| Review Article | ||
Open Vet. J.. 2025; 15(9): 4007-4023 Open Veterinary Journal, (2025), Vol. 15(9): 4007-4023 Review Article Polysulfated glycosaminoglycan as a treatment for osteoarthritis in veterinary medicine: Summary of the pharmacological, laboratory, and clinical dataGary W. White*GCT Consulting Services, Inc., Sallisaw, USA *Corresponding Author: Gary W. White. GCT Consulting Services, Inc., Sallisaw, USA. Email: gwhite [at] ipa.net Submitted: 07/04/2025 Revised: 10/08/2025 Accepted: 25/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
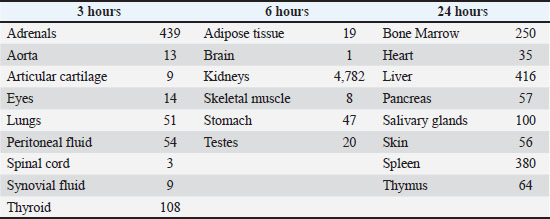
ABSTRACTPolysulfated glycosaminoglycan (PSGAG) is an antiarthritic drug that has been used in veterinary medicine for many years. PSGAG is rapidly distributed to diseased joint tissue after intraarticular or intramuscular administration, as shown in pharmacological studies conducted on a variety of animal species. In diseased joint tissue, PSGAG stimulates: 1) its own incorporation into the cartilage matrix, 2) inhibition of catabolic enzymes, 3) anabolic effects in the synovial and cartilage tissue, and 4) anti-inflammatory effects. Laboratory and clinical studies in humans, rabbits, horses, and dogs have shown reduced severity of clinical signs and beneficial biochemical and morphological effects in inflamed or damaged joints. The drug has minimal side effects and adverse reactions in horses and dogs. Due to the above findings, PSGAG has been classified as a disease modifying osteoarthritis drug (DMOAD), and the drug remains a popular treatment for synovial inflammation and osteoarthritis in horses and dogs. Herein, we review the experimental and clinical evidence that led PSGAG to its classification as a DMOAD. Keywords: Polysulfated glycosaminoglycan, Osteoarthritis, Cartilage, Veterinary, Horse, and dog. IntroductionPolysulfated glycosaminoglycan (PSGAG) is a semi–synthetic glycosaminoglycan (GAG), prepared by extracting GAGs from bovine tracheal cartilage (McIlwraith et al., 2001). GAGs are polysaccharides composed of repeating disaccharide units. After their extraction, these GAGs undergo sulfate esterification of certain sugar hydroxyl groups (McIlwraith et al., 2001). The GAG present in PSGAG is principally chondroitin sulfate containing 3–4 sulfate esters per disaccharide unit, making this molecule approximately 13% sulfur (Burkhardt and Ghosh, 1987). The molecular weight of PSGAG ranges from 2,000 to 16,000 Daltons. The average molecular weight for PSGAG used in the manufacturing of Adequan is 3,500 to 8,000 Daltons (Burkhardt and Ghosh, 1987). PSGAG was originally prepared over 50 years ago as a synthetic heparinoid (Eylau, 1959). Its use as a treatment for human arthritis was first described in 1959 (Eylau, 1959). The drug’s use in dogs and horses was first reported in 1966 (Kubitza, 1966). Although alternative osteoarthritis drugs have emerged over the years, PSGAG is still the most popular DMOAD in the US market (Ferris et al., 2011; Zanotto and Frisbie, 2021; “Equine Market Mega Study V. Product and Market Insights,” 2022). The pharmacology and clinical use of PSGAG have been extensively studied in man and other species. Herein, beginning with the initial descriptions of the pharmacokinetic properties of PSGAG in various animal species, we review the evidence, accumulated over five decades, of its anti-osteoarthritis properties. A body of work that led to the present classification of PSGAG as a DMOAD. Absorption, Distribution, Metabolism, and Excretion of PSGAG After Intramuscular and Intraarticular InjectionThe absorption and distribution of PSGAG after intramuscular injection has been studied in many species, including rats, rabbits, humans, horses, and dogs (Panse et al., 1976; Bach et al., 1977; Burba et al., 1993; Collier et al., 1998). In rabbits, the maximum blood concentrations of PSGAG occurs 20–40 minutes after intramuscular administration (Panse et al., 1976; Bach et al., 1977). If the dose of PSGAG injected is within the range of 1.79–7.50 mg/kg, the concentration of PSGAG in the blood is linearly proportional to the dose administered (Bach et al., 1977). PSGAG levels reach a peak in the superficial digital flexor tendon 2 hours after intramuscular administration and remain detectable for 192 hours (Walesby et al., 2000). The endogenous distribution of radiolabeled PSGAG after intramuscular (Panse et al., 1976) and intraarticular (Jikuya and Doi, 1975) injection was assessed at various times, from 2 hours up to 10 days post-injection. The two routes of administration produced comparable results. After intraarticular injection, PSGAG was found throughout all tissues and body fluids investigated, reaching peak concentrations at various times in various organs (Table 1). Table 1. Maximum concentration of PSGAG (all values in dpm/mg or dpm/ml) reached in the various organs in rabbits after an intraarticular injection of 1 mg/kg. Adapted from (Jikuya and Doi, 1975).
In human osteoarthritis patients, PSGAG absorption after intramuscular administration is similar to that in the rabbits, reaching maximum serum concentrations at 30 minutes post injection (Muller et al., 1983). PSGAG is bound to serum proteins in human blood. Thirty to forty percent of the drug binds to both albumin and chi- and beta-globulins (Muller et al., 1981). Thus, the drug exists in both the bound and free form in the blood stream. After peaking at about 30 minutes from the intramuscular injection, PSGAG blood levels decrease rapidly for about 24 hours to a level that stays relatively constant for several days (Muller et al., 1981, 1983). This can be explained by the distribution of the unbound drug to tissues and the persistence of the bound drug in the blood (Muller et al., 1981, 1983). Because of its relatively low molecular weight, the synovial membrane is not a significant barrier to the passage of the drug from the blood stream to the synovial fluid (Panse et al., 1976; Gallacchi and Muller, 1979; Muller et al., 1981, 1983). The distribution from the synovial fluid to the cartilage takes place by diffusion (Iwata et al., 1980). After a single intramuscular injection of PSGAG (125 mg 3HPSGAG), maximum drug concentrations reach all layers of the articular cartilage 24–48 hours after injection and decrease steadily from 48 to 96 hours (Muller et al., 1983). The uptake of the drug in cartilage appears to vary in the various layers of cartilage, with the highest uptake by the more superficial layer and the lowest uptake in the layer nearest subchondral bone. A summary of these data is shown in Table 2. Once in the articular cartilage, the drug is deposited into the cartilage matrix and appears to be bound to macromolecules; perhaps proteoglycans (Iwata et al., 1980) or other non-collagenous proteins (Burkhardt and Ghosh, 1987). Table 2. PSGAG Concentrations in cartilage after intramuscular injection of 125 mg to human patients (μg/g). Adapted from (Muller et al., 1983).
In horses, the mean concentration of PSGAG in the cartilage, 96 hours after receiving 500 mg 3HPSGAG by intramuscular injection, was approximately 0.3 μg/g (Burba et al., 1993). The distribution curves in serum and synovial fluid were similar to those of human and rabbit at similar doses (Iwata et al., 1980; Burba et al., 1993). In dogs, the mean concentration of PSGAG in the cartilage, 72 hours after intramuscular injection of 2 mg 3HPSGAG/lb body weight, was approximately 0.15 μg/g. Uptake of PSGAG in joint tissues is higher if the tissue is inflamed or diseased. Using an adjuvant model of inflammation in the paw of the rat, a study showed higher concentrations of PSGAG in inflamed joint tissue (Muller et al., 1981). In horses, peak synovial fluid levels of PSGAG were approximately 30% higher in joints with surgically induced full-thickness cartilage defects than in non-traumatized control joints (Burba et al., 1993). In the dog, the cartilage from joints with adjuvant-induced synovitis had 80% higher levels of PSGAG than the cartilage from normal joints, 72 hours after intramuscular injection of 2 mg 3HPSGAG/lb body weight (Collier et al., 1998). The levels of PSGAG achieved in the joint tissues of horses and dogs after intramuscular injection were sufficient to provide antidegenerative and anabolic effects in those tissues (Burba et al., 1993; Collier et al., 1993, 1998). Levels of PSGAG sufficient to achieve such effects were determined in the course of several in vivo studies (Burba et al., 1993; Collier et al., 1993, 1998). The metabolism of PSGAG in humans and rabbits involves desulfation and depolymerization, which give rise to sulfate, oligosaccharides, and monosaccharides as metabolites (Jikuya and Doi, 1975; Panse et al., 1976; Muller et al., 1983). In rabbits, metabolism takes place in the liver, spleen, and bone marrow and may also occur in the kidney (Jikuya and Doi, 1975). Although it is known that GAGs are metabolized by chondrocytes, no metabolism of the drug has been observed in the knee joint of rats (Iwata et al., 1980). It has been postulated that PSGAG can be degraded in granulocytes and that extracellular degradation may also take place (Kruze et al., 1976; Greiling, 1979). In rabbits, metabolism of PSGAG begins within 3 hours of intraarticular injection, and metabolites may be excreted for several days (Jikuya and Doi, 1975). A similar development occurs after intramuscular injection (Panse et al., 1976). PSGAG is administered intramuscularly and not protein bound or bound to other tissues and is excreted (Jikuya and Doi, 1975). This excretion is primarily via the kidneys, with a small proportion in the feces. In rabbits, 55.3% of intramuscularly administered PSGAG was excreted in the urine and 1.7% in the feces within 2 days (Panse et al., 1976). The rate of degradation of PSGAG in humans is reported to be similar to that in rabbits (Muller et al., 1981, 1983). In humans 35%–40% of a 50 mg intramuscular dose of PSGAG was excreted via the kidneys within 12 hours of administration (Muller et al., 1983). Both the metabolized and the unmetabolized drug are excreted, and the proportion of metabolites increases with time (Jikuya and Doi, 1975; Panse et al., 1976; Muller et al., 1981, 1983). Incorporation of PSGAG in cartilage matrixVarious authors have reported the incorporation of PSGAG in the cartilage matrix (Dustmann et al., 1974; Gallacchi and Muller, 1979; Golding and Ghosh, 1983). PSGAG binds to macromolecules in the extracellular matrix of cartilage; the identity of the binding site is thought to be either a proteoglycan or other non-collagenous protein. (Dustmann et al., 1974; Burkhardt and Ghosh, 1987). This activity in a damaged cartilage with a depleted proteoglycan content has been described as a repair process (Baici et al., 1980; Altman et al., 1989). It is possible that the binding of PSGAG improves the biochemical properties of damaged cartilage matrix, such as water binding capacity, or protect damaged matrix components from further enzymatic degradation (Baici et al., 1980; Altman et al., 1989). Biochemical Effects of PSGAG in Degenerative Joint DiseasesAt therapeutic doses, PSGAG has several biochemical effects on injured or degenerative joints. The sum of these effects is called chondroprotection: the inhibition of degradation and effects favorable to the repair of articular cartilage (Burkhardt and Ghosh, 1987). Drugs having these effects are classified as chondroprotective. Inhibition of catabolic enzymesThe ability of PSGAG to inhibit a broad range of catabolic enzymes has been reported both in vitro and in vivo (Burkhardt and Ghosh, 1987; May et al., 1988; Altman et al., 1989). This is significant because the enzymatic degradation of collagen, proteoglycans, and hyaluronic acid is a critical element of the pathogenesis of degenerative joint disease (Burkhardt and Ghosh, 1987). Many catabolic enzymes are inhibited by fluid or tissue concentrations of PSGAG as low as 0.1–2 μg/ml (Panse et al., 1976; Burkhardt and Ghosh, 1987). The mechanism of enzyme inhibition varies with the type of enzyme. For many of the glycosidases, which degrade GAGs and hyaluronic acid, the drug acts as a competitive substrate (Greiling, 1979). For elastase and other lysosomal peptidases, the inhibition appears to be both competitive and noncompetitive. In the case of elastase, electrostatic binding of the highly positive enzyme molecule to the negatively charged PSGAG molecule inactivates the enzyme (Baici et al., 1980). In the case of certain neutral proteases PSGAG appears to inhibit the conversion of the enzyme from inactive to active form (Altman et al., 1989). The inhibition of more than a dozen catabolic enzymes by PSGAG has been reported. The most important examples of this inhibitory effect are specifically described below. Elastase inhibition has been reported in vitro at concentrations as low as 0.5 μg/ml (Kruze et al., 1976; Baici et al., 1980; Stephens et al., 1980; Walton et al., 1981). Elastase breaks down elastin, collagen, proteoglycans, and structural glycoproteins (Baici et al., 1980). In addition to elastase, other collagen-degrading enzymes, including cathepsin B1, can be inhibited by PSGAG (Kruze et al., 1976; Stancikova et al., 1977). Metalloproteases are highly effective in the degradation of proteoglycans. Stromelysin is a neutral protease (designated matrix metalloprotease 3 or MMP-3) that degrades proteoglycans at low concentrations. In nutritionally deprived equine synoviocyte cultures, PSGAG was the only drug able to significantly inhibit stromelysin at concentrations readily achievable in equine joint tissues (May et al., 1988). An in vitro study of purified rat stromelysin, showed that PSGAG inhibited its activity at concentrations of 0.05–0.5 μg/ml (Nethery et al., 1992). In bovine chondrocytes treated with IL1α, to induce the expression of matrix metalloproteases, the addition of PSGAG reduced collagenase and proteoglycanase activities and inhibited the expression of MMP-1 and MMP-3 (Sadowski and Steinmeyer, 2002). The authors concluded that PSGAG could help reduce cartilage degradation. An in vitro study of equine gelatinase MMPs (MMP-2 and MMP-9) showed that PSGAG significantly inhibited the degrading activity of these two enzymes, but at concentrations that are not achievable in vivo (Clegg et al., 1998). An in vivo study in dogs with transected anterior cruciate ligaments showed that treatment with PSGAG (4 mg/kg by intramuscular injection twice weekly for 4 weeks) lowered the levels of active metalloprotease in their articular cartilage compared to the cartilage of control dogs (Altman et al., 1989). Loss of hyaluronic acid in synovial fluid and in the proteoglycan complexes of cartilage matrix due to enzymatic activity are significant mechanism in the pathogenesis of degenerative joint diseases (Burkhardt and Ghosh, 1987). PSGAG inhibits enzymes which degrade hyaluronic acid; the glycanohydrolases or “hyaluronidases”, beta-glucuronidase, and beta-N-acetyl-glycosaminidases (Greiling and Kaneko, 1973; Dustmann et al., 1974; Greiling, 1974; Momburg, 1976; Greiling, 1979; Verbruggen and Veys, 1979). Thus, PSGAG inhibits enzymes that destroy hyaluronic acid, proteoglycans, and collagen. Degenerative joint disease is characterized by a net loss of these critical components (Burkhardt and Ghosh, 1987). Anabolic effects in damaged or diseased joint tissuePSGAG stimulates the synthesis of collagen, proteoglycans, and hyaluronic acid by chondrocytes or synoviocytes (Verbruggen and Veys, 1980; Burkhardt and Ghosh, 1987; Glade, 1990). PSGAG induces increased amounts of proteoglycan and hyaluronate with higher molecular weight, in cultured human chondrocytes (Verbruggen and Veys, 1980) and also in lapine chondrocytes (Burkhardt and Ghosh, 1987). Increased synthesis of proteoglycans and collagen has been demonstrated in the cartilage of embryonic chicken, in cultures of osteoarthritic human cartilage, and in cultures of arthritic equine cartilage (Von der Mark, 1980; Glade, 1990). However, in a study of mildly osteoarthritic equine cartilage explants, cultures showed that PSGAG failed to stimulate sulfated proteoglycan synthesis at the doses studied. In fact, these doses (0.025 and 25 PSGAG mg/ml) reduced proteoglycan synthesis (Caron et al., 1993). PSGAG induced changes in proteoglycan metabolism and DNA content of normal and osteoarthritic cartilage, in a study using canine cartilage explants (Sevalla et al., 2000). When treated with PSGAG, articular cartilage explants maintained or increased DNA content at the expense of proteoglycan synthesis. Following MMP activation, proteoglycan degradation was inhibited by PSGAG in both osteoarthritic and control explants. It is interesting to note that the osteoarthritic cartilage was responsive to PSGAG treatment at a 100-fold lower concentration than control cartilage. These authors concluded that, if these in vitro results are also occurring in vivo, PSGAG may modify the progression of osteoarthritis in articular cartilage by supporting chondrocyte viability or stimulating chondrocyte division and protecting against proteoglycan degradation. Stimulation of hyaluronic acid synthesis by synoviocytes has been demonstrated both in vitro and in vivo. In a synovial membrane explant culture, PSGAG stimulated hyaluronic acid synthesis at concentrations as low as 0.2 μg/ml (Verbruggen and Veys, 1977). Other investigations in synovioblast cultures have confirmed these findings (Verbruggen and Veys, 1980; Nishikawa et al., 1985). A study using the inflamed subcutaneous air pouch model in rats showed that injections of PSGAG, into the air pouch for 7 days, resulted in the production of higher molecular weight hyaluronan in the pouch fluid, as compared to the non-treated pouch (Francis et al., 1993). An increase in synovial fluid hyaluronate concentration with PSGAG therapy has been demonstrated in humans, pigs (Brennan et al., 1987), and horses (Burba et al., 1993). The combination of the PSGAG incorporation into the cartilage matrix, the inhibition of catabolic enzymes, and the stimulation of endogenous anabolic pathways in joint tissue decreases the net loss of collagen and proteoglycans that characterizes degenerative joint disease. These mechanisms are the hallmark of a chondroprotective drug. More recently, the term “disease modifying osteoarthritis drug” or DMOAD has been proposed for a treatment that can modify, retard, or reverse the morphological changes of osteoarthritic articular cartilage in vivo. The above data, taken as a whole, support the designation of PSGAG as both a chondroprotective drug and as a DMOAD. Anti-inflammatory effectsIn addition to the biochemical effects previously described, PSGAG has also been shown to be anti-inflammatory in at least three important pathways. In vitro, PSGAG inhibits the biosynthesis of the eicosanoid prostaglandin E2 (Egg, 1983). Concentrations of PSGAG achievable via intraarticular injection inhibit prostaglandin E2 synthesis in cultured equine synoviocytes (Frean and Lees, 2000). Prostaglandin E2 plays a key role in pain and inflammation in joint disease (Burkhardt and Ghosh, 1987). PSGAG also decreases in a dose-dependent manner the release of toxic oxygen radicals from human neutrophils (Tsuboi et al., 1988). Oxygen radicals are implicated in the early breakdown of hyaluronic acid in the synovial fluid of inflamed joints (Burkhardt and Ghosh, 1987). Furthermore, PSGAG inhibits in vitro both equine (Rashmir-Raven et al., 1992) and human complement cascade (Loos and Heinz, 1982; de Messias et al., 1994). The inhibition of complement may contribute to the drug’s anti-inflammatory effect and has been reported in the plasma of horses, dogs, donkeys, and pigs (Zhou et al., 2012). These anti-inflammatory effects may account in part for the relief of pain and inflammation seen as a result of PSGAG treatment of degenerative joint diseases. The Benefits of PSGAG in the Treatment of Experimental and Clinical Joint DiseasesStudies in laboratory animalsEarly studies investigated the effects of PSGAG treatment using laboratory animals (Dustmann et al., 1974; Golding and Ghosh, 1983; Brennan et al., 1987). A more recent study using the blunt impact patellofemoral joint rabbit model concluded that intramuscular PSGAG mitigated the stiffness in the cartilage of the impacted patellae characteristically induced by the model (Ewers and Haut, 2000). Clinical trials in humansPSGAG was first reported as a treatment for human degenerative joint diseases in 1959 (Eylau, 1959). Several clinical trials of PSGAG in the treatment of osteoarthritis have been conducted since. Arthritis and degenerative joint diseases in humans have pathologic features that are strikingly similar to those in other species. Therefore, a summary of the important findings from these human trials seems appropriate in the context of this review. – PSGAG is indicated for the treatment of degenerative joint disorders and is most effective as long as there is no extensive full-thickness loss of articular cartilage (Richter, 1970; Dettmer, 1979; Siegmeth and Radi, 1983). – PSGAG may be given intraarticularly, intramuscularly, subcutaneously, or by local infiltration (Richter, 1970; Dettmer, 1979). The intramuscular and intraarticular routes are said to be equally effective (Dettmer, 1979). The drug should not be administered intraarticularly in the presence of active joint inflammation. The intramuscular treatment regimen in humans is 50 mg twice weekly for up to 15 injections. – PSGAG-induced clinical improvement may persist for 6 months or more after the end of treatment (Siegmeth and Radi, 1983). – PSGAG may be effective in the treatment of work-related but not sports-related lateral humeral epicondylalgia (Akermark et al., 1995). In small clinical trials, PSGAG was superior to placebo for the treatment of chondromalacia patella (Raatikainen et al., 1990) but was no more effective than conservative treatment for human patellofemoral pain syndrome (Kannus et al., 1992). Despite these promising preliminary results, there remains a need for large-scale, well-designed clinical trials to fully evaluate the efficacy of PSGAG in treating human arthritis. Experimental and clinical trials in horsesPSGAG has been approved for the treatment of lameness secondary to traumatic and/or degenerative joint dysfunction of the equine carpus in the U. S. since 1984 (Adequan IA NADA 136-383). The intramuscular version was approved in 1989 (Adequan IM NADA 140-901). The efficacy of both routes of administration has been shown in experimental and clinical trials. The optimal intraarticular dose of PSGAG in the equine carpus was established using an experimental model (FDA, 1984; Hamm et al., 1984). The model was created by the injection of a chemical irritant (Complete Freund’s Adjuvant) into the carpal joint of 30 healthy horses, creating a synovitis. The horses were given weekly injections of placebo, 5, 125, 250, or 500 mg PSGAG into the affected joint. Parameters of response included maximum permitted flexion, stride length at rest and after exercise, joint circumference, joint volume, and synovial fluid protein. Horses receiving the 250 and 500 mg treatment had statistically significant improvement in all parameters, compared to the other groups, but were themselves not significantly different (Hamm et al., 1984). The 250 mg dose at weekly intervals for up to 5 weeks was selected as the optimal intraarticular dose. This was the smallest dose that led to significant improvements in the clinical and laboratory parameters listed above. The same experimental model was used to find the optimal intramuscular dose in the horse (Hamm and Jones, 1988; FDA, 1996). Doses of 500 and 1,000 mg twice weekly for 4 weeks led to significant improvement in the clinical and laboratory parameters. The best regime was a 500 mg dose every 4 days for 7 doses. Based on these findings on experimentally induced carpitis, the efficacy of the 250 mg intraarticular dose was tested on naturally occurring, traumatic or degenerative, carpal joint diseases. In a field trial conducted in the U.S., 109 horses with joint disease were treated at eight centers with weekly intraarticular injections of Adequan. Cases were selected based on clinical examination and synovial fluid parameters. Treatment induced a significant improvement in synovial fluid protein levels, lameness, pain, swelling, and heat in the affected joints. The investigators judged the overall clinical response as good or excellent in 90% of cases (FDA, 1984; Hamm et al., 1984). The efficacy of intraarticular PSGAG was confirmed in a subsequent experimental study of eight horses with a physical defect created in one carpus and a chemical defect created in the contralateral carpus (Yovich et al., 1987). Four horses received PSGAG injections (250 mg intraarticular) and four received placebo injections. Horses were necropsied at 8 weeks after model induction. The carpal joints with physical defects did not develop the degree of degenerative change seen in the carpal joints injured chemically. In the chemically induced defective joints, PSGAG treatment resulted in a significant increase in GAG content in the articular cartilage, less chondrocyte degeneration, and less erosion than the control group. This study supported the chondroprotective actions of PSGAG in treating equine carpal joint disorders. The study was repeated using PSGAG via the intramuscular route, but the drug failed to show the same benefits as did the intraarticular study (Trotter et al., 1989). A retrospective clinical study examined 128 cases of lameness that responded positively to intraarticular anesthesia of the distal interphalangeal joint (Kristiansen and Kold, 2007). The horses were treated with three intraarticular injections of PSGAG at 8-day intervals (Regime A) or a single intraarticular injection of methylprednisolone acetate (MPA) (Regime B). If the horses in Regime B were not sound enough to return to work in 4 weeks, three intraarticular injections of PSGAG were administered at 8-day intervals. A successful outcome was defined as the horse returning to his/her previous level of work and remaining sound for a minimum of 1 year. In the group receiving Regime A, 67% of the horses had a successful outcome compared to 46% of the horses receiving Regime B (p=0.02; univariate analysis). The authors concluded that PSGAG treatment with no MPA pretreatment increased the odds of a successful outcome in these cases. A randomized, blinded, controlled clinical trial comparing three weekly intraarticular injections of PSGAG, sodium hyaluronate or placebo was conducted in Standardbred trotters with moderate to severe traumatic arthritis in Norway (Gaustad and Larsen, 1995). There was a significantly superior effect of both PSGAG and sodium hyaluronate compared to placebo for reduction of lameness score during the treatment period (p=0.03), time to soundness (p < 0.04), and prevalence of soundness at the final examination (p < 0.01). Three studies involving a model of equine osteoarthritis induced by surgical creation of an osteochondral defect in the carpal joint and treatment with intraarticular PSGAG have been reported. In one study, the effect of exercise and intraarticular PSGAG on the repair of the osteochondral defect was examined (Todhunter et al., 1993a). The authors reported that exercise was beneficial to cartilage, and immediate post operative PSGAG treatment was detrimental to the development of cartilaginous repair tissue. It is interesting to note that there was a reduction in lameness in the PSGAG treated, exercised ponies versus the untreated exercised ponies. The question of whether this increase in lameness in the untreated ponies might have led these ponies to protect the carpus and decrease trauma by allowing repair to proceed was raised. A second study assessed the effects of exercise and intraarticular PSGAG treatment on the development of osteoarthritis in the same carpal osteochondral defect model (Todhunter et al., 1993b). Radiographic scores indicated that the exercised non-treated ponies had significantly (p < 0.05) more signs of osteoarthritis than exercised medicated ponies. PSGAG treatment improved carpal flexion from weeks 2–6 compared to non-medicated ponies. Treated ponies also had a significant reduction in combined joint capsule and synovial membrane thickness at weeks 4, 8, and 13 compared with non-medicated joints. There was also significantly less synovial membrane inflammation on histology for the exercised medicated joints. The authors concluded that PSGAG reduced the clinical signs of osteoarthritis in the exercised ponies. As in the earlier study, PSGAG treatment was associated with less optimal cartilage repair tissue compared to the untreated joints. The third study in this model used scintigraphy to assess the effects of exercise and intraarticular PSGAG on the created osteochondral defects (Todhunter et al., 1993a). Both exercise and PSGAG treatment without exercise were said to increase bone remodeling. The combination of exercise and PSGAG resulted in decreased bone remodeling (p < 0.05), indicating a protective effect against the development of osteoarthritis in exercised and medicated ponies. The effect of intraarticular PSGAG and sodium hyaluronate was evaluated for the treatment of equine osteoarthritis induced by creation of an osteochondral fragment in the middle carpal joint and subsequent exercise on a high-speed treadmill beginning on post-surgery day 15 and continuing for 5 days a week until the end of the study at post-surgery day 70 (Frisbie et al., 2009a). The horses were treated with intraarticular injection of sodium hyaluronate, PSGAG, or saline on post-surgical days 14, 21, and 28. PSGAG treatment reduced synovial effusion compared to the control horses. Histologically, the degree of synovial membrane vascularity and subintimal fibrosis was significantly reduced by PSGAG treatment compared with controls. Less cartilage fibrillation was seen in the hyaluronate-treated group compared to controls. The authors concluded that both intraarticular PSGAG and sodium hyaluronate had beneficial disease-modifying effects and are viable treatments for equine osteoarthritis. The efficacy of the intramuscular dose regimen was compared to the intraarticular route in a clinical field trial (Hamm and Jones, 1988; FDA, 1996). Horses were treated with either weekly intraarticular injections of 250 mg PSGAG (n=24) or 500 mg intramuscularly every 4 days for seven injections (n=19). All horses had naturally occurred, traumatic, or degenerative joint disease of the carpus. Both groups showed improvements of similar magnitude in lameness, pain, heat, and swelling. The investigators rated the response good or excellent in 90% of the horses treated through the intraarticular route and 89% of the horses treated through the intramuscular route. There was no statistical or clinical difference in response between the 2 groups (Hamm and Jones, 1988; FDA, 1996). An additional 42 horses with naturally occurring carpal joint disease were treated with intramuscular PSGAG at the recommended dose and treatment regimen. These horses were treated at 10 centers, and most were in active race training. The investigators judged the response good or excellent in 76% of these cases (FDA, 1996). The distribution of PSGAG in joint tissues after intramuscular injection in the horse was examined in two studies. In the first study, eight horses with a surgically induced full thickness cartilage lesion in one carpus received a single intramuscular injection of 500 mg tritium-labeled PSGAG (Burba et al., 1993). Serum and carpal joint synovial fluid samples were collected at 0, 2, 4, 8, 12, 24, 48, and 96 hours post injection. Serum and synovial fluid drug levels followed patterns similar to those seen in man and rabbits. (Panse et al., 1976; Muller et al., 1983) Synovial fluid levels peaked at 2 hours, and therapeutic levels (0.1–2.0 μg/ml) were achieved. At 96 hours post injection articular cartilage was harvested from the carpal joints and subjected to scintillation analysis. Drug levels were maintained throughout the 96-hour period. There were no differences in the cartilage levels of the drug in the damaged versus the control carpal joints at 96 hours; however, peak synovial fluid drug levels were approximately 30% higher in the damaged carpi. The synovial fluid hyaluronic acid levels in the carpal joints were significantly increased by 24 hours post injection and remained increased through 96 hours. This finding suggests that the achieved drug levels were therapeutic and that an anabolic effect upon hyaluronate production was probable. The second study examined the distribution of the drug in the equine carpus, fetlock, hock, and distal interphalangeal (coffin) joints in eight normal horses receiving a single intramuscular injection of 500 mg of tritium-labeled PSGAG (Collier et al., 1993). In all joints, mean peak synovial fluid drug levels occurred 2 hours post injection and exceeded therapeutic levels. The levels in the larger and more proximal carpus and hock joints tended to be higher than the levels in the fetlock and coffin joints. In a large clinical study in the Scandinavian countries, the efficacy of intramuscular PSGAG was compared to the efficacy of intraarticular sodium hyaluronate in a multicenter, blinded controlled field trial (White, 1997). Test subjects were 210 performance horses with aseptic arthritis of one or both carpal or fetlock joints. Horses were randomly assigned to treatment groups: seven intramuscular injections of PSGAG or an intraarticular injection of 20 mg sodium hyaluronate. Efficacy was evaluated at 3, 5, and 12 weeks after treatment. The percentage of horses recovered from the lameness was compared for each group at each time point. For all horses completing the protocol, about 28%, 62% and 68% of the horses in the PSGAG group were judged recovered at weeks 3, 5, and 12, respectively. Results were similar for subgroups regardless of the number of joints, carpus joints or fetlock joints, and for all sodium hyaluronate groups. In this study, intramuscular PSGAG was said to be the therapeutic equivalent to intraarticular sodium hyaluronate. Additional studies comparing intramuscular PSGAG to oral joint supplements (White et al., 1994) and to compounded acetyl-d-glucosamine and injectable chondroitin sulfate (White et al., 2003) using the Complete Freund’s Adjuvant carpitis model confirmed the efficacy of intramuscular PSGAG in this model, and the results for the PSGAG-treated group were consistent with prior studies using this model. Intramuscular PSGAG-treated horses had a significant decrease in lameness score and carpal circumference and an increased carpal flexion and stride length compared to the oral supplement and the injectable glucosamine and chondroitin sulfate. The efficacy of intramuscular PSGAG was evaluated using the previously described induced carpal chip and treadmill exercise model (Frisbie et al., 2009a; Kawcak et al., 2011). Intramuscular PSGAG was used as a positive control in the evaluation of the efficacy of extracorporeal shock wave treatment on the model (Frisbie et al., 2009b). The authors reported no significant disease-modifying effect for either the shock wave or PSGAG-treated group but did report a significant decrease in lameness for the shock wave treated group compared to placebo or PSGAG-treated group. Further reports from these studies showed no effect of PSGAG on biomarkers of subchondral bone remodeling. (Kawcak et al., 2011) The effect of intramuscular PSGAG on articular cartilage from equine joints injected with MPA was evaluated in ponies (Fubini et al., 1993). One group of ponies was injected with intraarticular MPA followed by intramuscular saline injections. The other group received intraarticular MPA injections followed by intramuscular PSGAG injections. The MPA treatment led to lower GAG concentrations in articular cartilage compared to controls not treated with MPA. In this study, PSGAG did not have a significant protective effect against proteoglycan loss from the articular cartilage. The authors speculated that the mechanism of proteoglycan depletion after MPA injection might be different from the depletion that occurs in degenerative joint disease. A survey of equine practitioners (Caron et al., 1996) asked questions regarding the perceived efficacy of PSGAG for the treatment of equine joint disease. Over 90% of the responding veterinarians reported using PSGAG, and these respondents believed PSGAG was moderately effective for the treatment of four joint disease conditions. These respondents also considered PSGAG to be more effective for the treatment of subacute degenerative joint disease than sodium hyaluronate but considered sodium hyaluronate more effective for the treatment of idiopathic joint effusion or acute synovitis. In a more recent survey, PSGAG was the most frequently used disease-modifying drug in horses (62.8%) and was used by 78.3% of survey responders as a preventive measure to joint pathology in high-performance horses (Ferris et al., 2011). There is interest in the use of PSGAG as a preventative for joint disease in horses and dogs. In a review of PSGAG use in the treatment of osteoarthritis the authors proposed that the inhibitory action of PSGAG on activation and synthesis of metalloproteases responsible for cartilage degradation and the drug’s positive effect on synovitis might make PSGAG more effective in the prevention of osteoarthritis than in its treatment (Todhunter and Lust, 1994). A study of the successful prevention of the development canine hip dysplasia in susceptible pups is described later in this review. A prophylactic study of the effect of PSGAG on performance and joint injuries was conducted on a group of 2-year-old racing Quarter horses (White, 1998). The horses were randomly assigned to receive intramuscular treatment series of PSGAG or control (saline) beginning in March of the 2-year-old year (prior to racing) and continuing through September. The trainers were blinded to the treatment assignments. The incidence of joint injuries was similar for both groups but the horses in the PSGAG group stayed in training longer, started more races, and had higher earnings than the control horses. PSGAG attenuated the effect of joint injuries on the performance of the horses treated (White, 1998). Treatment with PSGAG has been studied to prevent or mitigate the effects of developmental orthopedic diseases in growing foals (White et al., 2007). In the first study, 75 foals on a central Kentucky thoroughbred breeding farm began intramuscular PSGAG treatments at 2 months of age. These treatment series continued until November of the yearling year. Incidences of clinically significant osteochondrosis dissecans (OCDs) and other developmental joint lesions and surgery for developmental joint lesions were compiled and compared to historical controls (the foals from the 3 previous years at the same farm). The treated foals had a significant reduction in the incidence of clinically significant joint lesions (p=0.014) and surgery for OCD lesions (p=0.006). Due to the high cost of the above-described treatment regimen, a second study looked at a more targeted regimen (White and Fregin, 2008). At the same farms, 85 foals began a 12-week loading dose of intramuscular PSGAG at 3 months of age, followed by a maintenance dose until 1 year of age. This more targeted regimen significantly reduced the number of surgeries for joint lesions (p=0.04) and the number of surgeries for hock and stifle OCD lesions (p=0.03) versus the historical control. The longer treatment regimen described previously reduced total joint surgeries significantly compared to the shorter targeted regimen (p=0.03). The use of PSGAG for the treatment of equine flexor tendinitis has also been described. A study of collagenase-induced core lesions in the equine superficial digital flexor tendon demonstrated that horses treated with intramuscular PSGAG showed a more rapid reduction in the size of the core lesion (p < 0.03) compared to saline-treated control (Booth et al., 1999). Histological exam confirmed the improved repair of the core lesions in the treated group. Another study using a similar model examined the effect of five intralesional injections of PSGAG compared to intralesional saline injections (Moraes et al., 2009). Histological evaluation of the tendons was done 150 days after model induction. Significantly more (p < 0.001) organized bundles of collagen were observed in the PSGAG-treated tendons. Intralesional injection of PSGAG in collagenase induced tendinitis has also been studied in rabbits (Oryan et al., 2008). In this study, the authors suggested that PSGAG might be effective in restoring the morphological, biochemical, and biomechanical properties of injured connective tissues and, therefore, may be of clinical value in the treatment of acute tendon injuries. In a clinical study of athletic horse’s tendon injuries, the injuries were treated conservatively, with laser irradiation and intralesional or intramuscular PSGAG (Marr et al., 1993). Seventy-six percent of the PSGAG horses returned to work compared to 50% of the laser treated and 46% of the conservatively treated horses. These findings were not statistically significant. Clinical and experimental studies in the dogSeveral studies of the chondroprotective mechanisms of PSGAG have employed the dog as an experimental animal. These studies have examined the effect of the drug on various experimental models of arthritis and, in one case, in a spontaneous, naturally occurring syndrome. These studies demonstrated that the chondroprotective effects of PSGAG seen in humans, horses, swine, and laboratory animals also occur in the dog (Ueno, 1976; Altman et al., 1989; Lust et al., 1992). Ueno (Ueno, 1976) studied the effects of PSGAG in severe surgically induced arthritis in the dog. The lateral menisci and lateral tibial joint surface of both stifles were resected. Beginning 7 days post-surgery, 13 dogs received intramuscular injections of 25 mg/kg PSGAG every third day for 10 injections, then every fourth day for an additional 10 injections. Dogs were euthanized three months post-surgery. Fourteen untreated dogs served as controls. Gross pathological examination, radiography, and histological examinations all showed a decrease in cartilage destruction of the lateral femoral condyle in the treated group. The author concluded that intramuscular injections of PSGAG prevented cartilage destruction in this experimental model. Hannan et al. (1987) examined the effect of injections of PSGAG on a group of dogs subjected to bilateral medial meniscectomy. Six dogs received subcutaneous injections of 2 mg/kg PSGAG three times weekly for 3 weeks and then twice weekly for 23 weeks. Untreated dogs and sham operated dogs served as controls. Dogs were subjected to postmortem examination. Based upon histological and biochemical examination of articular cartilage, the authors reported that PSGAG treatment provided a protective effect to the articular cartilage in the meniscectomies compartment. Treatment reduced surface fibrillation and chondrocyte cloning and helped keep a normal level of proteoglycans in the medial joint compartment. The authors concluded that systemically administered PSGAG provided partial protection from the articular cartilage damage secondary to medial meniscectomy. Altman et al. (1989) studied the effect of PSGAG injections on cartilage lesions produced by transection of the anterior cruciate ligament in dogs in 2 experiments. The first experiment examined the prophylactic effects of intraarticular PSGAG in this model. Starting 2 days after model induction, the dogs received twice weekly intraarticular injections of 4 mg/kg PSGAG for 4 weeks. Dogs were subjected to postmortem 4 weeks after the end of the treatment period. Saline-treated dogs served as controls. Gross and histological examination revealed lesser lesions of the medial femoral condyle in the treated dogs. Uronic acid and hydroxyproline levels in cartilage (a measure of GAG levels) were significantly higher in the PSGAG-treated dogs. Levels of collagenase were lower in the treated group and cartilage swelling, an indicator of collagen network integrity, remained near normal in the PSGAG treated dogs. The authors proposed that this prophylactic effect might be related to the drug’s ability to decrease collagen degradation. The second experiment examined the therapeutic effect of intramuscular injections of PSGAG on the same model. Starting 4 weeks after model induction dogs received 4 mg/kg PSAGAG twice weekly for 4 weeks by intramuscular injection. Saline treated dogs served as controls. Dogs were euthanized 12 weeks after model induction. Cartilage from the medial femoral condyles was subjected to histological and biochemical analysis. Condylar cartilage from the PSGAG treated dogs demonstrated less cartilage swelling, less total and active metalloprotease enzyme levels, and improved histological scores (Mankin scores). The authors proposed that the therapeutic effect on articular cartilage shown in this experiment was due to the drug’s suppressions of metalloprotease enzyme activity. The effect of intramuscular PSGAG on serum cartilage oligomeric matrix protein (COMP), C-reactive protein concentrations (CRP), and matrix metalloproteinase (MMP) 2 and-9 activities and lameness in dogs with naturally occurring osteoarthritis was studied (Fujiki et al., 2007). Lameness scores in the PSGAG treated dogs improved significantly (p=0.001) over the study period. Serum COMP concentrations were also significantly decreased (p=0.001) in the PSGAG treated dogs. Improvement in lameness and reduction in serum COMP appeared to be correlated. PSGAG treatment had no effect on serum CRP or MMP levels. The authors concluded that PSGAG is effective for inhibition of COMP degradation, and this appears to correlate with an improvement in lameness. COMP inhibition may be an important mechanism of action of PSGAG in canine osteoarthritis. In a study of the effects of PSGAG on chondrocyte differentiation of canine bone marrow derived stem cells PSGAG was shown to prevent chondrocyte hypertrophy and to promote a fibrocartilage phenotype in three-dimensional alginate cultures (Bwalya et al., 2017). Canine hip dysplasia is a common cause of degenerative joint disease in dogs. Radiographic changes in the congruity of the hip joints may be detected as early as 2 months of age in susceptible pups. Lust et al. (1992) examined the effect of PSGAG injections on a group of pups highly susceptible to the development of hip dysplasia in 2 experiments. In the first experiment, two groups of seven susceptible pups were randomly selected from 2 litters. One group received PSGAG at 2.5 mg/kg twice weekly beginning at 6 weeks of age and continuing until 8 months of age; the other group received twice weekly saline injections. The pups were examined radiographically at 8 months of age, and the Norberg angle, a measure of coxofemoral congruity, was recorded. The mean Norberg angle of the treated group was significantly improved by 4 degrees (102 in controls; 106 in treated). In the second experiment, the same regimen was followed in two groups of eight pups selected at random from 2 litters. In this study, the treated group received 5 mg/kg PSGAG by twice weekly intramuscular injection from 6 weeks of age to 8 months of age. The dogs were examined radiographically at 8 months then necropsied. In the treated group, none of the eight dogs had radiographic signs of subluxation, while four of the eight control dogs had radiographic subluxation. The mean Norberg angle in the treated dogs was 109.6° compared to 101.5° in the control dogs; a statistically significant improvement of over 8°. The mean pathologic score for the treated group was 1.6 versus 3.3 in the control dogs. Mean cartilage fibronectin content, a measure of cartilage degeneration in the dog, was significantly lower in the treated group (0.59 μg/mg) versus the control group (2.19 μg/mg). The authors concluded that PSGAG treatment resulted in less subluxation and degenerative change through 8 months of age in pups susceptible to the development of hip dysplasia. The efficacy of various doses of intramuscular PSGAG for the treatment of canine hip dysplasia was studied to establish an optimal dose in the dog (de Haan et al., 1994). This was a blinded, randomized, placebo controlled multicenter field trial. Dogs 2–10 years old (n=84) with radiographic evidence of subluxation and degenerative joint disease secondary to canine hip dysplasia were selected for the study. These dogs had to have minimum initial lameness scores, deficits in range of motion and joint pain to be included in the study. Dogs were assigned to 1 of 4 treatment groups receiving twice weekly intramuscular injections for 4 weeks. The groups were placebo and 1, 2, or 4 mg/lb body weight. All dogs were assessed prior to treatment and at 3, 5, 7, and 9 weeks. Using subjective scoring scales, a total orthopedic score was determined. There was improvement in total orthopedic scores for all treated groups with the 2 mg showing the highest degree of improvement. None of the findings was statistically significant. This result was possibly related to the severe nature of the disease in this test population, the subjective nature of the scoring system and variability between investigators. An additional controlled field trial of intramuscular PSGAG was conducted in dogs with naturally occurring osteoarthritis in one or two joints (Food and Drug Administration, 1997). Dogs were required to have minimum lameness and disability scores along with pain on joint manipulation and limited range of motion. This was a multicenter, blinded, randomized controlled field trial. Each group received intramuscular injections twice weekly for 4 weeks of either 4.4 mg/kg PSGAG or an equivalent volume of saline. Lameness scores, disability scores, pain and range of motion were evaluated prior to treatment and at the time of injection 5 and 1 week after the final injection. A total orthopedic score was calculated for each exam period. PSGAG treated dogs showed significant improvement in total orthopedic scores and range of motion scores (p < 0.05) versus the control dogs. Based on this study and others the optimal intramuscular dose as approved by the US FDA was 2 mg/lb (4.4 mg/kg) body weight twice weekly for 4 weeks. In three cases of pemphigus foliaceus, PSGAG given as an adjunctive therapy, was reported to reduce the need for corticosteroids (Simpson et al., 2019). Other speciesIn a survey of veterinarians about the treatment of chronic osteoarthritis pain in cats, 61.9% of respondents reported using PSGAG (Adrian et al., 2019). Presently, there are no well controlled study on the efficacy and safety of PSGAG in cats. Fatal hemorrhage occurred in 3 of 4 birds given a dose of 10 mg/kg PSGAG by intramuscular injection (Anderson et al., 2013). Other authors have reported a dose of 1 mg/kg PSGAG can be safely administered to avian species (Wonn et al., 2022). Potential Side Effects/Adverse Reactions of PSGAG TherapyIn manIn man, adverse reactions typically seen following intraarticular injection of any drug are also seen after intraarticular injection of PSGAG. Synovial inflammation has been reported in a few cases and septic arthritis can also occur. The incidence of such reactions after intraarticular injection of PSGAG is similar to that of other intraarticular drugs (Ishikawa et al., 1982). A less than 1% incidence of side effects, after intramuscular PSGAG, was recorded in a group of over 5,000 patients, receiving 15 injections of 50 mg PSGAG, twice weekly for 8 weeks (Eckenberger, 1983). Sensitization reactions have been reported at higher intramuscular doses (250 mg three times weekly) (Verbruggen and Veys, 1982). This reaction is thought to be due to a sensitization of platelet membrane proteins which interact with PSGAG and other heparinoids to induce IgG production (Wolf et al., 1983). The symptoms of this type of reaction include headache, sweating, drowsiness, paresthesia of the extremities, angina symptoms and rarely circulatory collapse (Dettmer et al., 1983). Considering the chemical similarity between PSGAG and heparin, disturbances of blood coagulation were expected. Heparin is about twice as potent an anticoagulant as PSGAG in vivo (Thomas et al., 1977). Injection site hematomas have been reported in man after intramuscular administration (Verbruggen and Veys, 1982; Siegmeth and Radi, 1983). At higher concentrations (100–400 μg/ml), PSGAG was shown to inhibit protein synthesis in vitro (Verbruggen and Veys, 1977; Greiling, 1979; Egg, 1983). This phenomenon, however, has not been linked to any adverse reactions or side effects to PSGAG treatment in humans. PSGAG also shows antimitotic effects at high concentrations (50–400 μg/ml) in vitro (Verbruggen and Veys, 1980). A reversible loss of hair was observed in human patients receiving 250 mg PSGAG by intramuscular injection for long periods of time. Potential PSGAG drug interactions are comparable to those of heparin. PSGAG might interact with: – Drugs that inhibit blood coagulation – Drugs which bind with plasma proteins (competition of binding sites) – Drugs which inhibit mitosis The listed contraindications to PSGAG therapy in man include: – Conditions which increase the risk of hemorrhage including coagulation disorders and prolonged use of non-steroidal anti-inflammatory drugs. – Severe hepatic or renal dysfunction. Protamine sulfate is listed as the antidote to the heparin-like disturbances of blood coagulation associated with PSGAG therapy in man. In horsesSide effects or adverse reactions to intraarticular injections of PSGAG in the horse related to disturbances in blood coagulation, inhibition of protein synthesis or inhibition of mitosis have not been reported. In general, two types of adverse reactions have been observed: – Non septic post-injection synovial inflammation. This phenomenon is usually self-limiting and resolves with symptomatic therapy. Potential causes include mechanical trauma to the joint capsule during injection, exceeding the recommended dose or frequency of injection, and the mixing of PSGAG with other drugs or solvents (data on file Luitpold Pharmaceuticals, Inc.). The possibility of sensitization to the drug also must be considered. Septic arthritis. Gustafson et al. have shown that PSGAG can induce overt septic arthritis when a marginally sub infective challenge of Staphylococcus is administered intraarticularly (Gustafson et al., 1989a,b). Thus, strict adherence to sterile injection technique with PSGAG is critical. The mechanism of this potentiation is not understood; PSGAG has been shown to inhibit the certain neutrophil functions such as release of toxic oxygen radicals (Tsuboi et al., 1988) and to inhibit the complement cascade (Loos and Heinz, 1982; Rashmir-Raven et al., 1992). The inhibition of these two defense mechanisms may contribute to this phenomenon. Nonetheless, the reported incidence of septic arthritis following intra articular injections of PSGAG with amikacin has been low (Smith et al., 2019). The only contraindication to the intraarticular injection of PSGAG in the horse is sensitivity to PSGAG. Side effects to the intramuscular injection of PSGAG have been rare. There are no known contraindications to intramuscular PSGAG in the horse. In the dogNo drug related side effects or adverse reactions have been reported in the dog at or near the proposed optimal clinical dose regimen. In experimental studies no side effects have been reported even at intramuscular doses as high as 25 mg/kg 2–3 times weekly for 10 weeks (Ueno, 1976; Hannan et al., 1987; Altman et al., 1989) A transient decrease in activated partial thromboplastin time was observed in dogs receiving 2.5 or 5 mg/kg by intramuscular injection (Lust et al., 1992). These values returned to normal by 27 hours post injection. In a subacute toxicity study, dogs received placebo or twice weekly intramuscular doses of 5, 15, or 50 mg PSGAG per kilogram body weight for 13 weeks (Food and Drug Administration, 1997). At doses close to the proposed optimal clinical dose (5 mg/kg or 2.27 mg/lb), no significant side effects or pathological lesions were reported. At the 50 mg/kg dose, one dog developed a large injection site hematoma at week 12, platelet counts, and prothrombin times were increased slightly at week 12, and liver and kidney weights were significantly increased (also at 15 mg/kg dose). Microscopic lesions were noted in the 15 and 50 mg/kg dose groups. These changes included macrophages with eosinophilic foamy cytoplasm in the lymph nodes, Kupffer cells with eosinophilic foamy cytoplasm in the liver, and swollen and foamy cells in the proximal convoluted tubules of the kidneys. A dose related inflammation and degeneration of the muscle was seen at the injection sites. In the dog, the subcutaneous administration of PSGAG was well tolerated with only mild self-limiting adverse events. Efficacy of the subcutaneous route of administration has not been studied in the dog (Varcoe et al., 2021). Systemic sensitivity reactions to PSGAG have not yet been recognized in the dog but the possibility of such a phenomenon must be kept in mind. Based upon these findings, the following contraindications for PSGAG therapy in dogs may be relevant: – Disturbances of blood coagulation – Prolonged therapy with salicylates of other non-steroidal anti-inflammatory drugs – Renal or hepatic insufficiencies – Evidence of sensitivity to PSGAG DiscussionA chondroprotective drug is one that is said to be able to reduce the net loss of cartilage matrix components in injured or inflamed joints. This net loss of proteoglycans, collagen, and hyaluronate is the most important pathological feature of osteoarthritis. PSGAG has been studied extensively in both laboratory and clinical studies. Albeit with some inconsistencies, the data taken tend to support the classification of the drug as a chondroprotective. This classification is based on the demonstration of the following actions: – Incorporation of the drug into cartilage tissue – Inhibition of important catabolic enzymes – Stimulation of anabolic activity (synoviocytes and chondrocytes) PSGAG has been designated as a DMOAD in the horse (McIlwraith, 1996). This is clinically significant because a DMOAD is a drug which can prevent, retard, or reverse the morphologic cartilage lesions of osteoarthritis in vivo. The role of PSGAG as a DMOAD has been confirmed by published survey data. The drug is perceived to prevent, slow down, or reverse morphological changes in cartilage lesions caused by osteoarthritis (Reis et al., 2024). There are clinical and experimental data from laboratory animals, man, horses, and dogs that clearly support the classification of PSGAG as a DMOAD. The ultimate success or failure of a therapeutic agent for joint disease in veterinary medicine is judged by relief of lameness and return to function. The anti-inflammatory properties of PSGAG further enhance the drug’s ability to achieve a positive clinical response in cases of traumatic or degenerative joint diseases. There are newer products, recently introduced on the market, that show great promise for the treatment of equine joint diseases. These include polyacrylamide gels, biologically based anti-inflammatory/regenerative products such as platelet-rich plasma, Interleukin-1 Receptor-Antagonist Protein, and stem cells. Surveys indicate that these products are gaining market share, although PSGAG remains one of the most popular treatments for equine joint disease in the US (Zanotto and Frisbie, 2021; “Equine Market Mega Study V. Product and Market Insights,” 2022). PSGAG is effective by both the intraarticular and intramuscular routes. The efficacy of the intraarticular route of administration has been shown in man and horses. Pharmacokinetic studies in laboratory animals, man, horses, and dogs have all shown that the drug rapidly reaches therapeutic concentrations in joint tissue including articular cartilage and these therapeutic concentrations persist for 72–96 hours after intramuscular injection. Intramuscular efficacy has also been demonstrated in several studies of laboratory animals including studies on dogs. Experimental model studies in the horse have not consistently shown efficacy of intramuscular PSGAG. Since no experimental model can exactly replicate the complex and multi-factorial clinical disease in horses, results of studies in experimental equine joint disease may not always accurately reflect clinical efficacy. In many species including man and dogs, instability and degenerative changes are often seen prior to synovial and joint capsule pain. In the horse, synovial inflammation is most often seen as a primary lesion and degenerative changes are secondary to synovitis and capsulitis. The clinical studies of intramuscular PSGAG in naturally occurring equine joint diseases have uniformly shown significant positive results. The safety of intraarticular and intramuscular PSGAG has been well-established, both in safety studies and over many years of clinical use in man, horses, and dogs. Side effects for the intramuscular administration are rare, mild, and self-limiting. Severe sensitivity reactions have not been reported in horses and dogs. Adverse reactions and side effects to intraarticular injections of PSGAG are like those found for other intraarticular medications. In horses intraarticular PSGAG may potentiate contamination of the joint with a sub-infective dose of bacteria which may lead to septic arthritis. Under field conditions, this can be effectively prevented by concurrent administration of amikacin (Gustafson et al., 1989b). PSGAG is unique among the various products available for the treatment of traumatic and degenerative joint diseases in horses and dogs and remains an integral part of the medical therapy for these diseases. AcknowledgmentsThe author wishes to acknowledge the late Professor E Wynn Jones FRCVS, PhD, and the late Dr Doyne Hamm DVM for their contributions to my research career in the field of veterinary medicine and for their contributions to research on PSGAG in veterinary medicine. Thanks to Giampaolo Greco, Avi Blake and Michelle Swartz PhD of American Regent, Inc. for assistance in the preparation of this manuscript. FundingFunds for the preparation of this manuscript and open access publication fees were provided by American Regent, Inc Animal Health. DisclosureThe author was previously employed by American Regent, Inc. (formerly Luitpold Pharmaceuticals, Inc.) as a researcher and research consultant. The department of Animal Health at American Reagent, Inc. provided support for the preparation and publication of this manuscript. ReferencesAdrian, D.E., Rishniw, M., Scherk, M. and Lascelles, B.D.X. 2019. Prescribing practices of veterinarians in the treatment of chronic musculoskeletal pain in cats. J. Feline Med. Surg. 21(6), 495–506; doi:10.1177/1098612X18787910 Akermark, C., Crone, H., Elsasser, U. and Forsskåhl, B. 1995. Glycosaminoglycan polysulfate injections in lateral humeral epicondylalgia: a placebo-controlled double-blind trial. Int. J. Sports Med. 16(3), 196–200; doi:10.1055/s-2007-972991 Altman, R.D., Dean, D.D., Muniz, O.E. and Howell, D.S. 1989. Therapeutic treatment of canine osteoarthritis with glycosaminoglycan polysulfuric acid ester. Arthritis Rheum. 32(10), 1300–1307; doi:10.1002/anr.1780321016 Anderson, K., Garner, M.M., Reed, H.H., Cook, K., Aguilar, R., Horton, S., Case, A.L. and Wolf, K.N. 2013. Hemorrhagic diathesis in avian species following intramuscular administration of polysulfated glycosaminoglycan. J. Zoo Wildl. Med. 44(1), 93–99; doi:10.1638/1042-7260-44.1.93 Bach, G.L., Panse, P. and Zeiller, P. 1977. Glycosaminoglycanpolysulfate (GAGPS, Arteparon) for basic therapy of arthrosis. III. Biochemical-diagnostic and clinical studies on the intramuscular use of GAGPS. Z. Rheumatol. 36(7-8), 269–274. Baici, A., Salgam, P., Fehr, K. and Böni, A. 1980. Inhibition of human elastase from polymorphonuclear leucocytes by a glycosaminoglycan polysulfate (Arteparon). Biochem. Pharmacol. 29(12), 1723–1727; doi:10.1016/0006-2952(80)90131-8 Booth, L.C., Pool, R.R. and Redding, W.R. 1999. The effects of polysulphated glycosaminoglycan on the healing of collagenase induced tendinitis. Vet. Comparative Orthopaedics Traumatol. 12, 48–55. Brennan, J.J., Aherne, F.X. and Nakano, T. 1987. Effects of glycosaminoglycan polysulfate treatment on soundness, hyaluronic acid content of synovial fluid and proteoglycan aggregate in articular cartilage of lame boars. Can. J. Vet. Res. 51(3), 394–398. Burba, D.J., Collier, M.A., Default, L.E., Hanson-Painton, O., Thompson, H.C. and Holder, C.L. 1993. In vivo kinetic study on uptake and distribution of intramuscular tritium-labeled polysulfated glycosaminoglycan in equine body fluid compartments and articular cartilage in an osteochondrial defect model. J. Equine Vet. Sci. 13(12), 696–703. Burkhardt, D. and Ghosh, P. 1987. Laboratory evaluation of antiarthritic drugs as potential chondroprotective agents. Seminars Arthritis Rheumatism17(2), 3–34. Bwalya, E.C., Kim, S., Fang, J., Wijekoon, H.M.S., Hosoya, K. and Okumura, M. 2017. Effects of pentosan polysulfate and polysulfated glycosaminoglycan on chondrogenesis of canine bone marrow-derived mesenchymal stem cells in alginate and micromass culture. J. Vet. Med. Sci. 79(7), 1182–1190; doi:10.1292/jvms.17-0084 Caron, J.P., Kaneene, J.B. and Miller, R. 1996. Results of a survey of equine practitioners on the use and perceived efficacy of polysulfated glycosaminoglycan. J. Am. Vet. Med. Assoc. 209(9), 1564–1568. Caron, J.P., Toppin, D.S. and Block, J.A. 1993. Effect of polysulfated glycosaminoglycan on osteoarthritic equine articular cartilage in explant culture. Am. J. Vet. Res. 54(7), 1116–1121. Clegg, P.D., Jones, M.D. and Carter, S.D. 1998. The effect of drugs commonly used in the treatment of equine articular disorders on the activity of equine matrix metalloproteinase-2 and 9. J. Vet. Pharmacol. Ther. 21(5), 406–413; doi:10.1046/j.1365-2885.1998.00157.x Collier, M.A., Clark, D., DeBaull, L., White, G.W., Jones, E.W., Walls, R. and Hamm, J. 1998. The distribution of radiabeled PSGAG in canine synovial fluid and articular cartilage after intramuscular injection of 3HPSGAG. Canine Practice(September/October). Canine Pract. 23(5), 6–9. Collier, M., Haugland, L., DeBault, L., Walls, R. and Siqueria, L.D. 1993. 3H-PSGAG concentrations in the synovial fluid of the equine carpal, forefetlock, hock, and coffin joints following a 500-mg intramuscular injection.J. Equine Vet. Sci. 15(6), 274–278. de Haan, J.J, Goring, R.L. and Beale, B.S. 1994. Evaluation of polysulfated glycosaminoglycan for the treatment of hip dysplasia in dogs. Vet. Surg. 23(3), 177–181; doi:10.1111/j.1532-950x.1994.tb00468.x De Messias, I.T., Mohren, D. and Kajdacsy-Balla, A. 1994. Inhibition of the classical and alternative pathways of the human complement system by glycosaminoglycan polysulfate. J. Investig. Allergol. Clin. Immunol. 4(4), 172–176. Dettmer, N., Nowack, H. and Raake, W. 1983. Thrombozyten Aggregation Nach Heparin und Arteparon. Munch. Med. Wschr. 125(25), 540–542. Dettmer, N. 1979. The therapeutic effect of glycosaminoglycan polysulfate (Arteparon) in arthroses depending on the mode of administration (intraarticular or intramuscular). Z. Rheumatol. 38(5-6), 163–181. Dustmann, H.O., Puhl, W. and Martin, K. 1974. Intra-articular injections of arteparon for arthrosis. Animal experiments (author’s transl). Z. Orthop. Ihre Grenzgeb. 112(6), 1188–1196. Eckenberger, H.P. 1983. The therapy of gonarthrosis with Arteparon. X Europ Cong Rheumatol Moscow. Egg, D. 1983. Effects of glycosaminoglycan-polysulfate and two non-steroidal anti-inflammatory drugs on prostaglandin E2 synthesis in Chinese hamster ovary cell cultures. Pharmacol. Res. Commun. 15(8), 709–717; doi:10.1016/s0031-6989(83)80001-0 Equine Market Mega Study V. 2022. Product and Market Insights. Greensboro, NC: Brakke Consulting. Ewers, B.J. and Haut, R.C. 2000. Polysulphated glycosaminoglycan treatments can mitigate decreases in stiffness of articular cartilage in a traumatized animal joint. J. Orthop. Res. 18(5), 756–761; doi:10.1002/jor.1100180512 Eylau, O. 1959. Intra-articular heparin therapy of genuine arthrosis deformans of the knee joint Intraarticulare Heparin-Behandlung der genuinen Arthrosis deformans der Kniegelenke. Med. Klin. 54(4), 145. FDA. 1984. Adequan IA NADA 136-383 Freedom of Information summary. Available via https://animaldrugsatfda.fda.gov/adafda/views/#/foiDrugSummaries#foiApplicationInfo FDA. 1996. Adequan IM NADA 140-901 Freedom of Information summary. Amendment. Available via https://animaldrugsatfda.fda.gov/adafda/views/#/foiDrugSummaries#foiApplicationInfo Ferris, D.J., Frisbie, D.D., Mcilwraith, C.W. and Kawcak, C.E. 2011. Current joint therapy usage in equine practice: a survey of veterinarians 2009. Equine Vet. J. 43(5), 530–535; doi:10.1111/j.2042-3306.2010.00324.x Food and Drug Administration. 1997. Adequan Canine (Polysulfated Glycosaminoglycan) NADA 141-038 (Freedom of information summary, Issue. Rome: Food and Drug Administration. Francis, D.J., Hutadilok, N., Kongtawelert, P. and Ghosh, P. 1993. Pentosan polysulphate and glycosaminoglycan polysulphate stimulate the synthesis of hyaluronan in vivo. Rheumatol. Int. 13(2), 61–64; doi:10.1007/BF00307735 Frean, S.P. and Lees, P. 2000. Effects of polysulfated glycosaminoglycan and hyaluronan on prostaglandin E2 production by cultured equine synoviocytes. Am. J. Vet. Res. 61(5), 499–505; doi:10.2460/ajvr.2000.61.499 Frisbie, D.D., Kawcak, C.E. and Mcilwraith, C.W. 2009b. Evaluation of the effect of extracorporeal shock wave treatment on experimentally induced osteoarthritis in middle carpal joints of horses. Am. J. Vet. Res. 70(4), 449–454; doi:10.2460/ajvr.70.4.449 Frisbie, D.D., Kawcak, C.E., Mcilwraith, C.W. and Werpy, N.M. 2009a. Evaluation of polysulfated glycosaminoglycan or sodium hyaluronan administered intra-articularly for treatment of horses with experimentally induced osteoarthritis. Am. J. Vet. Res. 70(2), 203–209; doi:10.2460/ajvr.70.2.203 Fubini, S.L., Boatwright, C.E., Todhunter, R.J. and Lust, G. 1993. Effect of intramuscularly administered polysulfated glycosaminoglycan on articular cartilage from equine joints injected with methylprednisolone acetate. Am. J. Vet. Res. 54(8), 1359–1365. Fujiki, M., Shineha, J., Yamanokuchi, K., Misumi, K. and Sakamoto, H. 2007. Effects of treatment with polysulfated glycosaminoglycan on serum cartilage oligomeric matrix protein and C-reactive protein concentrations, serum matrix metalloproteinase-2 and -9 activities, and lameness in dogs with osteoarthritis. Am. J. Vet. Res. 68(8), 827–833; doi:10.2460/ajvr.68.8.827 Gallacchi, G. and Muller, W. 1979. Incorporation of intramuscularly injected glycosaminoglycan polysulfate in human joint cartilage. Proc. 9th Eur. Cong. Rheumat. 199, 102. Gaustad, G. and Larsen, S. 1995. Comparison of polysulphated glycosaminoglycan and sodium hyaluronate with placebo in treatment of traumatic arthritis in horses. Equine Vet. J. 27(5), 356–362; doi:10.1111/j.2042-3306.1995.tb04070.x Glade, M.J. 1990. Polysulfated glycosaminoglycan accelerates net synthesis of collagen and glycosaminoglycans by arthritic equine cartilage tissues and chondrocytes. Am. J. Vet. Res. 51(5), 779–785. Golding, J. and Ghosh, P. 1983. Drugs for osteoarthrosis II: the effects of a glycosaminoglycan polysulphate ester (Arteparon) on proteoglycan aggregation and loss from articular cartilage of immobilized rabbit knee joints. Curr. Therapeutic Res. 34(1), 67–80. Greiling, H. 1979. Biochemical investigations of the mode of action of Arteparon. Proc 9th Europ Cong Rheumat. 11, 18. Greiling, H. and Kaneko, M. 1973. Inhibition of lysosomal enzymes by glycosaminoglycanpolysulfate. Therapy of chronic joint diseases using compounds with antidegenerative properties; biochemistry. Arzneimittelforschung 23(4), 593–597. Greiling, H. 1974. Biorheological Properties and the Proteo Hyaluronate content of Synovial Fluid.In Biopolymere und Biomechanik von Bindegewebssystemen. Berlin: Springer-Verlag Berlin. Gustafson, S.B., Mcilwraith, C.W. and Jones, R.L. 1989a. Comparison of the effect of polysulfated glycosaminoglycan, corticosteroids, and sodium hyaluronate in the potentiation of a subinfective dose of Staphylococcus aureus in the midcarpal joint of horses. Am. J. Vet. Res. 50(12), 2014–2017. Gustafson, S.B., Mcilwraith, C.W., Jones, R.L. and Dixon-White, H.E. 1989b. Further investigations into the potentiation of infection by intra-articular injection of polysulfated glycosaminoglycan and the effect of filtration and intra-articular injection of amikacin. Am. J. Vet. Res. 50(12), 2018–2022. Hamm, D. and Jones, W. 1988. Intra-articular (IA) and intramuscular (IM) treatment of noninfectious equine arthritis (DJD) with polysulfated glycosaminoglycan (PSGAG). J. Equine Vet. Sci. 8(6), 456–459. Hamm, D., Goldman, L. and Jones, E.W. 1984. Polysulfated glycosaminoglycan: a new intra-articular treatment for equine lameness. Vet. Med. 79, 811–816. Hannan, N., Ghosh, P., Bellenger, C. and Taylor, T. 1987. Systemic administration of glycosaminoglycan polysulphate (arteparon) provides partial protection of articular cartilage from damage produced by meniscectomy in the canine. J. Orthop. Res. 5(1), 47–59; doi:10.1002/jor.1100050108 Ishikawa, K., Kitagawa, T., Tanaka, T., Terayama, K., Kuriya, N., Iwata, H., Niwa, S. and Sakurai, M. 1982. Clinical testing of intra-articularly injected glycosaminoglycan polysulfate in gonarthrosis (a controlled multicenter double-blind study). Z. Orthop. Ihre Grenzgeb. 120(5), 708–716; doi:10.1055/s-2008-1051383 Iwata, H., Kaneko, M., Kawai, K., Kajino, G. and Nakagawa, M. 1980. Uptake of glycosaminoglycan polysulfate by articular and meniscus cartilage: a biochemical and autoradiographic investigation. Clin. Orthop. Relat. Res. 153(153), 265–272. Jikuya, K. and Doi, R. 1975. Studies on the fate of mucopolysaccharides polysulfate in animals. Shikoku Acta Medica 31(1), 99–110. Kannus, P., Natri, A., Niittymäki, S. and Järvinen, M. 1992. Effect of intraarticular glycosaminoglycan polysulfate treatment on patellofemoral pain syndrome. A prospective, randomized double-blind trial comparing glycosaminoglycan polysulfate with placebo and quadriceps muscle exercises. Arthritis Rheum. 35(9), 1053–1061; doi:10.1002/art.1780350910 Kawcak, C.E., Frisbie, D.D. and Mcilwraith, C.W. 2011. Effects of extracorporeal shock wave therapy and polysulfated glycosaminoglycan treatment on subchondral bone, serum biomarkers, and synovial fluid biomarkers in horses with induced osteoarthritis. Am. J. Vet. Res. 72(6), 772–779; doi:10.2460/ajvr.72.6.772 Kristiansen, K.K. and Kold, S.E. 2007. Multivariable analysis of factors influencing outcome of 2 treatment protocols in 128 cases of horses responding positively to intra-articular analgesia of the distal interphalangeal joint. Equine Vet. J. 39(2), 150–156; doi:10.2746/042516407x170094 Kruze, K., Fehr, K. and Boni, A. 1976. Effect of antirheumatic drugs on cathepsin B1 from bovine spleen. Z. Rheumatol. 35(3-4), 95–102. Kubitza, G. 1966. Zur Therapie degenerativer Gelenkerkrankungen bei Pferden und Hunden. Tierärztl. Umschau 21, 402–408. Loos, M. and Heinz, H.P. 1982. Wirkung eines mucopolysaccharidpolyschwefelsaureesters (MPS, arteparon) auf das komplement system. Schweiz.-Deutsch. Rheumatol. 3(5), 155. Lust, G., Williams, A.J., Burton-Wurster, N., Beck, K.A. and Rubin, G. 1992. Effects of intramuscular administration of glycosaminoglycan polysulfates on signs of incipient hip dysplasia in growing pups. Am. J. Vet. Res. 53(10), 1836–1843. Marr, C., Love, S., Boyd, J. and Mckellar, Q. 1993. Factors affecting the clinical outcome of injuries to the superficial digital flexor tendon in National Hunt and point-to-point racehorses. Vet. Rec. 132(19), 476–479; doi:10.1136/vr.132.19.476 May, S., Hooke, R. and Lees, P. 1988. The effect of drugs used in the treatment of osteoarthritis on stromelysin (proteoglycanase) of equine synovial cell origin. Brit. J. Pharmacol. 93, 281. McIlwraith, C.W. 1996. Intra-articular and systemic medications for the treatment of equine joint disease. In Proceedings American Association of Equine Practitioners 42, 101–125. McIlwraith, C.W., Frisbie, D.D. and Kawcak, C.E. 2001. Current treatments for traumatic synovitis, capsulitis, and osteoarthritis. In Proceedings of the Annual Convention of the AAEP. Momburg, H. 1976. Klinisch-Chemische Veranderungen in der Synovialflussigkeit nach intraartikularer Injektion eines Glykosaminoglykanpolysulfats. Verh. Dtsch. Ges. Rheumatol. 4, 383–390. Moraes, J.R.E., Facco, G.G., Moraes, F.R., Engracia Filho, J.R., Miyazato, L.G. and Beretta, D.C. 2009. Effects of glycosaminoglycan polysulphate on the organisation of collagen fibres in experimentally induced tendonitis in horses. Vet. Rec. 165(7), 203–205; doi:10.1136/vr.165.7.203 Muller, W., Dick, W. and Panse, P. 1981. Konzentrationen von Glykosaminoglykanpolysulfat im Serum, in der Synovia und im Knorpel nach intramuskulärer Injektion beim Menschen. Therapie-Woche 31, 5902–5914. Muller, W., Panse, P., Brand, S. and Staubli, A. 1983. In vivo study of the distribution, affinity for cartilage and metabolism of glycosaminoglycan polysulphate (GAGPS, Arteparon). Z. Rheumatol. 42(6), 355–361. Nethery, A., Giles, I., Jenkins, K., Jackson, C., Brooks, P., Burkhardt, D., Ghosh, P., Whitelock, J., O’Grady, R.L. and Welgus, H.G. 1992. The chondroprotective drugs, Arteparon and sodium pentosan polysulphate, increase collagenase activity and inhibit stromelysin activity in vitro. Biochem. Pharmacol. 44(8), 1549–1553; doi:10.1016/0006-2952(92)90471-t Nishikawa, H., Mori, I. and Umemoto, J. 1985. Influences of sulfated glycosaminoglycans on biosynthesis of hyaluronic acid in rabbit knee synovial membrane. Arch. Biochem. Biophys. 240(1), 146–153; doi:10.1016/0003-9861(85)90017-7 Oryan, A., Goodship, A.E. and Silver, I.A. 2008. Response of a collagenase-induced tendon injury to treatment with a polysulphated glycosaminoglycan (Adequan). Connect. Tissue Res. 49(5), 351–360; doi:10.1080/03008200802325169 Panse, P., Zeiller, P. and Sensch, K.H. 1976. Distribution and excretion of a glycosaminopolysulfate in the rabbit after parenteral application (author’s transl). Arzneimittelforschung 26(11), 2024–2029. Raatikainen, T., Väänänen, K. and Tamelander, G. 1990. Effect of glycosaminoglycan polysulfate on chondromalacia patellae. A placebo-controlled 1-year study. Acta Orthop. Scand. 61(5), 443–448; doi:10.3109/17453679008993559 Rashmir-Raven, A.M., Coyne, C.P., Fenwick, B.W., Gaughan, E.M., Andrews, G.A. and Debowes, R.M. 1992. Inhibition of equine complement activity by polysulfated glycosaminoglycans. Am. J. Vet. Res. 53(1), 87–90. Reis, I.L., Lopes, B., Sousa, P., Sousa, A.C., Caseiro, A.R., Mendonça, C.M., Santos, J.M., Atayde, L.M., Alvites, R.D. and Maurício, A.C. 2024. Equine musculoskeletal pathologies: clinical approaches and therapeutical perspectives-a review. Vet. Sci. 11(5), 11; doi:10.3390/vetsci11050190 Richter, A. 1970. Therapy of joint diseases. Experimental and clinical experience with a mucopolysaccharide polysulfuric acid ester. Med. Monatsschr. 24(3), 121–125. Sadowski, T. and Steinmeyer, J. 2002. Effects of polysulfated glycosaminoglycan and triamcinolone acetonid on the production of proteinases and their inhibitors by IL-1alpha treated articular chondrocytes. Biochem. Pharmacol. 64(2), 217–227; doi:10.1016/s0006-2952(02)01073-0 Sevalla, K., Todhunter, R.J., Vernier-Singer, M. and Budsberg, S.C. 2000. Effect of polysulfated glycosaminoglycan on DNA content and proteoglycan metabolism in normal and osteoarthritic canine articular cartilage explants. Vet. Surg. 29(5), 407–414; doi:10.1053/jvet.2000.9139 Siegmeth, W. and Radi, I. 1983. Comparison of glycosaminoglycan polysulfate (Arteparon) and physiological saline solution in arthrosis of the large joints. Results of a multicenter double-blind study. Z. Rheumatol. 42(4), 223–228. Simpson, A., Rosychuck, R., Schissler, J. and Souza, C. 2019. Polysulfated glycosaminoglycan as a novel, adjunctive therapy for pemphigus foliaceus in three dogs. J. Am. Anim. Hosp. Assoc. 55(6), 318–322; doi:10.5326/JAAHA-MS-6750 Smith, L.C.R., Wylie, C.E., Palmer, L. and Ramzan, P.H.L. 2019. Synovial sepsis is rare following intrasynovial medication in equine ambulatory practice. Equine Vet. J. 51(5), 595–599; doi:10.1111/evj.13063 Stanciková, M., Trnavský, K. and Keilová, H. 1977. The effect of antirheumatic drugs on collagenolytic activity of cathepsin B1. Biochem. Pharmacol. 26(22), 2121–2124; doi:10.1016/0006-2952(77)90262-3 Stephens, R.W., Walton, E.A., Ghosh, P., Taylor, T.K., Gramse, M. and Havemann, K. 1980. A radioassay for proteolytic cleavage of isolated cartilage proteoglycan. 2. Inhibition of human leukocyte elastase and cathepsin G by anti-inflammatory drugs. Arzneimittelforschung 30(12), 2108–2112. Thomas, D.P., Lane, D.A., Michalski, R., Johnson, E.A. and Kakkar, V.V. 1977. A heparin analogue with specific action on antithrombin III. Lancet 1(8003), 120–122; doi:10.1016/s0140-6736(77)91708-1 Todhunter, R.J. and Lust, G. 1994. Polysulfated glycosaminoglycan in the treatment of osteoarthritis. J. Am. Vet. Med. Assoc. 204(8), 1245–1251. Todhunter, R.J., Altman, N.S., Kallfelz, F.A., Nersesian, P. and Lust, G. 1993a. Use of scintimetry to assess effects of exercise and polysulfated glycosaminoglycan on equine carpal joints with osteochondral defects. Am. J. Vet. Res. 54(7), 997–1006. Todhunter, R.J., Freeman, K.P., Yeager, A.E. and Lust, G. 1993b. Effects of exercise and polysulfated glycosaminoglycan on the development of osteoarthritis in equine carpal joints with osteochondral defects. Vet. Surg. 22(5), 330–342; doi:10.1111/j.1532-950x.1993.tb00409.x Trotter, G.W., Yovich, J.V., McIlwraith, C.W. and Norrdin, R.W. 1989. Effects of intramuscular polysulfated glycosaminoglycan on chemical and physical defects in equine articular cartilage. Can. J. Vet. Res. 53(2), 224–230. Tsuboi, I., Matsuura, T. and Shichijo, S. 1988. Effect of glycosaminoglycan-polysulfate on human neutrophil function. Japanese J. Inflammation 8(2), 131–135. Ueno, R. 1976. Results of intramuscular injection of glycosamino-glycanpolysaccharide (GAGPS) in experimental arthrosis of the knee in dogs (author’s transl). Z. Orthop. Ihre Grenzgeb. 114(1), 108–112. Varcoe, G., Tomlinson, J. and Manfredi, J. 2021. Owner perceptions of long-term systemic use of subcutaneous administration of polysulfated glycosaminoglycan. J. Am. Anim. Hosp. Assoc. 57(5), 205–211; doi:10.5326/JAAHAMS-7101 Verbruggen, G. and Veys, E. 1977. Influence of sulphated glycosaminoglycans upon proteoglycan metabolism of the synovial lining cells. Acta Rhumatol. Bel. 1(1-2), 75–92. Verbruggen, G. and Veys, E.M. 1979. Influence of an oversulphated heparinoid upon hyaluronate metabolism of the human synovial cell in vivo. J. Rheumatol. 6(5), 554–561. Verbruggen, G. and Veys, E.M. 1980. Proteoglycan metabolism of connective tissue cells. an in vitro technique and its relevance to in vivo conditions. In Degenerative joints, test tubes, tissues, models, man. Proceedings of the First Conference on Degenerative Joint Diseases. Application of Fundamental Knowledge in Human Pathology, Ghent, Belgium: Excerpta Medica. Verbruggen, G. and Veys, E. 1982. Treatment of chronic degenerative joint diseases with a glycosaminoglycan polysulfate. International Drug Symposium ArteparonR. Basle: Eular Publisher, Von der Mark, K. 1980. Collagen synthesis in cultures of chondrocytes as effected by arteparon. IX Eur Congr Rheumatol Basel Euler 39, 50. Walesby, H.A., Rosenbusch, R., Booth, L.C. and Riley, C.B. 2000. Uptake and distribution of tritium-labeled polysulfated glycosaminoglycan in serum, urine, and superficial digital flexor tendon of rabbits after intramuscular administration. Am. J. Vet. Res. 61(1), 20–23; doi:10.2460/ajvr.2000.61.20 Walton, E.A., Stevens, R.W., Ghosh, P. and Toylor, T.K.F. 1981. Nonsteroidal antiinflammatory drugs (NSAIDs) and articular cartilage integrity. Seminars. Arthritis Rheumatism 11(1), 147–149. White, G.W. 1997. Results of a positive controlled blinded multicenter field trial comparing intramuscular polysulfated glycosaminoglycan and intra-articular sodium hyaluronate M.I.C.E.M. Maastricht International Congress on Equine Medicine. In Proceedings of 10th International Congress of World Equine Veterinary Association Moscow Russia, Maastricht, Netherlands. White, G.W. 1998. Can We Attenuate the Effect of Joint Injuries on Young Racehorses by Administration of Intramuscular Polysulfated Glycosaminoglycan?. J. Equine Vet. Sci. 18(2), 786–788. White, G.W. and Fregin, G.F. 2008. Efficacy of a targeted regimen of polysulfated glycosaminoglycan on developmental joint lesions in thoroughbred foals. In WEVA - International Congress - World Equine Veterinary Association, Moscow. White, G.W., Fregin, G.F. and Selden, J.R. 2007. Effect of prophylactic intramuscular administration of polysulfated glycosaminoglycan on developmental and traumatic joint injuries in thoroughbred foals. J. Equine Vet. Sci. 27(3), 107–111; doi:10.1016/j.jevs.2007.01.011 White, G.W., Jones, E.W., Hamm, J. and Sanders, T. 1994. The efficacy of orally administered sulfated glycosaminoglycan in chemically induced equine synovitis and degenerative joint disease. J. Equine Vet. Sci. 14, 350–353. White, G.W., Stites, T., Jones, E.W. and Jordan, S. 2003. Efficacy of intramuscular chondroitin sulfate and compounded acetyl-d-glucosamine in a positive controlled study of equine carpitis. J. Equine Vet. Sci. 23, 295–300. Wolf, H., Nowack, H. and Wick, G. 1983. Detection of antibodies interacting with glycosaminoglycan polysulfate in patients treated with heparin or other polysulfated glycosaminoglycans. Int. Arch. Allergy Appl. Immunol. 70(2), 157–163; doi:10.1159/000233315 Wonn, A.M., Brooks, M.B., Hu, H. and Gamble, K.C. 2022. Hypocoagulability effect of adequan in domestic chickens (Gallus Gallus) and chilean flamingos (Phoenicopterus Chilensis). J. Zoo Wildl. Med. 53(1), 126–132; doi:10.1638/2021-0052 Yovich, J.V., Trotter, G.W., Mcilwraith, C.W. and Norrdin, R.W. 1987. Effects of polysulfated glycosaminoglycan on chemical and physical defects in equine articular cartilage. Am. J. Vet. Res. 48(9), 1407–1414. Zanotto, G.M. and Frisbie, D.D. 2021. Current joint therapy usage in equine practice: changes in the last 10 years. Equine Vet. J. , doi:10.1111/evj.13489 Zhou, Z.H., Rajabi, M., Chen, T., Karnaukhova, E. and Kozlowski, S. 2012. Oversulfated chondroitin sulfate inhibits the complement classical pathway by potentiating C1 inhibitor. PLos One 7(10), e47296; doi:10.1371/journal.pone.0047296 | ||
| How to Cite this Article |
| Pubmed Style Gary W. White. Polysulfated glycosaminoglycan as a treatment for osteoarthritis in veterinary medicine: Summary of the pharmacological, laboratory, and clinical data. Open Vet. J.. 2025; 15(9): 4007-4023. doi:10.5455/OVJ.2025.v15.i9.6 Web Style Gary W. White. Polysulfated glycosaminoglycan as a treatment for osteoarthritis in veterinary medicine: Summary of the pharmacological, laboratory, and clinical data. https://www.openveterinaryjournal.com/?mno=251210 [Access: November 22, 2025]. doi:10.5455/OVJ.2025.v15.i9.6 AMA (American Medical Association) Style Gary W. White. Polysulfated glycosaminoglycan as a treatment for osteoarthritis in veterinary medicine: Summary of the pharmacological, laboratory, and clinical data. Open Vet. J.. 2025; 15(9): 4007-4023. doi:10.5455/OVJ.2025.v15.i9.6 Vancouver/ICMJE Style Gary W. White. Polysulfated glycosaminoglycan as a treatment for osteoarthritis in veterinary medicine: Summary of the pharmacological, laboratory, and clinical data. Open Vet. J.. (2025), [cited November 22, 2025]; 15(9): 4007-4023. doi:10.5455/OVJ.2025.v15.i9.6 Harvard Style Gary W. White (2025) Polysulfated glycosaminoglycan as a treatment for osteoarthritis in veterinary medicine: Summary of the pharmacological, laboratory, and clinical data. Open Vet. J., 15 (9), 4007-4023. doi:10.5455/OVJ.2025.v15.i9.6 Turabian Style Gary W. White. 2025. Polysulfated glycosaminoglycan as a treatment for osteoarthritis in veterinary medicine: Summary of the pharmacological, laboratory, and clinical data. Open Veterinary Journal, 15 (9), 4007-4023. doi:10.5455/OVJ.2025.v15.i9.6 Chicago Style Gary W. White. "Polysulfated glycosaminoglycan as a treatment for osteoarthritis in veterinary medicine: Summary of the pharmacological, laboratory, and clinical data." Open Veterinary Journal 15 (2025), 4007-4023. doi:10.5455/OVJ.2025.v15.i9.6 MLA (The Modern Language Association) Style Gary W. White. "Polysulfated glycosaminoglycan as a treatment for osteoarthritis in veterinary medicine: Summary of the pharmacological, laboratory, and clinical data." Open Veterinary Journal 15.9 (2025), 4007-4023. Print. doi:10.5455/OVJ.2025.v15.i9.6 APA (American Psychological Association) Style Gary W. White (2025) Polysulfated glycosaminoglycan as a treatment for osteoarthritis in veterinary medicine: Summary of the pharmacological, laboratory, and clinical data. Open Veterinary Journal, 15 (9), 4007-4023. doi:10.5455/OVJ.2025.v15.i9.6 |