| Research Article | ||
Open Vet. J.. 2025; 15(7): 3290-3299 Open Veterinary Journal, (2025), Vol. 15(7): 3290-3299 Research Article Protective efficacy of commercially available infectious bronchitis vaccination regimes against heterologous IBV QX challengeWael K. Elfeil1,2*†, Abdallah Makahleh3†, Farrah C. Phillips3 and Magdy F. Elkady41Avian and Rabbit Medicine Department, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt 2MEVAC - Middle East for Vaccines, El-Salihya El-Gededa, Egypt 3Kemin Industries, Inc., Des Moines, IA 4Poultry Diseases Department, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef, Egypt *Corresponding Author: Wael K. Elfeil. Avian and Rabbit Medicine Department, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt. Email: Elfeil [at] vet.suez.edu.eg †These authors contributed equally to this manuscript. Submitted: 01/05/2025 Revised: 08/06/2025 Accepted: 10/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
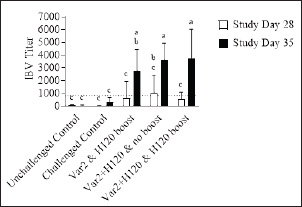
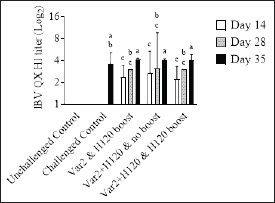
ABSTRACTBackground: Infectious bronchitis (IB), caused by the IB virus (IBV), is a highly contagious respiratory disease of poultry that causes widespread global economic losses within the poultry industry. The high mutation rate of IBV and continual emergence of new serotypes present a major challenge for disease control which is mainly focused on vaccination. Aim: The objective of the current study was to evaluate the efficacy of two commercially available vaccines, MEVACTM IB H120 and MEVACTM IB VAR 2, against challenge with a heterologous virus, IBV QX in specific pathogen-free chickens. Methods: Serological responses to IBV QX challenge were evaluated using Enzyme-Linked Immunosorbent Assay (ELISA) and hemagglutination inhibition (HI). Results: By day 35, all vaccinated groups showed a significant increase in mean antibody titers to IBV QX. At days 14 and 28, all vaccinated birds demonstrated the presence of HI antibodies against IBV QX. A reduction in viral shedding was observed in all vaccinated groups. All vaccinated groups exhibited a clear reduction in ciliostasis scores, indicating that the vaccines provided protection against IBV QX challenge. Histopathological analysis further confirmed the beneficial effects of vaccination in reducing tracheal damage, with all vaccinated birds exhibiting lower scores than the control group. The study demonstrates that a combination of variant (MEVACTM IB VAR 2) and classical (MEVACTM IB H120) vaccine strains provided cross-protection against IBV QX heterologous challenge. Conclusion: In so doing, the study contributes important insights to help guide the development of future vaccination programs in combating the threat that IBV poses to the poultry industry. Keywords: Infectious bronchitis, Infectious bronchitis virus, IBV QX Challenge, Vaccine efficacy, Poultry respiratory pathogen. IntroductionInfectious bronchitis (IB) is a highly contagious respiratory disease of poultry caused by the IB virus (IBV), a gammacoronavirus belonging to the Coronaviridae family. First reported in 1931 (Schalk and Hawn, 1931; Cavanagh and Naqi, 2003; Jackwood, 2012; Swayne and Boulianne, 2020), the disease affects the respiratory, urinary, and reproductive systems of infected birds. IBV typically induces respiratory signs, poor feed conversion ratio, and reduced weight gain in broilers, while in layers, it leads to significant drops in egg production and quality in layers, resulting in widespread global economic losses within the poultry industry (Ignjatović and Sapats, 2000; Cavanagh and Britton, 2008). IBV is an enveloped, single-stranded RNA virus that encodes three virus-specific proteins: the spike, membrane, and nucleocapsid glycoproteins (Rottier et al., 1984; Maier et al., 2015). Among these, the spike protein holds particular importance for both the virus’s infectivity and vaccine efficacy. The spike protein consists of two glycopolypeptides, S1 and S2, which extend from the virus surface, facilitating attachment to host cell surfaces and entry into host cells (Cavanagh et al., 1997; Sultan et al., 2019). The S1 glycoprotein plays a crucial role in determining serotype and binding of the virus to the host cell receptor. It contains a number of epitopes (or antigenic determinants) to which antibodies attach, and most hemagglutination inhibition (HI) and serum neutralization antibodies are directed against this glycoprotein (Butcher et al., 2009; Legnardi et al., 2020). Based on the spike protein full sequence, there is a new nomenclature for IBV split viruses belonging to IBV genotype-I into 29 subtypes from GI-1 to GI-29 (Valastro et al., 2016). Clinical signs of IB depend on the tissue tropism of the infecting virus. Nephropathogenic strains, with a renal tropism, often exhibit the highest mortality rates, with IBV replicating in renal tubular epithelial cells, leading to structural changes in the kidneys. Unvaccinated flocks may experience morbidity close to 100 percent (Jordan, 2017; Sultan et al., 2019), with the disease being more severe in young birds. Efforts to control IB primarily revolve around vaccination. However, despite the widespread use of vaccines dating back to the 1950s, the disease continues to circulate widely, severely impacting the poultry industry and complicated with several secondary infections (Diab et al., 2019). This lack of progress in disease control is largely due to the inherent high mutation rate of IBV, which results in continuous genetic and antigenic changes of the circulating virus, leading to the emergence of new strains or serotypes (Cook et al., 2012). Most of these genetic mutations occur in the S1 glycoprotein, where even minor changes in the amino acid sequence can give rise to new serotypes (Jackwood and de Wit, 2013). This constant change makes the successful control of IBV by vaccination challenging (Hoerr, 2021). As a consequence, no single vaccine can protect against all emerging serotypes (Sultan et al., 2019). However, research has shown that a heterologous vaccine virus may provide cross-protection (Elhady et al., 2018). In other words, the vaccine confers protective immunity against an IB serotype that shares cross-reacting antigens with the vaccine virus (Sultan et al., 2019). This cross-protection is possible, because despite the frequent mutations in the S1 protein, much of the viral genome, including some S1 epitopes, remains unchanged. Additionally, the S2 protein, being genetically more stable than S1, also has epitopes that may contribute to the production of protective antibodies. Given the dynamic nature of IBV in the field, an alternative to the use of homologous vaccines is necessary for successful disease control and to minimize the complications with other pathogens in poultry flocks (Mahmoud et al., 2022; Zanaty et al., 2023). Protectotyping, a concept suggested previously (Lohr, 1989), involves combining vaccine virus strains that are antigenically different into a vaccination program. Typically, a classical strain is combined with a variant strain, to produce a synergistic effect and an enhanced level of protection compared to using two strains separately. Studies have demonstrated that this approach, utilizing two or more live attenuated vaccines, can confer protection against heterologous serotypes (Cook et al., 1999). The major losses associated with the IBV infection come from the complications of the IBV infection with other poultry pathogens such as Avian influenza H9N2 viruses, adenovirus, infectious bursal disease virus, and reovirus (Talat et al., 2020; Badr et al., 2022). The co-infection of the poultry flocks with the multi-drug-resistant strains from Escherichia coli, Salmonella, and Pasteurella infections either in the presence of low pathogenic avian influenza virus or immunosuppressive pathogens such as reovirus and adenovirus exaggerates the losses associated with the infection (Elfeil et al., 2020; Sultan et al., 2022; Ezzat et al., 2023; Mahmoud et al., 2023; Salama et al., 2023). To determine the optimum vaccine or combination of vaccines, a degree of trial-and-error may be used in the field. However, identifying prevalent serotypes in the region and incorporating these antigenically dominant strains into vaccination programs maximizes the chances of success, as significant cross-protection is likely between emerging serotypes. The objective of this study was to evaluate the efficacy of two live attenuated commercially available vaccines, MEVACTM IB H120 and MEVACTM IB VAR 2, against challenge with a heterologous virus, IBV QX. Materials and MethodsAnimals and ethical statementSpecific pathogen free (SPF) chicken eggs were obtained from VALO BioMedia GmbH (Osterholz-Scharmbeck, Germany) as 0-day-old embryos and were raised in the study site hatchery (Izegem, Belgium). On day zero (D0), 125 healthy days of hatch SPF birds were enrolled in the study. The clinical condition of the birds was assessed as previously described (Gussem et al., 2013). Any birds that did not meet the inclusion criteria were excluded from the study and humanely euthanized in adherence to ethical guidelines. Enrolled birds were randomly assigned to one of five treatment groups (Table 1). The study was conducted at Wovenhof Poulpharm animal center (Izegem, Belgium). Treatment groups were housed in separate isolators measuring 1 m2, with a height of 1 m. Inside each isolator, a commercial pan feeder with a feed reservoir was hung, and three drinking nipples were provided for the birds’ access to ad libitum food and water. The birds were exposed to appropriate lighting conditions and the environmental temperature was maintained using infrared lamps. The study was performed according to Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Ethical considerations were strictly adhered to, and all efforts were made to minimize animal suffering and stress, with humane euthanasia performed when necessary. Investigational vaccinesTwo commercially available live attenuated vaccines, MEVAC IB Var 2 (MEVAC, a Kemin company, Cairo, Egypt, Lot 2106100401) and MEVAC IB H120 (MEVAC, a Kemin company, Cairo, Egypt, Lot 2107080401), were used in the study. The vaccines were stored and administered following the manufacturer’s label recommendations. The investigational vaccines were administered via eye drops to the birds in vaccination groups on days D0 and D14 (Table 1). A calibrated pipette was used to administer 0.05 ml of the reconstituted vaccine individually to each bird. The doses used were 3.710 EID50 (MEVAC IB Var 2) and 4.010 EID50 (MEVAC IB H120). Birds in the unvaccinated control groups received 0.05 ml of sham vaccine on D0 (Table 1). Table 1. Treatment groups.
Challenge virus strainAll birds, except the unchallenged control group, were challenged on D28 with the IBV QX strain, GI-19 genotype (Poulpharm BV, Izegem, Germany, 2019 isolate; Table 1). Each bird received a dose of 0.1 ml containing 104 EID50 of the challenge virus via eye-drop administration. Sampling and measurementsClinical signsGeneral health observations were conducted and recorded by an experienced animal caretaker at least once daily, starting from hatching and continuing until the conclusion of the study. Specific clinical observations for signs associated with IBV were recorded daily D28–D35. The scoring system for clinical signs was previously described (Sultan et al., 2019; Kilany et al., 2025). Serological responses against IBVOn D0, four blood samples of at least 1 ml were collected from ten, unenrolled hatchmates. These samples were subjected to serological analysis using the Enzyme-Linked Immunosorbent Assay (ELISA) method (Biocheck BV, The Netherlands, Cat. CK119IBV) to confirm that the flock of SPF chickens was free from antibodies against IBV. At days 14, 28, and 35, blood samples were collected via wing vein from ten animals per group to determine the serological responses to the IBV QX challenge. Serum was separated by centrifugation and analyzed by both ELISA and HI assay. IBV antibody titers measured by ELISA below 833 were considered negative. IBV QX antigen (Poulpharm, Izegem, Germany) was used in the HI assay to assess the presence of inhibitory antibodies against IBV. All HI titers were expressed as the log2 of the reciprocal of the highest serum dilution showing complete HI. Viral shedding after challengeOn D31, D33, and D35, tracheal swabs were taken from ten birds per group (the same ten birds that were used for blood sampling throughout the study) and stored in phosphate-buffered saline. Samples were processed for quantitative real-time polymerase chain reaction (RT-qPCR) with a commercial kit specific for the IBV QX (IBV QX, Cat. 31095, Kylt, Höltinghausen, Germany) according to the manufacturer’s instructions. Simultaneously, 10-fold dilutions of the vaccine were analyzed with the same kit, and the Ct values from the qPCR analysis were correlated with the known titer (EID50) of the diluted vaccine samples. The qPCR data were represented as an IBV QX titer (Log10EID50/ml) based on this correlation with live infectious virus. Ciliostasis scoringOn day 33 and day 35, seven birds from each group were humanely euthanized by intravenous injection of an overdose of a 300 mg/ml solution of pentobarbital sodium (Release®). Separate birds were used for ciliostasis scoring from those used for tracheal swabbing. The tracheas were collected and placed in individual 15 ml sterile conical tubes containing 10 ml pre-warmed minimum essential medium (MEM) Hanks’ salt. Tracheal samples were transferred to sterile Petri dishes containing 5 ml of MEM Hanks’ salt, and any excessive fat and connective tissue were removed using a scalpel. Each trachea explant was flushed twice with MEM Hanks’ salt. Ten thin transverse tracheal sections were cut using a scalpel, with three sections obtained from the upper, four from the middle, and three from the lower part of the trachea. These sections were observed under a light microscope at 10× and 100× magnification within 2 hours of sampling to assess ciliary activity. The activity of the cilia in each tracheal section was scored for ciliary movement (Table 2). The scoring criteria for determining normal ciliary activity were based on the European Pharmacopoeia monograph 07/2021:0442, Avian IB vaccine (live). Specifically, ciliary activity in a given tracheal section was considered normal if at least 50% of the internal ring exhibited vigorous ciliary activity, in other words when the ring received a score of two or less. To be deemed normal, a bird needed to have a minimum of nine out of ten rings showing normal ciliary activity. To calculate the ciliostasis score for each bird, the scores for all 10 tracheal sections were summed, and the mean score per tracheal ring was evaluated for each treatment group. Table 2. Ciliostasis scoring for tracheal sections.
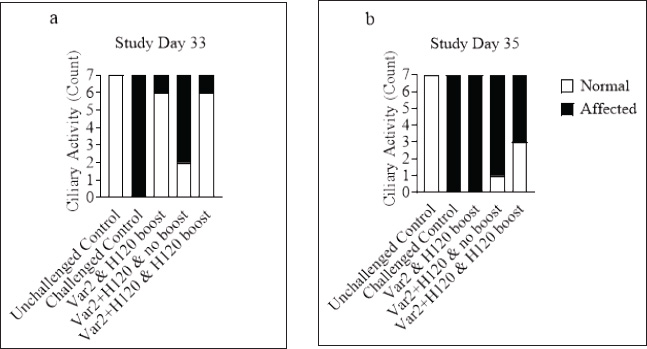
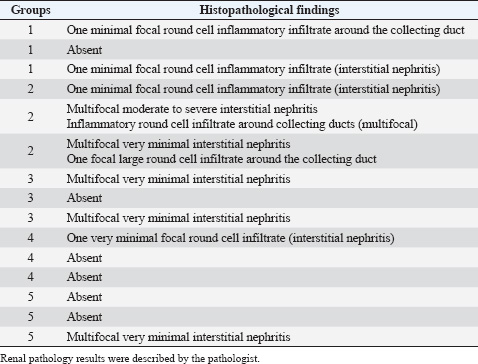
Histopathological analysisOn D35, three birds from each group were humanely euthanized by intravenous injection of an overdose of a 300 mg/ml solution of pentobarbital sodium (Release®). Tracheal and renal samples were fixed in 4% formaldehyde at a 2–1 ratio and stored at room temperature. Two days later, the samples were embedded in paraffin, sectioned, stained with hematoxylin and eosin (H&E), and examined by a pathologist. In the renal tissue, histopathological findings were recorded in a descriptive manner. For the tracheal tissue, histological parameters were assessed (Table 3). Statistical analysisGraphs were generated and statistical analyses were performed using GraphPad Prism version 10.5.0 for Windows, GraphPad Software, Boston, Massachusetts USA, www.graphpad.com. ResultsClinical signs, adverse events, and mortalitiesThroughout the study, no clinical signs associated with IBV were observed and all animals consistently scored as normal on all occasions including the challenge control group (data not shown). During the study, there were three mortalities and two were reported as adverse events. One bird in group 3 died on D24 and did not have macroscopic lesions or renal abnormalities. However, the histopathological analysis of tracheal samples indicated significant tracheal damage. The second mortality was also a bird in group 3, which collapsed after blood sampling on day 14 and was humanely euthanized. No abnormalities in the renal or tracheal tissues were observed by histology. Lastly, a bird from group 2 died on study day 11 due to peritonitis and hepatitis. Serological response to vaccinationSerum samples from D0 and D14 from all groups were negative for IBV antibodies (data not shown). By D28 IBV antibodies were detected in all three vaccinated groups, but not in the control groups (Fig. 1). By D35, the unvaccintated challenge control group had detectable antibodies, but the average was below the positive threshold cutoff of 833 (Fig. 1). Statistically, all three vaccine groups generated the same antibody titers on both D28 and D35 (Fig. 1). However, the group that received the combination of Var2+H120 and no boost exhibited the highest mean titer on D28, and the group that received this combination plus a boost of H120 on D14 had the highest average titer overall (D35, Fig. 1). The HI assay detected QX-specific antibodies as early as D14 in the vaccinated groups (Fig. 2). Conversely, the challenged control group of birds did not generate detectable HI titers until D35 (Fig. 2). Like what was observed by ELISA, there were no statistically significant differences in HI titers between groups with detectable titers measured on the same day (Fig. 2). However, the HI titers increased significantly in all three of the vaccinated groups from D14 to D35 (Fig. 2). As expected, the unchallenged control group of birds remained negative for IBV antibodies detected by either ELISA or HI assay throughout the study (Figs. 1 and 2). Viral shedding after challengeChallenge virus shedding was measured by RT-qPCR of tracheal swabs 3, 5, and 7 days post challenge (DPC) in all treatment groups. In the unvaccinated challenge control group and the birds that received the Var2 prime and H120 boost, shedding peaked 5 DPC (Fig. 3). Shedding peaked 3 DPC in the two groups that received Var2+H120 as the prime dose (Fig. 3). All three vaccine groups had significantly lower detectable IBV QX on 5 and 7 DPC compared to the unvaccinated control group (Fig. 3). There were no statistically significant differences in challenge virus shedding between any of the vaccinated groups on any of the days tested, but the birds that received Var2+H120 as the prime dose had slightly lower levels of challenge virus on 5 and 7 DPC compared to the vaccinate group that received only Var2 as the prime (Fig. 3). Ciliostasis and histopathologyCiliostasis scoring means were compared on D33 and D35 for all groups. The challenged control group had a mean of ~4 (maximum score) on both days (Fig. 4). The scores in the vaccinated groups were significantly lower than the challenged control on both days (Fig. 4). The group that received Var2+H120 on day 0 and a boost with H120 on day 14 had significantly lower ciliostasis scores on D35 than the other vaccine groups (Fig. 4). Dichotomous analysis of the ciliary activity in the birds (“normal” vs. “affected”) indicated that on D33 the two vaccine groups that received booster doses exhibited the best outcome (Fig. 5a). On D35, the group with the best outcome was again, and the birds that received Var2+H120 on day 0 and a boost with H120 on day 14 (Fig. 5b). However, on D35, more than half the birds’ ciliary activity in all groups, except the unchallenged control, were affected by the IB QX challenge (Fig. 5b). Histopathological analysis of the trachea corroborated the ciliostasis scoring results. The birds in group 5 (Var2+H120 on day 0 and a boost with H120 on day 14) had the lowest numerical cumulative score, and all vaccinated birds had significantly lower scores than the challenged control group (Fig. 6). The histopathological analysis of the kidneys was descriptive as opposed to quantitative. As expected from a virulent challenge, the unvaccinated challenge control group had moderate to severe, multifocal nephritis or interstitial nephritis in all three birds’ kidneys (Table 4). Two of the three birds in the vaccinated group that received only Var2 as the prime (group 3) had multifocal very minimal interstitial nephritis. The groups that received both Var2+H120 on day 0 only had one bird out of three with any interstitial nephritis reported, which was about the same as what was reported in the kidneys from the unchallenged birds in group 1 (Table 4). Table 3. Histological scoring criteria for trachea.
Fig. 1. IBV antibody titers. On D0, D14, D28, and D35 ten birds from each group were bled. Serum samples were analyzed with a commercial ELISA kit. Samples from D0 and D14 were negative and were excluded for clarity. Bars represent the mean ± SD (n=10). The dotted line represents the 833 minimum titer for a sample to be considered positive. Two-way ANOVA followed by Šídák multiple comparisons test. Bars not connected by the same letter are significantly different (p < 0.05).
Fig. 2. IBV QX HI titers. On D14, D28, and D35, 10 birds from each group were bled. Serum samples were analyzed by HI assay using an IBV QX-specific antigen. Bars represent the mean ± SD (n=10). Two-way ANOVA followed by Šídák multiple comparisons test. Bars not connected by the same letter are significantly different (p < 0.05).
Fig. 3. IBV QX shedding after challenge. On D31, D33, and D35, tracheal swabs were collected from 10 birds from each group and analyzed by qPCR. Ct values of tracheal swab samples were correlated with dilutions of live virus to generate a standard curve to correlate the results with a titer EID50/ml (Log10). Bars represent the mean ± SD (n=10). Two-way ANOVA followed by Šídák multiple comparisons test. Bars not connected by the same letter are significantly different (p < 0.05). DPC=days post challenge. DiscussionIn this study, the efficacy of different vaccination regimes combining IB H120 and IB Var 2 vaccine strains in prime-boost vaccination strategy has been implemented against a virulent IBV QX challenge was investigated. The challenges posed by the numerous IB serotypes and the high mutation rate of the IBV spike 1 glycoprotein make disease control difficult using conventional vaccination approaches. However, the results of the study demonstrate that protectotyping, which involves combining antigenically different strains in a vaccination program, may offer a viable strategy for overcoming these challenges and providing broader coverage through cross-protection. Moreover, a program that combines a classical IBV strain, such as IB H120 with a variant strain, such as IB VAR 2, may offer the best protection. The study used a commercially available ELISA to monitor humoral responses to IBV QX challenge. The absence of pre-existing IBV antibodies in the flock at day 0 showed that the flock was free of prior infection with IBV. By day 35, all vaccinated groups showed a significant increase in antibody titers compared to the challenge control group (Fig. 1) and thus matches with previous results by Sultan et al. (2019), who evaluated the immune response for the prime-boost strategy of two vaccines from lineage-I, subtype G-23 and GI-1 (Sultan et al., 2019). Although there were no significant differences between the vaccinated groups on either D28 or D35, the group that received the Var2+H120 prime followed by a boost with H120 had the highest mean antibody titer measured by ELISA on D35 (Fig. 1), and this results aligned with previous reports who mentioned using the Var2+ H120 then boosting with H120 induce potent immune response (Houta et al., (2024)). Vaccinated birds generated IBV QX-specific antibodies as early as D14, whereas the unvaccinated challenge control did not have measurable titers until D35 (Fig. 2), which come with alignment with previous studies on the implementation of the Var2 and H120 and recorded the induction of immune response 10–14 days post first vaccine (Elhady et al., 2018; Sultan et al., (2019)). There were no significant differences in HI titers between the vaccinated groups on any day (Fig. 2) as the ELISA kits measure the general immune response and thus come with alignment with Houte et al. 2024, who mentioned that different vaccination regimes are capable to induce measurable immune responses by ELISA kit (Shao et al., 2020; Houta et al., 2024). The HI assay demonstrated that all vaccine regimens generated antibodies that neutralize the hemagglutinating activity of the IBV QX spike protein using global testing standards (WOAH, 2018). Birds from the unchallenged control group remained negative throughout the study, confirming the absence of antibodies to IBV and robust biosecurity measures. To assess viral replication and shedding, RT-qPCR was used on tracheal swab samples. Unsurprisingly, the unvaccinated control group showed the highest level of viral replication, while all unvaccinated groups exhibited a significant reduction in viral shedding (Fig. 4), thus come with alignment with the Sultan et al. 2019, who recorded the reduction in virus shedding following implementing prime-boost vaccination program for IBV, These findings suggest that the vaccines were able to dampen the replication of the challenge virus in the respiratory tract (Sultan et al., 2019). Tracheal damage following IBV QX challenge was evaluated using ciliostasis scoring, a well-recognized method to determine the extent of tracheal damage and the degree of protection provided by a vaccine (Jackwood et al., 2015). Impaired mucociliary clearance due to infection of the tracheal mucosa is a feature of infection with IBV and may predispose birds to secondary bacterial infections (Hoerr, 2021). The challenged control group displayed the highest mean ciliostasis score per tracheal ring, indicating successful IBV QX challenge, which come with alignment with previous records evaluating challenge with IBV-Var2 strain (Sultan et al., 2019). All vaccinated groups exhibited a significant reduction in ciliostasis scores compared to the challenged control group on D33 and D35 (Fig. 4), similar to the results obtained by the challenge module with IBV Var2 using the prime-boost vaccination strategy (Houta et al., 2024). The vaccinated group that did not receive a booster dose of vaccine on D14 (group 4) had significantly higher ciliostasis score on D33 compared to the other two vaccine groups (Fig. 4), thus emphasizing the potential role of the booster dose of the IBV vaccine in the vaccination strategy as previous recorded (Sultan et al., 2019; Eid et al., 2024). Additionally, the group that received a combination of two vaccines on D0 and the H120 boost on D14 (group 5) had significantly lower ciliostasis score than the other two vaccine groups on D35, suggesting that the combination of Var2+H120 on D0 provided better protection than the other vaccine regimens (Fig. 4); this protection against the IBV QX (GI-19) results come in alignment with previous protection challenge modules but against IBV-Vra2 (GI-23), which highlighted the add value of the prime boost vaccination of different IBV vaccine lineage as an efficient tool to provide cross-protection against different IBV serotypes (Houta et al., 2024). Similarly, the Var2+H120 and H120 boost group (group 5) exhibited less impact on ciliary activity than the other two vaccine regimens on D35 further supporting the efficacy of this vaccine schedule in protecting against IBV QX challenge (Fig. 5b), which match the previous record in using prime-boost with a combination of different serotypes as an efficient tool to control IBV (Houta et al., 2024). Although the tracheal pathology score in group 5 was not significantly lower than the other two vaccine groups, it was numerically lower (Fig. 6). It is important to acknowledge that no one single vaccine combination can provide complete protection against all IB serotypes. However, by identifying the serotypes that are prevalent in a region, protectotyping can be used to provide broad coverage through cross-protection as previously recorded for different IB serotypes (Sultan et al., 2019; Eid et al., 2024; Bataille et al., 2025; Brimer et al., 2025). This study demonstrated that a combination of variant (MEVACTM IB VAR 2) and classical (MEVACTM IB H120) vaccine strains provided protection against IBV QX challenge, even in the absence of the specific challenge strain in the vaccine virus portfolio. Further studies are needed to establish the optimum vaccination protocol; however, this approach is likely to play a pivotal role in the control of IB in the poultry industry, both now and in the future. Further large-scale studies are warranted to corroborate these findings and understand the potential of protectotyping to control IBV infections in poultry.
Fig. 4. Ciliostasis scores. On D33 and D35, seven birds from each group were euthanized and 10 tracheal explant sections were scored for ciliostasis. Bars represent the mean ± SD (n=70). Two-way ANOVA followed by Šídák multiple comparisons test. Bars not connected by the same letter are significantly different (p < 0.05).
Fig. 5. Ciliary activity. On D33 (a) and D35 (b), seven birds from each group were euthanized and 10 tracheal explant sections were scored for ciliostasis For a given tracheal section, ciliary activity was considered normal when at least 50% of the internal ring showed vigorous ciliary movement, e.g., when the ring was given a score ≤ 2. A bird was considered normal when ≥9 out of 10 rings showed normal ciliary activity.
Fig. 6. Tracheal histopathology scores. On D35, tracheal samples from three birds from each group were analyzed by a pathologist according to 6-category scoring criteria on a scale of 0–3. Bars represent the mean ± SD (n=33). One-way ANOVA followed by Tukey’s multiple comparisons test. Bars not connected by the same letter are significantly different (p < 0.05). Scoring criteria and scale are described in Table 3. Table 4. Histopathological analysis of the kidneys. On D35, kidney samples from three birds from each group were analyzed by a pathologist.
AcknowledgementsThe authors thank Davy VanGaver and Isaura Christiaens for their contributions to this work. Conflict of interestThere is no conflict of interest to declare. Ethical declarationThe study was performed according to Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Ethical considerations were strictly adhered to, and all efforts were made to minimize animal suffering and stress, with humane euthanasia performed when necessary. Authors’ contributionsAll authors contributed equally to the current investigation. Data availabilityAll data were included within this manuscript, ReferencesBadr, H., Roshdy, H., Kilany, W., Elfeil, W., Sedik, A., Mohammed, W. and Shalaby, A. 2022. Isolation and molecular identification of avibacterium paragallinarum in suspected cases of poultry. J. Adv. Vet. Res. 12, 253–258. Bataille, H., Jan, M.R., Gustavo, S., Marcelo, Z. and De Wit, S. 2025. The combination of infectious bronchitis virus BR1 and mass vaccines provides broad protection. Avian Pathol. 54, 234–240. Brimer, S.K, Fischer, E.A.J, Beckstead, R., White, J., Cazaban, C., Tatár-Kis, T., Velkers, F.C., Elattrache, J. and Stegeman, A. 2025. A vaccine programme comprising GA08 (GI-27) and mass (GI-1) strains prevents DMV1639 (GI-17) infectious bronchitis virus transmission among broiler chickens. Avian Pathol. 54, 83–95. Butcher, G.D., Shapiro, D.P. and Miles, R.D. 2009. Infectious bronchitis virus: classical and variant strains. EIDS J. 2003(16), 1–5. Cavanagh, D. and Britton, P. 2008. Coronaviruses: general features. Encycloped. Virol. 2008, 549–554. Cavanagh, D. and Naqi, S. 2003. Infectious bronchitis. Dis. Poult. 11, 101–119. Cavanagh, D., Elus, M.M. and Cook, J.K. 1997. Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross-protection in vivo. Avian Pathol. 26, 63–74. Cook, J.K., Jackwood, M. and Jones, R. 2012. The long view: 40 years of infectious bronchitis research. Avian Pathol. 41, 239–250. Cook, J.K., Orbell, S.J., Woods, M.A. and Huggins, M.B. 1999. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 28, 477–485. Diab, M.S., Abd El Hafez, M.S., Ashry, M.A. and Elfeil, W.K. 2019. Occurrence of avian influenza H5N1 among chicken, duck farms and human in Egypt. Amer. J. Anim. Vet. Sci. 14, 26–32. Eid, A.A.M., Mahmoud, A.M., Hamouda, E.E., Metwally, M., Ezz-Eldin, R.M.M. and Elbakrey, R.M. 2024. The efficacy of simultaneous successive classic and variant infectious bronchitis virus vaccines versus circulating variant II Egyptian field virus. Open Vet. J. 14, 90–107. Elfeil, W.K., Ezzat, M.E., Fathi, A., Alkilany, M.A.A. and Abouelmaatti, R.R. 2020. Prevalence and genotypic analysis and antibiotic resistance of salmonella species isolated from imported and freshly slaughtered chicken. Amer. J. Anim. Vet. Sci. 15, 134–144. Elhady, M.A., Ali, A., Kilany, W.H., Elfeil, W.K., Ibrahim, H., Nabil, A., Samir, A. and Sayed, M.E. 2018. Field efficacy of an attenuated infectious bronchitis variant 2 virus vaccine in commercial broiler chickens. Vet. Sci. 5, 49. Ezzat, M., Rady, M., Elfeil, W.M., Abdufadel, M. and El-Tarabili, R.M. 2023. Disinfectant and multidrug-resistant Gram-negative bacteria in chicks. J. Adv. Vet. Res. 13, 2112–2117. Gussem, M., Van Middelkoop, K., Van Mullem, K. and Veer-Luiten, E. 2013. Broiler signals: a practical guide to broiler focused management. Zutphen, Netherlands: Roodbont Publishers. Hoerr, F.J. 2021. The pathology of infectious bronchitis. Avian Dis. 65, 600–611. Houta, M.H., Hassan, K.E., Kilany, W.H., Shany, S.A.S., El-Sawah, A.A., Elkady, M.F., Abdel-Moneim, A.S. and Ali, A. 2024. Evaluation of different heterologous-homologous vaccine regimens against challenge with GI-23 lineage infectious bronchitis virus. Virology 598, 110193. Ignjatović, J. and Sapats, S. 2000. Avian infectious bronchitis virus. Rev. Sci. Tech. 19, 493–508. Jackwood, M.W. 2012. Review of infectious bronchitis virus around the world. Avian Dis. 56, 634–641. Jackwood, M.W. and De Wit, S. 2013. Infectious bronchitis. Dis. Poult. 4, 139–159. Jackwood, M.W., Jordan, B.J., Roh, H.J., Hilt, D.A. and Williams, S.M. 2015. Evaluating protection against infectious bronchitis virus by clinical signs, ciliostasis, challenge virus detection, and histopathology. Avian Dis. 59, 368–374. Jordan, B. 2017. Vaccination against infectious bronchitis virus: a continuous challenge. Vet. Microbiol. 206, 137–143. Kilany, W.H., Zain El-Abideen, M.A., Hisham, I., Van Gaver, D., Makahleh, A., Christiaens, I., Vlerick, L. and Elkady, M.F. 2025. Laboratory safety and immunogenicity evaluation of live attenuated avian infectious bronchitis GI-23 virus vaccine. Vaccine 45, 126659. Legnardi, M., Tucciarone, C.M., Franzo, G. and Cecchinato, M. 2020. Infectious bronchitis virus evolution, diagnosis and control. Vet. Sci. 7, 79. Lohr, J. 1989. Lohr, J.E., 1989. Differentiation of IBV strains. In Proceedings of the First International Symposium on infectious bronchitis, Rauischholzhausen, West Germany, 1989, pp. 199–207. Mahmoud, E., El-Kholi, S.A., Rady, M.A., El-Tarabili, R.M., Hashem, M.A. and Elfeil, W.M.K. 2023. Molecular characterization of virulence genes among MDR and XDR avian pathogenic E. coli. J. Adv. Vet. Res. 13, 2014–2018. Mahmoud, S.I., Zyan, K.A., Hammoud, M.M., Khalifa, E., Dardir, S., Khalifa, R., Kilany, W.H. and Elfeil, W.K. 2022. Effect of co-infection of low pathogenic avian influenza H9N2 virus and avian pathogenic E. coli on H9N2 vaccinated commercial broiler chickens. Front. Vet. Sci. 9, 918440. Maier, H.J., Bickerton, E. and Britton, P.W. 2015. Coronaviruses: methods and protocols. New York: Humana Press: Springer. Rottier, P.J.M., Zeijst, B.A.M., Spaan, W.J.M. and Horzinek, M.C. 1984. Molecular biology and pathogenesis of coronaviruses. Boston, MA: Springer US: Imprint: Springer. Salama, E., Hamed, D.M., Zanaty, A., Rady, M. and Elfeil, W.M. 2023. Investigation on fowl adenovirus outbreaks in some broiler and broiler breeders’ flocks in Egypt. J. Adv. Vet. Res. 13, 2063–2067. Schalk, A.F. and Hawn, M.C. 1931. An apparently new respiratory disease of baby chicks. J. Am. Vet. Med. Assoc. 8, 413–423. Shao, L., Zhao, J., Li, L., Huang, X., Yang, H., Cheng, J., Liu, C. and Zhang, G. 2020. Pathogenic characteristics of a QX-like infectious bronchitis virus strain SD in chickens exposed at different ages and protective efficacy of combining live homologous and heterologous vaccination. Vet. Res. 51, 86. Sultan, H.A., Ali, A., El Feil, W.K., Bazid, A.H.I., Zain El-Abideen, M.A. and Kilany, W.H. 2019. Protective efficacy of different live attenuated infectious bronchitis virus vaccination regimes against challenge with IBV variant-2 circulating in the Middle East. Front. Vet. Sci. 6, 341. Sultan, H.A., Elfeil, W.K., Nour, A.A., Tantawy, L., Kamel, E.G., Eed, E.M., El Askary, A. and Talaat, S. 2022. Efficacy of the Newcastle disease virus genotype VII.1.1-matched vaccines in commercial broilers. Vaccines 10, 29. Swayne, D.E. and Boulianne, M. 2020. Diseases of poultry. 14th ed. Hoboken, NJ: Wiley Blackwell. Talat, S., Abouelmaatti, R.R., Almeer, R., Abdel-Daim, M.M. and Elfeil, W.K. 2020. Comparison of the effectiveness of two different vaccination regimes for avian influenza H9N2 in broiler chicken. Animals (Basel) 10, 1–12. Valastro, V., Holmes, E.C., Britton, P., Fusaro, A., Jackwood, M.W., Cattoli, G. and Monne, I. 2016. S1 gene-based phylogeny of infectious bronchitis virus: an attempt to harmonize virus classification. Infection Gen. Evol. 39, 349–364. WOAH. 2018. International Office of Epizootics Biological Standards Commission. Manual of diagnostic tests and vaccines for terrestrial animals: (mammals, birds and bees), Paris: Office international des épizooties. Zanaty, A., Mosaad, Z., Elfeil, W.M.K., Badr, M., Palya, V., Shahein, M.A., Rady, M. and Hess, M. 2023. Isolation and genotypic characterization of new emerging avian reovirus genetic variants in Egypt. Poultry 2, 174–186. | ||
| How to Cite this Article |
| Pubmed Style Elfeil WK, Makahleh A, Phillips FC, Elkady MF. Protective efficacy of commercially available infectious bronchitis vaccination regimes against heterologous IBV QX challenge. Open Vet. J.. 2025; 15(7): 3290-3299. doi:10.5455/OVJ.2025.v15.i7.40 Web Style Elfeil WK, Makahleh A, Phillips FC, Elkady MF. Protective efficacy of commercially available infectious bronchitis vaccination regimes against heterologous IBV QX challenge. https://www.openveterinaryjournal.com/?mno=255496 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i7.40 AMA (American Medical Association) Style Elfeil WK, Makahleh A, Phillips FC, Elkady MF. Protective efficacy of commercially available infectious bronchitis vaccination regimes against heterologous IBV QX challenge. Open Vet. J.. 2025; 15(7): 3290-3299. doi:10.5455/OVJ.2025.v15.i7.40 Vancouver/ICMJE Style Elfeil WK, Makahleh A, Phillips FC, Elkady MF. Protective efficacy of commercially available infectious bronchitis vaccination regimes against heterologous IBV QX challenge. Open Vet. J.. (2025), [cited January 12, 2026]; 15(7): 3290-3299. doi:10.5455/OVJ.2025.v15.i7.40 Harvard Style Elfeil, W. K., Makahleh, . A., Phillips, . F. C. & Elkady, . M. F. (2025) Protective efficacy of commercially available infectious bronchitis vaccination regimes against heterologous IBV QX challenge. Open Vet. J., 15 (7), 3290-3299. doi:10.5455/OVJ.2025.v15.i7.40 Turabian Style Elfeil, Wael K., Abdallah Makahleh, Farrah C. Phillips, and Magdy F. Elkady. 2025. Protective efficacy of commercially available infectious bronchitis vaccination regimes against heterologous IBV QX challenge. Open Veterinary Journal, 15 (7), 3290-3299. doi:10.5455/OVJ.2025.v15.i7.40 Chicago Style Elfeil, Wael K., Abdallah Makahleh, Farrah C. Phillips, and Magdy F. Elkady. "Protective efficacy of commercially available infectious bronchitis vaccination regimes against heterologous IBV QX challenge." Open Veterinary Journal 15 (2025), 3290-3299. doi:10.5455/OVJ.2025.v15.i7.40 MLA (The Modern Language Association) Style Elfeil, Wael K., Abdallah Makahleh, Farrah C. Phillips, and Magdy F. Elkady. "Protective efficacy of commercially available infectious bronchitis vaccination regimes against heterologous IBV QX challenge." Open Veterinary Journal 15.7 (2025), 3290-3299. Print. doi:10.5455/OVJ.2025.v15.i7.40 APA (American Psychological Association) Style Elfeil, W. K., Makahleh, . A., Phillips, . F. C. & Elkady, . M. F. (2025) Protective efficacy of commercially available infectious bronchitis vaccination regimes against heterologous IBV QX challenge. Open Veterinary Journal, 15 (7), 3290-3299. doi:10.5455/OVJ.2025.v15.i7.40 |