bovine mastitis. Microorganisms 9, 1497. Kaczorek, E., Małaczewska, J., Wójcik, R., Rękawek, W. and Siwicki, A.K. 2017. Phenotypic and genotypic antimicrobial susceptibility pattern of Streptococcus spp. isolated from cases of clinical mastitis in dairy cattle in Poland. J. Dairy Sci. 100, 6442–6453. Kadlec, K., Feßler, A.T., Hauschild, T. and Schwarz, S. 2012. Novel and uncommon antimicrobial resistance genes in livestock-associated methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infection 18, 745–755. Kakooza, S., Eneku, W., Nabatta, E., Wampande, E.M., Ssajjakambwe, P., Wanyana, M., Munyiirwa, D.F.N., Ndoboli, D., Namuyinda, D., Athieno, G., Kayaga, E., Okwasiimire, R., Tsuchida, S., Ushida, K., Sakurai, K. and Mutebi, F. 2024. Integrating multi-wet laboratory diagnostics to study staphylococci in animals in Uganda. BMC. Microbiol. 24, 298. Kang, K.N. 2022. Mechanistic insights into Gram-negative envelope remodeling for protection against severe outer membrane defects. Arlington, TX: The University of Texas at Arlington. Kang, S., Lee, S. and Choi, S. 2014. Prevalence and molecular characterization of quinolone antibiotic resistance in Escherichia coli isolates from raw bulk milk in Gyeonggi-do. Korean J. Microbiol. 50, 185–190. Karzis, J., Petzer, I.M., Naidoo, V. and Donkin, E.F. 2021. The spread and antimicrobial resistance of Staphylococcus aureus in South African dairy herds – a review. Onderstepoort J. Vet. Res. 88, 1937. Khanal, S., Boonyayatra, S. and Awaiwanont, N. 2022. Prevalence of methicillin-resistant Staphylococcus aureus in dairy farms: a systematic review and meta-analysis. Front. Vet. Sci. 9, 947154. Kovačević, Z., Samardžija, M., Horvat, O., Tomanić, D., Radinović, M., Bijelić, K., Vukomanović, A.G. and Kladar, N. 2022. Is there a relationship between antimicrobial use and antibiotic resistance of the most common mastitis pathogens in dairy cows?. Antibiotics 12(3), 3. Lade, H. and Kim, J.S. 2023. Molecular determinants of β-lactam resistance in methicillin-resistant Staphylococcus aureus (MRSA): an updated review. Antibiotics 12, 1362. Lan, T., Liu, H., Meng, L., Xing, M., Dong, L., Gu, M., Wang, J. and Zheng, N. 2020. Antimicrobial susceptibility, phylotypes, and virulence genes of Escherichia coli from clinical bovine mastitis in five provinces of China. Food Agricult. Immunol. 31, 406–423. Lee, G.Y., Lee, S.I., Kim, S.D., Park, J.H., Kim, G.B. and Yang, S.J. 2022. Clonal distribution and antimicrobial resistance of methicillin-susceptible and -resistant Staphylococcus aureus strains isolated from broiler farms, slaughterhouses, and retail chicken meat. Poultry Sci. 101, 102070. León Saldaña, E.G. 2025. Caracterización molecular de los agentes bacterianos causantes de mastitis bovina asociados a resistencia de antibióticos en vacas lecheras de la Provincia de Cajamarca. Li, L. and Zhao, X. 2018. Characterization of the resistance class 1 integrons in Staphylococcus aureus isolates from milk of lactating dairy cattle in Northwestern China. BMC Vet. Res. 14, 1–7. Li, P., Liu, D., Zhang, X., Tuo, H., Lei, C., Xie, X., Gu, J. and Zhang, A. 2019. Characterization of plasmid-mediated quinolone resistance in gram-negative bacterial strains from animals and humans in China. Microb. Drug Resist. 25, 1050–1056. Li, Z., Wright, A.D.G., Yang, Y., Si, H. and Li, G. 2017. Unique bacteria community composition and co-occurrence in the milk of different ruminants. Sci. Rep. 7, 40950. Liang, M., Ge, X., Xua, H., Ma, K., Zhang, W., Zan, Y., Efferth, T., Xue, Z. and Hua, X. 2022. Phytochemicals with activity against methicillin-resistant Staphylococcus aureus. Phytomedicine 100, 154073. Lima, S.F., Bicalho, M.L.S. and Bicalho, R.C. 2018. Evaluation of milk sample fractions for characterization of milk microbiota from healthy and clinical mastitis cows. PLoS One 13, e0193671. Liu, G., Thomsen, L.E. and Olsen, J.E. 2022. Antimicrobial-induced horizontal transfer of antimicrobial resistance genes in bacteria: a mini-review. J. Antimicrob. Chemother. 77, 556–567. Lopardo, H.A., Vigliarolo, L., Bonofiglio, L., Gagetti, P., García Gabarrot, G., Kaufman, S., Mollerach, M., Toresani, I. and Von Specht, M. 2022. Beta-lactam antibiotics and viridans group Streptococci. Rev. Argent. Microbiol. 54, 335–343. Ma, X., Chen, H., Wang, F., Wang, S., Wu, Y., Ma, X., Wei, Y., Shao, W. and Zhao, Y. 2023. Molecular characterisation and antimicrobial resistance of Streptococcus agalactiae isolates from dairy farms in China. J. Vet. Res. 67, 161–167. Mahdavi, F., Zaboli, F. and Khoshbakht, R. 2019. Characteristics of erythromycin resistance in methicillin-resistant Staphylococcus aureus isolated from raw milk. Int. J. Enteric Pathog. 7, 121–125. Mahdavi, M., Fathi, B., Jamshidi, A. and Khoramian, B. 2022. Evaluation of resistance to fluoroquinolones and determination of mutations in gyrA and parC genes in Escherichia coli isolated from raw milk of dairy cows with coliform mastitis in Khorasan Razavi province, Iran. Iranian J. Vet. Sci. Technol. 1423–31. Majumder, S., Jung, D., Ronholm, J. and George, S. 2021. Prevalence and mechanisms of antibiotic resistance in Escherichia coli isolated from mastitic dairy cattle in Canada. BMC. Microbiol. 21, 222. Majumder, S., Sackey, T., Viau, C., Park, S., Xia, J., Ronholm, J. and George, S. 2023. Genomic and phenotypic profiling of Staphylococcus aureus isolates from bovine mastitis for antibiotic resistance and intestinal infectivity. BMC. Microbiol. 23, 43. Manimaran, A., Desingu, P.A., Kumaresan, A., Singh, P., Subramanya, K., Dodamani, P. and Dineshbhai, P.A. 2025. The metagenomic and whole-genome metagenomic detection of multidrug-resistant bacteria from subclinical mastitis-affected cow’s milk in India. Front. Cell. Infect. Microbiol. 15, 1549523. Martins, K.B., Faccioli-Martins, P.Y., Riboli, D.F.M., Pereira, V.C., Fernandes, S., Oliveira, A.A., Dantas, A., Zafalon, L.F. and Cunha, M.D.L.R.D.S.D. 2015. Clonal profile, virulence and resistance of Staphylococcus aureus isolated from sheep milk. Braz. J. Microbiol. 46, 535–543. Masschalck, B., Deckers, D. and Michiels, C.W. 2003. Sensitization of outer-membrane mutants of Salmonella typhimurium and Pseudomonas aeruginosa to antimicrobial peptides under high pressure. J. Food Prot. 66, 1360–1367. Massé, J., Vanier, G., Fairbrother, J.M., De Lagarde, M., Arsenault, J., Francoz, D., Dufour, S. and Archambault, M. 2023. Description of antimicrobial-resistant Escherichia coli and their dissemination mechanisms on dairy farms. Vet. Sci. 10, 242. Massella, E., Giacometti, F., Bonilauri, P., Reid, C.J., Djordjevic, S.P., Merialdi, G., Bacci, C., Fiorentini, L., Massi, P., Bardasi, L., Rubini, S., Savini, F., Serraino, A. and Piva, S. 2021. Antimicrobial resistance profile and ExPEC virulence potential in commensal Escherichia coli of multiple sources. Antibiotics 10, 351. Mbindyo, C.M., Gitao, G.C., Plummer, P.J., Kulohoma, B.W., Mulei, C.M. and Bett, R. 2021. Antimicrobial resistance profiles and genes of Staphylococci isolated from mastitic cow’s milk in Kenya. Antibiotics 10, 772. McDougall, S., Clausen, L., Ha, H.J., Gibson, I., Bryan, M., Hadjirin, N., Lay, E., Raisen, C., Ba, X., Restif, O., Parkhill, J. and Holmes, M.A. 2020. Mechanisms of β-lactam resistance of Streptococcus uberis isolated from bovine mastitis cases. Vet. Microbiol. 242, 108592. Mech, P. 2024. Modulation of antibacterial resistance with special reference to inhibition of efflux pump in Staphylococcus aureus. Bareilly, India: Indian Veterinary Research Institute. Meng, L., Liu, H., Lan, T., Dong, L., Hu, H., Zhao, S., Zhang, Y., Zheng, N. and Wang, J. 2020. Antibiotic resistance patterns of Pseudomonas spp. isolated from raw milk revealed by whole genome sequencing. Front. Microbiol. 11, 1005. Meng, L., Zhang, Y., Liu, H., Zhao, S., Wang, J. and Zheng, N. 2017. Characterization of Pseudomonas spp. and associated proteolytic properties in raw milk stored at low temperatures. Front. Microbiol. 8, 2158. Mlynarczyk-Bonikowska, B., Kowalewski, C., Krolak-Ulinska, A. and Marusza, W. 2022. Molecular mechanisms of drug resistance in Staphylococcus aureus. Int. J. Mol. Sci. 23, 8088. Mohamadi, S., Rezaee, R., Hashemi, M., Kiani, B., Ghasemi, S., Alizadeh Sani, M. and Afshari, A. 2023. Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Staphylococcus aureus (VRSA), and vancomycin-resistant Enterococci (VRE) contamination of food samples in Iran: a systematic review and meta-analysis. Iranian J. Med. Microbiol. 17, 135–149. Mollenkopf, D.F. 2017. Epidemiology of ß-lactamase-producing Enterobacteriaceae in humans and Livestock. Columbus, OH: The Ohio State University. Monistero, V., Hossain, D., Fusar Poli, S., De Medeiros, E.S., Cremonesi, P., Castiglioni, B., Biscarini, F., Graber, H.U., Mochettaz, G., Ganio, S., Gazzola, A., Addis, M.F., Roullet, C., Barberio, A., Deotto, S., Biasio, L., Ulloa, F., Galanti, D., Bronzo, V. and Moroni, P. 2025. Prevalence of variant GTRI Staphylococcus aureus isolated from dairy cow milk samples in the alpine grazing system of the Aosta valley and its association with AMR and virulence profiles. Antibiotics 14, 348. Morales-Ubaldo, A.L., Rivero-Perez, N., Valladares-Carranza, B., Velázquez-Ordoñez, V., Delgadillo-Ruiz, L. and Zaragoza-Bastida, A. 2023. Bovine mastitis, a worldwide impact disease: prevalence, antimicrobial resistance, and viable alternative approaches. Vet. Anim. Sci. 21, 100306. Murinda, S.E., Ibekwe, A.M., Rodriguez, N.G., Quiroz, K.L., Mujica, A.P. and Osmon, K. 2019. Shiga toxin-producing Escherichia coli in mastitis: an international perspective. Foodborne Pathog. Dis. 16, 229–243. Mushtaq, M., Agrawal, R., Bhat, M.A., Singh, R. and Pande, N. 2019. Antibiotic resistance gene typing in Staphylococcus aureus isolated from bovine mastitis. Indian J. Anim. Sci. 89, 1188–1191. Muzammil, I., Ijaz, M., Saleem, M.H. and Ali, M.M. 2023. Molecular characterization of vancomycin-resistant Staphylococcus aureus isolated from bovine milk. Zoonoses Public Health 70, 420–433. Mwasinga, W., Shawa, M., Katemangwe, P., Chambaro, H., Mpundu, P., M’Kandawire, E., Mumba, C. and Munyeme, M. 2023. Multidrug-resistant Escherichia coli from raw cow milk in Namwala District, Zambia: public health implications. Antibiotics 12, 1421. Na, S., Intanon, M., Srithanasuwan, A., Chaisri, W. and Suriyasathaporn, W. 2025. Evidence of vancomycin-resistant Staphylococcus aureus, multidrug-resistant S. aureus, and Enterococcus faecium-causing mastitis in Thailand and Cambodia. Vet. World 18, 202–209. Naderi, M., Yousefi Nojookambari, N., Talebi, S., Mohammadi, M.R. and Yazdansetad, S. 2025. Molecular characterization of aminoglycoside-modifying enzymes (AMEs) in aminoglycoside-resistant Staphylococcus aureus: a cross-sectional study in Northeastern Iran. Iranian J. Pathol. 20, 117–124. Nahar, A., Islam, A.K.M.A., Islam, M.N., Khan, M.K., Khan, M.S., Rahman, A.K.M.A. and Alam, M.M. 2023. Molecular characterization and antibiotic resistance profile of ESBL-producing Escherichia coli isolated from healthy cow raw milk in smallholder dairy farms in Bangladesh. Vet. World 16, 1333–1339. Nale, J.Y. and Mcewan, N.R. 2023. Bacteriophage therapy to control Bovine mastitis: a review. Antibiotics 12, 1307. Nandhini, P., Kumar, P., Mickymaray, S., Alothaim, A.S., Somasundaram, J. and Rajan, M. 2022. Recent developments in methicillin-resistant Staphylococcus aureus (MRSA) treatment: a review. Antibiotics 11, 606. Naranjo-Lucena, A. and Slowey, R. 2023. Invited review: antimicrobial resistance in bovine mastitis pathogens: a review of genetic determinants and prevalence of resistance in European countries. J. Dairy Sci. 106, 1–23. Neculai-Valeanu, A.S. and Ariton, A.M. 2022. Udder health monitoring for prevention of Bovine mastitis and improvement of milk quality. Bioengineering 9, 608. Nelli, A., Voidarou, C.C., Venardou, B., Fotou, K., Tsinas, A., Bonos, E., Fthenakis, G.C., Skoufos, I. and Tzora, A. 2022. Antimicrobial and methicillin resistance pattern of potential mastitis-inducing Staphylococcus aureus and coagulase-negative Staphylococci isolates from the mammary secretion of dairy goats. Biology 11, 1591. Nery Garcia, B.L., Dantas, S.T.A., Da Silva Barbosa, K., Mendes Mitsunaga, T., Butters, A., Camargo, C.H. and Nobrega, D.B. 2024. Extended-spectrum beta-lactamase-producing Escherichia coli and other antimicrobial-resistant gram-negative pathogens isolated from bovine mastitis: a one health perspective. Antibiotics 13, 391. Nesterova, E., Morozova, P., Gladkikh, M., Kazemzadeh, S. and Syromyatnikov, M. 2024. Molecular methods for detecting microorganisms in beverages. Beverages 10, 46. Nguyen, Q.D., Tsuruta, T. and Nishino, N. 2020. Examination of milk microbiota, fecal microbiota, and blood metabolites of Jersey cows in cool and hot seasons. Anim. Sci. J. 91, 13441. Ning, K., Zhou, R. and Li, M. 2023. Antimicrobial resistance and molecular typing of Staphylococcus aureus isolates from raw milk in Hunan Province. PeerJ 11, e15847. Oikonomou, G., Addis, M.F., Chassard, C., Nader-Macias, M.E.F., Grant, I., Delbès, C., Bogni, C.I., Le Loir, Y. and Even, S. 2020. Milk microbiota: what are we exactly talking about?. Front. Microbiol. 11, 60. Oliver, J.P., Gooch, C.A., Lansing, S., Schueler, J., Hurst, J.J., Sassoubre, L., Crossette, E.M. and Aga, D.S. 2020. Invited review: fate of antibiotic residues, antibiotic-resistant bacteria, and antibiotic resistance genes in US dairy manure management systems. J. Dairy Sci. 103, 1051–1071. Omwenga, I., Aboge, G.O., Mitema, E.S., Obiero, G., Ngaywa, C., Ngwili, N., Wamwere, G., Wainaina, M. and Bett, B. 2021. Antimicrobial usage and detection of multidrug-resistant Staphylococcus aureus, including methicillin-resistant strains in raw milk of Livestock from Northern Kenya. Microb. Drug Resist. 27, 843–854. Ouamba, A.J.K., Gagnon, M., Lapointe, G., Chouinard, P.Y. and Roy, D. 2022. Graduate student literature review: farm management practices: potential microbial sources that determine the microbiota of raw bovine milk. J. Dairy Sci. 105, 7276–7287. Ozavci, V., Dolgun, H.T.Y., Kirkan, S., Seferoglu, Y., Semen, Z. and Parin, U. 2023. Evaluation of Streptococcus species isolated from subclinical sheep mastitis by molecular methods and determination of virulence factors and antimicrobial resistance genes. Vet. Med. 68, 359–367. Pascu, C., Herman, V., Iancu, I. and Costinar, L. 2022. Etiology of mastitis and antimicrobial resistance in dairy cattle farms in the western part of Romania. Antibiotics 11, 57. Pérez, V.K.C., Costa, G.M.D., Guimarães, A.S., Heinemann, M.B., Lage, A.P. and Dorneles, E.M.S. 2020. Relationship between virulence factors and antimicrobial resistance in Staphylococcus aureus from bovine mastitis. J. Global Antimicrob. Resist. 22, 792–802. Piddock, L.J. 2006. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 19, 382–402. Pitta, D.W., Dou, Z., Kumar, S., Indugu, N., Toth, J.D., Vecchiarelli, B. and Bhukya, B. 2016. Metagenomic evidence of the prevalence and distribution patterns of antimicrobial resistance genes in dairy agroecosystems. Foodborne Pathog. Dis. 13, 296–302. Poirel, L., Madec, J.Y., Lupo, A., Schink, A.K., Kieffer, N., Nordmann, P. and Schwarz, S. 2018. Antimicrobial resistance in Escherichia coli. Microbiol. Spectr. 6(6.4), 14. Prescott, J.F. and Hardefeldt, L.Y. 2013. Other beta-lactam antibiotics: beta-lactamase inhibitors, carbapenems, and monobactams. Antimicrob. Therapy Vet. Med. 6, 175–187. Qu, Y., Zhao, H., Nobrega, D.B., Cobo, E.R., Han, B., Zhao, Z., Li, S., Li, M., Barkema, H.W. and Gao, J. 2019. Molecular epidemiology and distribution of antimicrobial resistance genes of Staphylococcus species isolated from Chinese dairy cows with clinical mastitis. J. Dairy Sci. 102, 1571–1583. Quigley, L., O’Sullivan, O., Stanton, C., Beresford, T.P., Ross, R.P., Fitzgerald, G.F. and Cotter, P.D. 2013. The complex microbiota of raw milk. FEMS Microbiol. Rev. 37, 664–698. Quintieri, L., Fanelli, F. and Caputo, L. 2019. Antibiotic resistant Pseudomonas spp. spoilers in fresh dairy products: an underestimated risk and the control strategies. Foods 8, 372. Rahi, A., Kazemeini, H., Jafariaskari, S., Seif, A., Hosseini, S. and Safarpoor Dehkordi, F. 2020. Genotypic and phenotypic-based assessment of antibiotic resistance and profile of Staphylococcal cassette chromosome mec in the methicillin-resistant Staphylococcus aureus recovered from raw milk. Infect. Drug Resist. 13, 273–283. Ramasamy, T., Keerthana, S., Srinivasan, M.R., Chandrasekar, D., Porteen, K., Borthakur, A., Elamaran, A. and Sriram, P. 2020. Molecular characterization of antibiotic resistance gene Pattern of Staphylococcus aureus and Escherichia coli in mastitis affected dairy cows. Indian J. Anim. Res. 1(6), 6. Ramirez, M.S. and Tolmasky, M.E. 2010. Aminoglycoside modifying enzymes. Drug Resist. Updates 13, 151–171. Reyes, J., Rodriguez-Lecompte, J.C., Blanchard, A., Mcclure, J.T. and Sánchez, J. 2019. Molecular variability of Streptococcus uberis isolates from intramammary infections in Canadian dairy farms from the Maritime region. Can. J. Vet. Res. 83, 168–176. Rocha, G.D., Nogueira, J.F., Gomes Dos Santos, M.V., Boaventura, J.A., Nunes Soares, R.A., José De Simoni Gouveia, J., Matiuzzi Da Costa, M. and Gouveia, G.V. 2022. Impact of polymorphisms in blaZ, blaR1 and blaI genes and their relationship with β-lactam resistance in S. aureus strains isolated from bovine mastitis. Microb. Pathog. 165, 105453. Rodriguez, Z., Lopez-Benavides, M., Gentilini, M.B. and Ruegg, P.L. 2025. Impact of training dairy farm personnel on milking routine compliance, udder health, and milk quality. J. Dairy Sci. 108, 1615–1624. Rubiola, S., Chiesa, F., Dalmasso, A., Di Ciccio, P. and Civera, T. 2020. Detection of antimicrobial resistance genes in the milk production environment: impact of host DNA and sequencing depth. Front. Microbiol. 11:1983. Ruegg, P.L. 2021. What is success? a narrative review of research evaluating outcomes of antibiotics used for treatment of clinical mastitis. Front. Vet. Sci. 8, 639641. Saed, H.A.E.R. and Ibrahim, H.M.M. 2020. Antimicrobial profile of multidrug-resistant Streptococcus spp. isolated from dairy cows with clinical mastitis. J. Adv. Vet. Anim. Res. 7, 186–197. Sapp, A.C., Nane, G.F., Amaya, M.P., Niyonzima, E., Hategekimana, J.P., Vansickle, J.J., Gordon, R.M. and Havelaar, A.H. 2023. Estimates of disease burden caused by foodborne pathogens in contaminated dairy products in Rwanda. BMC. Public Health 23, 657. Savaşan, S. and Sezener, M.G. 2022. The determination of antibiotic resistance and biofilm properties in Pseudomonas aeruginosa isolates from raw milk samples. J. Anatolian Environ. Anim. Sci. 7, 21–26. Scaria, J., Anupama, K.V. and Nidheesh, P.V. 2021. Tetracyclines in the environment: an overview on the occurrence, fate, toxicity, detection, removal methods, and sludge management. Sci. Total Environ. 771, 145291. Schneider, P., Salamon, H., Weizmann, N., Nissim-Eliraz, E., Lysnyansky, I. and Shpigel, N.Y. 2023. Immune profiling of experimental murine mastitis reveals conserved response to mammary pathogenic Escherichia coli, Mycoplasma bovis, and Streptococcus uberis. Front. Microbiol. 14, 1126896. Schwarz, S., Feßler, A.T., Loncaric, I., Wu, C., Kadlec, K., Wang, Y. and Shen, J. 2018. Antimicrobial resistance among Staphylococci of animal origin. Microbiol. Spectr. 6 e0010-2017. Sekhri, I., Chandra, M., Kaur, G., Narang, D., Gupta, D.K. and Arora, A.K. 2020. Prevalence of Pseudomonas aeruginosa and other microorganisms from mastitis milk and their antimicrobial resistance pattern. Indian J. Anim. Res. 55, 716–721. Sharifi, A., Sobhani, K. and Mahmoudi, P. 2023. A systematic review and meta-analysis revealed a high-level antibiotic resistance of bovine mastitis Staphylococcus aureus in Iran. Res. Vet. Sci. 161, 23–30. Sharma, C., Rokana, N., Chandra, M., Singh, B.P., Gulhane, R.D., Gill, J.P.S., Ray, P., Puniya, A.K. and Panwar, H. 2018. Antimicrobial resistance: its surveillance, impact, and alternative management strategies in dairy animals. Front. Vet. Sci. 4, 237. Shen, J., Wu, X., Yang, Y., Lv, Y., Li, X., Ding, X., Wang, S., Yan, Z., Yan, Y., Yang, F. and Li, H. 2021. Antimicrobial resistance and virulence factor of Streptococcus dysgalactiae isolated from clinical bovine mastitis cases in Northwest China. Infect. Drug. Resist. Volume 14, 3519–3530. Shi, H., Wang, L., Li, G., Li, D., Zhai, H., Ji, S., Hu, Y., Lv, T. and Yao, L. 2025. Characteristic profiles of molecular types, antibiotic resistance, antibiotic resistance genes, and virulence genes of Staphylococcus aureus isolates from caprine mastitis in China. Front. Cell. Infect. Microbiol. 15, 1533844. Shoaib, M., Xu, J., Meng, X., Wu, Z., Hou, X., He, Z., Shang, R., Zhang, H. and Pu, W. 2023. Molecular epidemiology and characterization of antimicrobial-resistant Staphylococcus haemolyticus strains isolated from dairy cattle milk in Northwest, China. Front. Cell. Infect. Microbiol. 13, 553. Silva, A.T.F., Gonçalves, J.L., Dantas, S.T.A., Rall, V.L.M., De Oliveira, P.R.F., Dos Santos, M.V., Peixoto, R.D.M. and Mota, R.A. 2023. Genetic and phenotypic characterization of subclinical mastitis-causing multidrug-resistant Staphylococcus aureus. Antibiotics 12, 1353. Singh, R., Arora, A.K., Rai, T.S. and Chandra, M. 2021. Isolation, antibiogram and molecular characterization of Group B Streptococci isolates from Bovine mastitis. Indian J. Anim. Res. 57, 114–119. Skočková, A., Bogdanovičová, K., Koláčková, I. and Karpíšková, R. 2015. Antimicrobial-resistant and extended-spectrum β-lactamase–producing Escherichia coli in raw cow’s milk. J. Food Prot. 78, 72–77. Speksnijder, D.C., Hopman, N.E.M., Kusters, N.E., Timmerman, A., Swinkels, J.M., Penterman, P.A.A., Krömker, V., Bradley, A.J., Botteldoorn, N., Gehring, R. and Zomer, A.L. 2022. Potential of ESBL-producing Escherichia coli selection in bovine feces after intramammary administration of first generation cephalosporins using in vitro experiments. Sci. Rep. 12, 15083. Srinivasan, A., Dick, J.D. and Perl, T.M. 2002. Vancomycin resistance in staphylococci. Clin. Microbiol. Rev. 15, 430–438. Srithanasuwan, A., Intanon, M., Chaisri, W. and Suriyasathaporn, W. 2023. In Vitro bacterial competition of Staphylococcus aureus, Streptococcus agalactiae, and Escherichia coli against coagulase-negative Staphylococci from Bovine mastitis milk. Antibiotics 12, 600. Stanek, P., Żółkiewski, P. and Januś, E. 2024. A review on mastitis in dairy cows research: current status and future perspectives. Agriculture 14, 1292. Stefańska, I., Kwiecień, E., Didkowska, A., Kizerwetter-Świda, M., Chrobak-Chmiel, D., Sałamaszyńska-Guz, A., Żmuda, P., Anusz, K. and Rzewuska, M. 2025. Genetic analysis reveals the genetic diversity and zoonotic potential of Streptococcus dysgalactiae isolates from sheep. Sci. Rep. 15, 3165. Sultana, T., Rahman, M.M., Rahaman, M., Arafat, K.Y., Haider, M.G., Rahman, A.N.M.A., Talukder, A.K., Das, Z.C. and Hoque, M.N. 2025. Genomic characterization of Pseudomonas asiatica as an emerging mastitis pathogen in dairy cows with resistance and virulence implications. J. Global Antimicrob. Resist. . Tartor, Y.H., Enany, M.E., Ismail, N.I., El-Demerdash, A.S., Eidaroos, N.H., Algendy, R.M., Mahmmod, Y. and Elsohaby, I. 2024. Vancomycin-resistant Staphylococcus aureus endangers Egyptian dairy herds. Sci. Rep. 14, 30606. Tewari, R., Mitra, S., Ganaie, F., Das, S., Chakraborty, A., Venugopal, N., Shome, R., Rahman, H. and Shome, B.R. 2019. Dissemination and characterisation of Escherichia coli producing extended-spectrum β-lactamases, AmpC β-lactamases and metallo-β-lactamases from livestock and poultry in Northeast India: a molecular surveillance approach. J. Glob. Antimicrob. Resist. 17, 209–215. Tian, X.Y., Zheng, N., Han, R.W., Ho, H., Wang, J., Wang, Y.T., Wang, S.Q., Li, H.G., Liu, H.W. and Yu, Z.N. 2019. Antimicrobial resistance and virulence genes of Streptococcus isolated from dairy cows with mastitis in China. Microb. Pathog. 131, 33–39. Todorović, D., Velhner, M., Grego, E., Vidanović, D., Milanov, D., Krnjaić, D. and Kehrenberg, C. 2018. Molecular characterization of multidrug-resistant Escherichia coli isolates from Bovine clinical mastitis and pigs in the Vojvodina Province, Serbia. Microb. Drug Resist. 24, 95–103. Tomanić, D., Samardžija, M. and Kovačević, Z. 2023. Alternatives to antimicrobial treatment in bovine mastitis therapy: a review. Antibiotics 12, 683. Tóth, A.G., Csabai, I., Krikó, E., Tőzsér, D., Maróti, G., Patai, A.V., Makrai, L., Szita, G. and Solymosi, N. 2020. Antimicrobial resistance genes in raw milk for human consumption. Sci. Rep. 10, 7464. Tsitou, V.M., Mitov, I. and Gergova, R. 2020. Relationship between MLSB resistance and the prevalent virulence genotypes among Bulgarian Staphylococcus aureus isolates. Acta Microbiol. Immunol. Hung. 68, 55. Tuzcu, N., Hadimli, H.H. and Padron, B. 2023. Determination of virulence genes and antibiotic resistance profiles of Streptococcus agalactiae isolated from buffalo milk. Turkish J. Vet. Anim. Sci. 47, 356–364. Uehara, Y. 2022. Current status of Staphylococcal cassette chromosome mec (SCCmec). Antibiotics 11, 86. Urban-Chmiel, R., Marek, A., Stępień-Pyśniak, D., Wieczorek, K., Dec, M., Nowaczek, A. and Osek, J. 2022. Antibiotic resistance in bacteria—a review. Antibiotics 11, 1079. Uyanik, T., Çadirci, O., Gücükoğlu, A. and Can, C. 2022. Investigation of major carbapenemase genes in ESBL-producing Escherichia coliand Klebsiella pneumoniae strains isolated from raw milk in Black Sea region of Turkey. Int. Dairy J. 128, 105315. Vander Elst, N. 2024. Bacteriophage-derived endolysins as innovative antimicrobials against bovine mastitis-causing streptococci and staphylococci: a state-of-the-art review. Acta Vet. Scand. 66, 20. Venkataraman, S., Shahgolzari, M., Yavari, A. and Hefferon, K. 2024. Delivery of therapeutics using bacteriophage vectors. Biol. Life Sci.. Vezina, B., Al-Harbi, H., Ramay, H.R., Soust, M., Moore, R.J., Olchowy, T.W.J. and Alawneh, J.I. 2021. Sequence characterisation and novel insights into bovine mastitis-associated Streptococcus uberis in dairy herds. Sci. Rep. 11, 3046. Wang, Y., Batra, A., Schulenburg, H. and Dagan, T. 2022. Gene sharing among plasmids and chromosomes reveals barriers for antibiotic resistance gene transfer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 377, 20200467. Weigel, L.M., Clewell, D.B., Gill, S.R., Clark, N.C., Mcdougal, L.K., Flannagan, S.E., Kolonay, J.F., Shetty, J., Killgore, G.E. and Tenover, F.C. 2003. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 302, 1569–1571. Wendlandt, S., Kadlec, K., Feßler, A.T. and Schwarz, S. 2015. Identification of ABC transporter genes conferring combined pleuromutilin-lincosamide-streptogramin A resistance in bovine methicillin-resistant Staphylococcus aureus and coagulase-negative staphylococci. Vet. Microbiol. 177, 353–358. Wendlandt, S., Lozano, C., Kadlec, K., Gómez-Sanz, E., Zarazaga, M., Torres, C. and Schwarz, S. 2013. The enterococcal ABC transporter gene lsa(E) confers combined resistance to lincosamides, pleuromutilins and streptogramin A antibiotics in methicillin-susceptible and methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 68, 473–475. Widodo, A., Lamid, M., Effendi, M.H., Khailrullah, A.R., Kurniawan, S.C., Silaen, O.S.M., Riwu, K.H.P., Yustinasari, L.R., Afnani, D.A., Dameanti, F.N.A.E.P. and Ramandinianto, S.C. 2023a. Antimicrobial resistance characteristics of multidrug resistance and extended-spectrum beta-lactamase producing Escherichia coli from several dairy farms in Probolinggo, Indonesia. Biodivers. J. Biol. Divers. 24, 539–546. Widodo, A., Lamid, M., Effendi, M.H., Tyasningsih, W., Raharjo, D., Khairullah, A.R., Kurniawan, S.C., Yustinasari, L.R., Riwu, K.H.P. and Silaen, O.S.M. 2023b. Molecular identification of blaTEM and blaCTX-M genes in multidrug-resistant Escherichia colifound in milk samples from dairy cattle farms in Tulungagung, Indonesia. J. Vet. Res. 67, 381–388. World Health Organization. 2017. Stop using antibiotics in healthy animals to prevent the spread of antibiotic resistance. Geneva: World Health Organization. Xu, C., Kong, L., Gao, H., Cheng, X. and Wang, X. 2022a. A review of current bacterial resistance to antibiotics in food animals. Front. Microbiol. 13, 822689. Xu, J., Shi, C., Song, M., Xu, X., Yang, P., Paoli, G. and Shi, X. 2014. Phenotypic and genotypic antimicrobial resistance traits of foodborne Staphylococcus aureus isolates from Shanghai. J. Food Sci. 79, M635–M642. Xu, T., Cao, W., Huang, Y., Zhao, J., Wu, X. and Yang, Z. 2022b. The prevalence of Escherichia coli derived from Bovine clinical mastitis and distribution of resistance to antimicrobials in Part of Jiangsu Province, China. Agriculture 13, 90. Yang, F., Shi, W., Meng, N., Zhao, Y., Ding, X. and Li, Q. 2023. Antimicrobial resistance and virulence profiles of staphylococci isolated from clinical bovine mastitis. Front. Microbiol. 14, 1190790. Yang, Y., Xie, S., He, F., Xu, Y., Wang, Z., Ihsan, A. and Wang, X. 2024. Recent development and fighting strategies for lincosamide antibiotic resistance. Clin. Microbiol. Rev. 37, 161. Yu, Z.N., Wang, J., Ho, H., Wang, Y.T., Huang, S.N. and Han, R.W. 2020. Prevalence and antimicrobial-resistance phenotypes and genotypes of Escherichia coli isolated from raw milk samples from mastitis cases in four regions of China. J. Global Antimicrob. Resist. 22, 94–101. Yuan, H., Han, S., Zhang, S., Xue, Y., Zhang, Y., Lu, H. and Wang, S. 2022. Microbial properties of raw milk throughout the year and their relationships to quality parameters. Foods 11, 3077. Zahid, P., Soliman, E. and Mahdy, Z. 2023. Molecular identification of Methicillin-resistant staphylococcus aureus (MRSA) and Vancomycin- resistant staphylococcus aureus (VRSA) strains isolated from milk and milk products. Benha Vet. Med. J. 45, 213–217. Zeng, J., Wang, Y., Fan, L., Yang, N., Pan, J., Han, Y., Wang, X., Li, Q., Guo, G., Zheng, J. and Zeng, W. 2022. Novel Streptococcus uberis sequence types causing bovine subclinical mastitis in Hainan, China. J. Appl. Microbiol. 132, 1666–1674. Zhang, S., Piepers, S., Shan, R., Cai, L., Mao, S., Zou, J., Ali, T., De Vliegher, S. and Han, B. 2018. Phenotypic and genotypic characterization of antimicrobial resistance profiles in Streptococcus dysgalactiae isolated from bovine clinical mastitis in 5 provinces of China. J. Dairy Sci. 101, 3344–3355. Zhang, T., Tao, L., Boonyayatra, S. and Niu, G. 2021. Antimicrobial resistance of Streptococcus uberis isolated from Bovine mastitis: a review. Indian J. Anim. Res. 56, 1435–1441. Zhang, Y., Zhang, N., Wang, M., Luo, M., Peng, Y., Li, Z., Xu, J., Ou, M., Kan, B., Li, X. and Lu, X. 2023. The prevalence and distribution of aminoglycoside resistance genes. Biosafety Health 5, 14–20. Zhang, Z., Chen, Y., Li, X., Wang, X. and Li, H. 2022. Detection of antibiotic resistance, virulence gene, and drug resistance gene of Staphylococcus aureus isolates from Bovine mastitis. Microbiol. Spectr. 10, 471. Zhao, Y., Shao, W., Wang, F., Ma, J., Chen, H., Wang, S., Wu, Y., Wang, C., Zheng, N. and Wang, J. 2022. Antimicrobial resistance and virulence genes of isolated from mastitis milk samples in China. J. Vet. Res. 66, 581–590. | ||
| How to Cite this Article |
| Pubmed Style Kazemzadeh S, Korneeva O, Shabunin S, Syromyatnikov M. Antibiotic resistance in mastitis-causing bacteria: Exploring antibiotic-resistance genes, underlying mechanisms, and their implications for dairy animal and public health. Open Vet. J.. 2025; 15(9): 3980-4006. doi:10.5455/OVJ.2025.v15.i9.5 Web Style Kazemzadeh S, Korneeva O, Shabunin S, Syromyatnikov M. Antibiotic resistance in mastitis-causing bacteria: Exploring antibiotic-resistance genes, underlying mechanisms, and their implications for dairy animal and public health. https://www.openveterinaryjournal.com/?mno=250397 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i9.5 AMA (American Medical Association) Style Kazemzadeh S, Korneeva O, Shabunin S, Syromyatnikov M. Antibiotic resistance in mastitis-causing bacteria: Exploring antibiotic-resistance genes, underlying mechanisms, and their implications for dairy animal and public health. Open Vet. J.. 2025; 15(9): 3980-4006. doi:10.5455/OVJ.2025.v15.i9.5 Vancouver/ICMJE Style Kazemzadeh S, Korneeva O, Shabunin S, Syromyatnikov M. Antibiotic resistance in mastitis-causing bacteria: Exploring antibiotic-resistance genes, underlying mechanisms, and their implications for dairy animal and public health. Open Vet. J.. (2025), [cited January 12, 2026]; 15(9): 3980-4006. doi:10.5455/OVJ.2025.v15.i9.5 Harvard Style Kazemzadeh, S., Korneeva, . O., Shabunin, . S. & Syromyatnikov, . M. (2025) Antibiotic resistance in mastitis-causing bacteria: Exploring antibiotic-resistance genes, underlying mechanisms, and their implications for dairy animal and public health. Open Vet. J., 15 (9), 3980-4006. doi:10.5455/OVJ.2025.v15.i9.5 Turabian Style Kazemzadeh, Shima, Olga Korneeva, Sergey Shabunin, and Mikhail Syromyatnikov. 2025. Antibiotic resistance in mastitis-causing bacteria: Exploring antibiotic-resistance genes, underlying mechanisms, and their implications for dairy animal and public health. Open Veterinary Journal, 15 (9), 3980-4006. doi:10.5455/OVJ.2025.v15.i9.5 Chicago Style Kazemzadeh, Shima, Olga Korneeva, Sergey Shabunin, and Mikhail Syromyatnikov. "Antibiotic resistance in mastitis-causing bacteria: Exploring antibiotic-resistance genes, underlying mechanisms, and their implications for dairy animal and public health." Open Veterinary Journal 15 (2025), 3980-4006. doi:10.5455/OVJ.2025.v15.i9.5 MLA (The Modern Language Association) Style Kazemzadeh, Shima, Olga Korneeva, Sergey Shabunin, and Mikhail Syromyatnikov. "Antibiotic resistance in mastitis-causing bacteria: Exploring antibiotic-resistance genes, underlying mechanisms, and their implications for dairy animal and public health." Open Veterinary Journal 15.9 (2025), 3980-4006. Print. doi:10.5455/OVJ.2025.v15.i9.5 APA (American Psychological Association) Style Kazemzadeh, S., Korneeva, . O., Shabunin, . S. & Syromyatnikov, . M. (2025) Antibiotic resistance in mastitis-causing bacteria: Exploring antibiotic-resistance genes, underlying mechanisms, and their implications for dairy animal and public health. Open Veterinary Journal, 15 (9), 3980-4006. doi:10.5455/OVJ.2025.v15.i9.5 |