| Original Article | ||
Open Vet. J.. 2022; 12(2): 264-272 Open Veterinary Journal, (2022), Vol. 12(2): 264–272 Original Research Clinical, molecular, and pathological investigations of ovine pulmonary adenocarcinoma in the middle of IraqFalah Abd Abass1 and Yahia Ismail Khudhair2*1Veterinary Hospital, Al-Diwaniyah, Al-Qadisiyah, Iraq 2Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Qadisiyah, Iraq *Corresponding Author: Yahia Ismail Khudhair. Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Qadisiyah, Iraq. Email: yahia.khudiar [at] qu.edu.iq Submitted: 06/12/2021 Accepted: 23/02/2022 Published: XX/03/2022 © 2022 Open Veterinary Journal
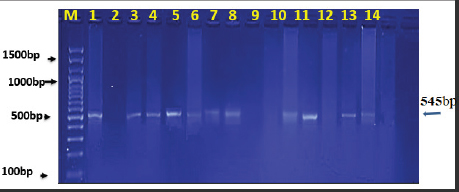
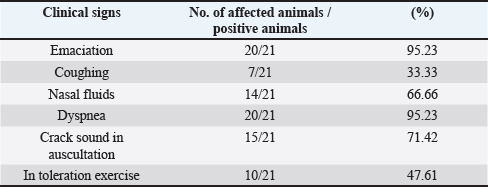
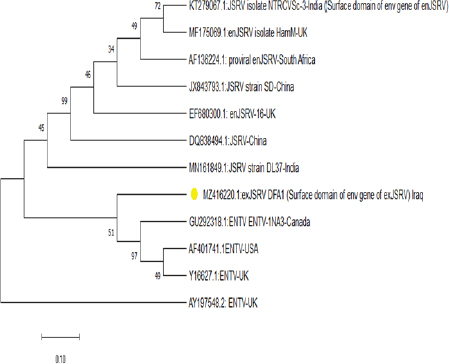
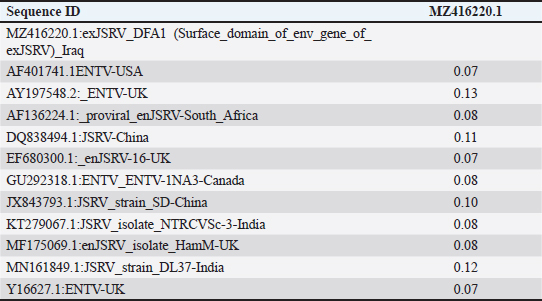
AbstractBackground: Ovine pulmonary adenocarcinoma (OPA), caused by Jaagsiekte sheep retrovirus (JSRV), is a contagious neoplastic disease in sheep characterized by chronic respiratory signs, inducing the transformation of secretory epithelial cells of the distal respiratory tract. Aims: To perform clinical, epidemiological, and molecular studies with evaluation of some predisposing factors at the herd level of OPA infection in sheep in Al-Qadisiyah Province, Iraq. Methods: The first step of the study was undertaken to evaluate the clinical cases of OPA in naturally infected sheep and correlation with observing respiratory signs. Seventy-five sheep with chronic respiratory signs were examined clinically, and by molecular and sequences analysis. The second step was the epidemiological part that was carried out on 195 randomly selected animals from 30 flocks, with the prevalence rate based on PCR; sex, age, and size of flocks were assessed, as well as macroscopic and microscopic features of the neoplastic lung. Deep nasal swabs and nasal secretion were collected from all animals. RNA extraction and RT-PCR were also carried out. Results: The results showed that 12 (16%) samples were positive for OPA, based on env gene-specific primers. Nucleotide sequences of partial 545 bp of the env gene showed (0.07–0.12) variations from global strains presented in the NCBI database. The prevalence rate of OPA was 21/195 (10.76%) with PCR. The epidemiological factors analysis showed that there was no effect of sex and herd size on the prevalence rates (p ≥ 0.01), whereas age was significantly affected and the age of 2–4 years was more susceptible (p ≥ 0.01). Gross and microscopic examinations were discussed with the confirmation of an OPA infection. Conclusion: The current study provides useful data about the clinical and epidemiological features of JSRV that is circulating in sheep of Iraq, and concludes that epidemiological studies and disease control may require multi-diagnostic assays. Keywords: Adenocarcinoma, OPA, Ovine, Pulmonary, Iraq. IntroductionOvine pulmonary adenocarcinoma (OPA) is a contagious neoplastic disease in sheep characterized by chronic respiratory symptoms and caused by the Jaagsiekte sheep retrovirus (JSRV), which causes secretory epithelial cells of the distal respiratory tract to change (Karagianni et al., 2019). The disease is transmitted through aerosols, which are the primary horizontal pathway for spreading the virus during inhalation of infectious aerosols; however, vertical transmission from the dams to their lambs via colostrum and milk were reported (Grego et al., 2008). The disease is widespread in most regions with large sheep populations and can be found in sheep-raising countries including Europe, Africa, Asia, and America (Quintas et al., 2021). Clinically, the disease is marked by progressive respiratory indications such as difficulty breathing, especially after exercise, emaciation, and signs of pneumonia that do not respond to antibiotic treatment (Shi et al., 2021). Progressive cases that cause neoplastic pulmonary lesions in infected sheep, with high rates of infection and death, result in a highly significant economic problem for sheep farmers in many countries (De las et al., 2021). The diagnosis of infected sheep and estimates of the prevalence of JSRV is mostly dependent on postmortem examination of the lungs, which is supplemented by clinical indicators and histopathology, particularly in advanced stages of the affected lung (Radad and Khalil, 2014; Lee et al., 2019). Where there are no detectable JSRV proteins outside the tumor and no circulating JSRV-specific antibodies during the preclinical stage, it is more difficult to identify infected animals (Lee et al., 2019; Ortin et al., 2019). The PCR) tests are informative for epidemiological studies and for identifying infected flocks (Voigt et al., 2007). There are very few studies concerning OPA in Iraq. Recently, Al-Husseiny et al. (2020) identified the OPA disease in Iraq. This study was designed to obtain the clinical, molecular, and histopathological investigations, as well as some epidemiological factors of OPA infection, to evaluate the genetic diversity of JSRV in Iraq. Material and MethodsSamplesThe first part of the study is based on the history of chronic respiratory infections, clinical symptoms and examination, including the positivity of the wheal-barrow test. A total number of 75 samples were obtained from 75 sheep suspected with OPA. These samples were tested using PCR for virus detection, sequencing, and phylogenetic analysis. In addition, gross and histopathological examinations of lung were done. The second part included the collection of 195 sheep samples of different ages (animals were allocated by age to three categories for further analysis: ≥ 2 years, 2–4 years, and ≤4 years) from 30 flock in a random model to evaluate the prevalence rate, and effect of sex, age, and flock size on infection rates. RNA ExtractionNasal swabs and nasal secretion were diluted with 2 ml phosphate buffer saline and filtered through 0.22 Millipore filter; then viral RNA was extracted using the RNA extraction kit (AccuZolTM RNA extraction kit Bioneer, Korea) following the manufacturer’s instructions. The extracted RNA samples were verified using Nanodrop spectrophotometer before being stored at −80°C until testing with the PCR. cDNA synthesisAccording to the manufacturer’s instructions, one script cDNA Synthesis SuperMix (Abm/Canada,) extracted RNA was transformed to synthesis cDNA, which was then used as a template in RT-PCR tests. PCR amplificationSpecific primers of the env gene were utilized; the forward primer sequence of the env gene was 5ʹ-GACCCCTCGACATTCCGTTT-3ʹ (6,222–6,241) and the reverse primer was 5ʹ-CAGCTATTTCAACGGGCAGC-3ʹ (6,757–6,777). The extracted RNA and cDNA were used as a template to identify JSRV by single PCR. One step RT-PCR Premix kit (INtRON Biotechnology, Inc., Korea) was used for PCR amplification of env gene with 20 μl reaction volume of 10 μl Premix, 1.5 μl each primer (10 pmol), 5 μl from an RNA template, and 2 μl of nucleus free water. Thermal cycling conditions of the env gene primer was denaturation at 94°C for 30 seconds; then 35 cycles of denaturation at 94°C for 30 seconds, 57°C for 30 seconds, and 72°C for 45 seconds, followed by one cycle of final extension at 72°C for 5 minutes and maintained at 4°C. The PCR products were visualized by ethidium bromide staining of 2% agarose gel with ethidium bromide in Tris-borate/EDTA buffer (90 mM Tris-borate and 2 mM EDTA). DNA was visualized using VISION Gel Documentation (Scie-Plas, Cambridge, UK). Sequence and phylogenetic analysisThe amplified DNA was sent to Macrogen (Seoul, Korea) to submit the Sanger sequence. All sequences were assembled and submitted to GenBank, NCBI. Multiple alignments and phylogenetics were analyzed by Mega X (version 6.0 Mega X). Microscopic examinationThe lungs of infected animals were taken immediately after slaughter and examined carefully and visually for any change in the color and by hand palpation, in addition to the examination of trachea and bronchi, to detect the presence of lung fluids. Three pieces of 1 cm3 tissue samples from infected sheep lungs were taken and fixed in 10% neutral buffered formalin. Hematoxylin and eosin staining (Leica Biosystems, England) and Masson’s trichrome staining (Masson’s Trichrome Stain Kit, Leica Biosystems, England) were used to stain sections of 4–5 μ thickness cut from paraffin blocks (Luna, 1986; Bancroft and Gamble, 2008). Statistical analysisStatistical analysis was calculated by using the OpenEpi website (using one of the epidemiological and statistical tools; https://www.openepi.com/SampleSize/SSPropor.htm (Noordzij et al., 2010). Ethical approvalThe review board of the University of Al-Qadisiyah, College of Veterinary Medicine, approved this study. Consent was obtained from the farm owners before animal examination and sampling. ResultsOut of 75 animals that were selected, depending on their pulmonary disease symptoms, 12 (16%) samples were positive with OPA using PCR test based on the env gene (Fig. 1). All sheep were clinically investigated (Table 1). The observed frequencies and percent of primary characteristic indications varied, and the majority of animals tested with respiratory symptoms were negative. Animals infected with OPA illness showed a variety of clinical signs, including emaciation, coughing, and dyspnea. A multiple alignment and phylogenetic analysis showed some variations from global strains of the NCBI database. The 545 nucleotide sequence analysis discloses that the current sequenced JSRV env gene has some genetic deviations from global isolates. High sequence identity of local JSRV isolates showed significant differences from the strains of UK and India (EF680300.1 JSRV, 0.07) (KT279067.1: JSRV_isolate_NTRCVSc-3), respectively (Fig. 2), with distances values as shown in Table (2). The results of the current study showed that the overall prevalence rate of 195 samples collected at random from different farms in Al-Qadisiyah province was 21/195 (10.76%) positive samples using PCR. JRSV-positive animals ranged in age from 6 months to ≤5 years, with the average age group of 2–4 years showing a slightly higher proportion of infected animals (Table 3) (χ2=0.1337, p < 0.01). JASV infection was insignificantly associated with animals’ sex (χ2=0.0137, p < 0.01).
Fig. 1. Ethidium bromide-stained agarose gel electrophoresis of 545 bp of JSRV env gene fragment amplified from OPA-infected sheep. (Lines 1–14 are positive samples, expect 2, 9, 10, and 12 were negative.) Table 1. Clinical signs of JSRV-infected sheep according to PCR results.
Fig. 2. Phylogenetic tree analysis of env region sequence in the local Iraqi isolate of JSRV of sheep; total genetic changes, 0.010%. Table 2. Multiple sequence alignment analysis of the homogeneity of substitution patterns among the Iraqi JSRV based on the env gene strain (partial region) sequence with various global JSRV and ANDV sequences from sheep and goat.
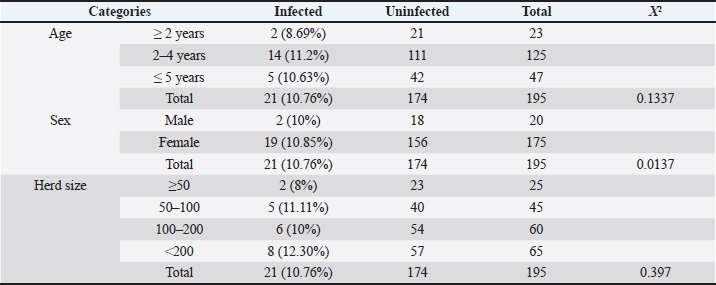
Table 3. Identification of OPA infection according to sheep age, gender, and herd size.
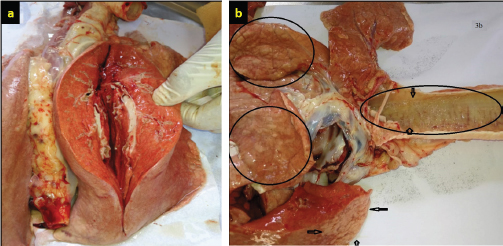
There was a significant association between JSRV infection and the herd size groups (χ2=0.397; p < 0.01). This study shows the correlation between the size of the herd and chance of animal infectivity, in ≤50 animals step up to ≥200 animals herd size, with a significant increase in the proportion of infected animals and an increase in herd size. Based on the χ2 test (Table 3), it was found that in herd size more than 200 heads, the probability of JSRV infection was 8 (12.30%) times higher than expected. In necropsied and slaughtered animals, gross examinations of lungs demonstrated and found that the dorsal region of the caudal lobes was the most affected region. The pathological changes were areas of diffuse consolidation and well-identifiable nodules, with many different sizes of white nodules forming cancer masses. Infected lungs were also heavier and larger when compared with healthy lungs. Cut sections of lungs showed a pale tint, a rubber consistency, and the impression of ribs on the lungs was visible, as well as fluid filling the thoracic cavity (Fig. 3a and b). On the other hand, cut sections of the consolidated area revealed mucus secretions and frothy fluids from the tumor lesions and bronchioles (Fig. 4a–c).
Fig. 3. (a) Gross appearances of the affected lung of sheep with OPA showing pink color with multiple nodules with a loss of spongy appearance; the incision surface is moist and frothy fluid is dripping from the tumor lesion. (b) The tumor lesion’s cutting surface is moist, and frothy fluids are flowing from the bronchioles.
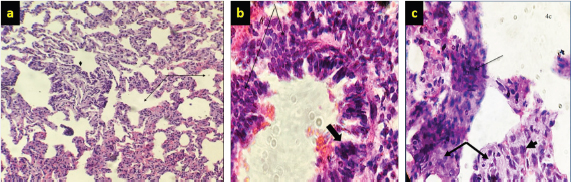
Fig. 4. (a) There is marked branching of the alveolar spaces (arrow) due to hyperplasia of alveolar cells and emphysema (thick arrow) (10×; H&E). (b) Higher magnification marked papillary projection toward the lumen of bronchioles (thick arrow) (40×; H&E). (c) Hypermagnification of marked hyperplasia epithelial cells (thick arrow) of bronchioles within infiltration of macrophages (arrow) in the mucosa of bronchioles (40×; H&E). Histopathological findings of lungs were mostly glandular transformation. Hyperplasia of alveolar cells causes branching of the alveolar spaces, as well as macrophage infiltration in the lung’s interstitial tissue and emphysema, was marked in some microsections with thickening of the interalveolar septa, alveolar exudative inflammation, and fibrosis with calcification (dystrophic calcification) (Fig. 6a and b). A necrosis-associated lymphocyte infiltration, resulting in marked hyperplasia of epithelial cells, especially lining of bronchioles, and desquamation of cells within the lumen of bronchioles is a consequence of this study. Furthermore, mediastinal lymph nodes showed substantial follicular atrophy compared with normal sections. Well-defined fibrous connective tissue was deposited, surrounded, and divided the cancer foci lesions; columnar and/or cuboidal cells surrounded the affected alveoli, forming papillary to acinar projections of altered neoplastic epithelial cells. Collagen deposition is expanded around blood vessels along with lymphoid tissue (Fig. 5a–c). DiscussionOPA has been reported in different countries around the world, including Iraq (Al-Husseiny et al., 2020). The disease is sporadic or endemic throughout the world, and it is recognized as a major disease in the international trade of sheep (Gomes et al., 2017; Jörger et al., 2017). There is a lack of accurate information about the prevalence of JSRV-related OPA in Iraq. Throughout this study, farms investigation, clinical examination, molecular diagnosis, and gross and histological alterations of JSRV were carried out in naturally infected sheep. A total of 10,000 various sheep of 30 herds were investigated in farms and slaughterhouses allocated by the Al-Diwaniyah Governorate. The first part of this study consisted of the examination of 75 samples of nasal fluids that were collected from sheep with respiratory problems with OPA suspicion. The results showed that only 12 (16%) samples were positive with PCR, whereas the majority of tested animals with respiratory symptoms were negative. These respiratory signs could be linked to other respiratory disorders, like a chronic bacterial infection, lung abscessation, or other lung lesions, that can be differentiated from OPA (Abass et al., 2019; Mosa and Zenad, 2020). These findings are consistent with earlier studies (Amini and Mostafa Tehrani, 2013; Mekibib et al., 2019; Toma et al., 2020). They revealed that the extensive pulmonary fibrosis and atelectasis related to chronic suppurative bacterial bronchopneumonia and verminous bronchopneumonia were the major differential diagnosis of OPA at farm levels.
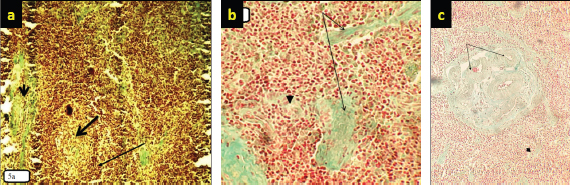
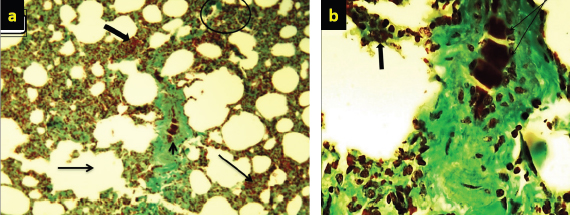
Fig. 5. (a) Marked necrosis in the lymphoid follicles of nodules (thick arrow) with depletion in these follicles (arrow) and deposition of collagen (arrow head) in the lymphoid tissue (10×; Masson’s staining). (b) Higher magnification. There is severe necrosis (arrow head) in the lymphoid cells, especially lymphocytes and reticular cells, with deposition of collagen (arrow) (40×; Masson’s staining). (c) Significant or marked collagen deposition (thick arrow) that is increased around the blood vessels and is also found along the lymphoid tissue. Also, there is depletion in the lymphoid follicles (arrow).
Fig. 6. (a) Multiple tumor masses (arrow) with papillary acinar structure (thick arrow) which extended within the lumen of alveoli (10×; H&E). (b) Papillary acinar structures with hyperplasia of alveolar calls (arrow) which lined the alveoli. Also, there is infiltration of macrophage (thick arrow) and lymphocytes (arrow head) in the pulmonary tissues (40×; H&E). However, the recorded clinical signs were nonspecific; major clinical signs were noted to give high suspicion to OPA; it usually reflects the extent of tumor development and devastation changes in the lungs. Marked dyspnea, mouth breathing (panting), and auscultation of moist rales were readily detected. Wheel-barrow test was carried out for assessed the accumulation of fluid within the respiratory tract by elevating the hindquarters and lowering the head of the animal, which lead to running frothy mucoid fluid from the nostrils (Ortín et al., 2019). The PCR results showed that 12 (16%) were positive, similar to the results of other studies (Vithana Dewage, 2020). The 545 nucleotide sequence analysis discloses that the current sequenced JSRV env gene has some genetic deviations from global isolates. The sequence of the local JSRV isolates showed significant differences from the strains of UK and India (EF680300.1 JSRV, 0.07) (KT279067.1: JSRV_isolate_NTRCVSc-3), respectively, and significant differences with strains from China (JX843793.1:JSRV_strain_SD and DQ838494.1:JSRV). Furthermore, the sequence showed significantly different values with JSRV of South Africa in sheep (AF153615.1) and the ENTV strain of China (MT254061.1) in goats that were 0.06 and 0.13, respectively. These results explained the relationship of Iraqi isolates with mutations in pro-gene structure but had no effect on the gene. The prevalence rate of the OPA disease study area was 12 (10.76%) out of 195 samples that were randomly selected from different herds. The results differed from those of a recent study conducted in Iraq (25%) (Mansour et al., 2019). The difference could be due to the later use of an unspecific primer in virus detection and the utilized lungs and lymph nodes tissues samples, which could lead to false positives with JSRV. Our study’s prevalence rate was similar to other studies in India (8%) (Sonawane et al., 2016), Iran (13.75%) (Bahari et al., 2016), and slightly lower from a study in Northwest Iran (18%) (Rezazadeh et al., 2012). There was a lot of diversity in the reports of OPA prevalence among Iraq and others countries which could be related to differences in location, source of material, nature of dissemination, management conditions, age of animal, season, and research duration. Furthermore, because OPA is a slow-moving virus with a prolonged incubation period, most animals infected with JSRV do not show clinical indications and remain subclinical or develop only a few lesions at the end of their life span. Most previous reports on the occurrence of OPA were purely based on gross and histological results, which could be one of the causes for our country’s low OPA rate (Radostits et al., 2017; He et al., 2017; Quintas et al., 2021). The results of this study indicated the association between the percentage of infection and the age of the investigated sheep, with the highest rate detected at 2–4 years, which is similar with other studies (Jassim et al., 2017; Beytut et al., 2009). Because OPA has a long incubation time, older sheep are more susceptible to infection than lambs. Singh et al. (2020) suggested that the sheep husbandry system and the period during which animals remained in direct contact, especially during the cooler months, may have a substantial impact on the occurrence of OPA. On postmortem examination, the dorsal portion of the caudal lobe of the lung was shown to be the most affected area of the lung. The general changes were similar to earlier OPA reports (Palmarini and Fan, 2001; Radad and Khalil, 2014; Belalmi et al., 2020; Toma et al., 2020; Mustafa et al., 2021). The histological examination was considered to have multiple foci of neoplastic proliferation involving alveolar and bronchiolar epithelium that were seen in sections from the consolidated areas of tumor nodules. In early lesions, the affected alveoli were highly irregular in shape and only involved a few alveoli. The histological abnormalities observed in all OPA-affected lungs in this investigation were consistent with previous findings (Belalmi et al., 2020; Toma et al., 2020; Mustafa et al., 2021). There were essentially two types of tumor cell development patterns observed: papillary and acinar or glandular. The alveolar wall was enlarged in a papillary pattern, and papillary projections of altered neoplastic epithelial cells, supported by a thin connective tissue stalk, partially or totally filled the alveolar lumen, which is in agreement with previous research (De las et al., 2021; Quintas et al., 2021). So, the nature of the virus and its influence on TYPE II pneumocytes, as well as the proliferation of these cells and the establishment of malignant tumors, is explained by changes in the infected lung tissue, as well as the infiltration of inflammatory cells in the area surrounding the tumors. The current study would provide useful data about surveillance and epidemiological features of JSRV circulating in the sheep population in Iraq, and concludes that epidemiological studies and control of disease may require multi-diagnostic methods, clinical signs, PCR, and gross and histopathological examinations to detect the presence of JSRV in infected animals with OPA. Authors’ ContributionsYahia I. Khudhair: structured the study design and conducted bioinformatics analyses. Falah A. Abass: animals’ examination, samples collection, hematological hypothesis, molecular study working and results’ interpretation, data collection, literature review, and manuscript preparation. All the authors contributed to manuscript preparation, and worded, read, and approved the final manuscript. AcknowledgmentsThe authors would like to thank Professor M.A.A. Alfatlawi, Head of the Department of Veterinary Microbiology (Al-Qadisiyah University), for kindly facilitating work in the laboratory of parasitology. They also thank the animal herds’ owners for examination and sampling of animals. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAbass, K.S., Mohammed, N.S., Taleb, M. and Raheem, Z.S. 2019. Study of bovine and ovine pulmonary and hepatic abscessation at Kirkuk abattoir. Plant Arch. 19(2), 1640–1644. Al-Husseiny, S., Jassim, A. and Mansour, K.A. 2020. Phylogenetic analysis of Jaagsiekte sheep retrovirus (JSRV) in Iraqi Awassi sheep. IJVS. 34(2), 351–355. Amini, F. and Mostafa-Tehrani, A. 2013. A five-year survey (2002–2007) on ovine pulmonary adenomatosis in a mixed-breed sheep flock. Bulg. J. Vet. Med. 16, 139-142. Bahari, A., Ghannad, M.S., Dezfoulian, O., Rezazadeh, F. and Sadeghi-Nasab, A. 2016. Detection of Jaagsiekte sheep retrovirus in apparently healthy sheep by real-time TaqMan PCR in comparison with histopathological findings. J. Vet. Res. 60, 7–12. Bancroft, J.D. and Gamble, M. 2008. Theory and Practice of Histological Techniques, 6th ed. China: Churchill Livingstone, Elsevier. Belalmi, N.E.H., Sid, N., Bennoune, O., Ouhida, S., De Las Heras, M. and Leroux, C. 2020. Evidence of jaagsiekte sheep retrovirus-induced pulmonary adenocarcinoma in Ouled Djellal breed sheep in Algeria. Vet. Res. Forum. Faculty of Veterinary Medicine, Urmia University, Urmia, Iran. 11(1), 93. Beytut, E., Sözmen, M. and Erginsoy, S. 2009. Immunohistochemical detection of pulmonary surfactant proteins and retroviral antigens in the lungs of sheep with pulmonary adenomatosis. J. Comp. Pathol. 140(1), 43–53. De las Heras, M., Borobia, M. and Ortín, A. 2021. Neoplasia-Associated Wasting Diseases with Economic Relevance in the Sheep. Industry Animals (Basel) 11, 381. Gomes, M., Archer, F., Girard, N., Gineys, B., Dolmazon, C., Erny, A.B. and Leroux, C. 2017. Blocked expression of key genes of the angiogenic pathway in JSRV-induced pulmonary adenocarcinomas. Vet. Res. 48(1), 1–16. Grego, E., De Meneghi, D., Álvarez, V., Benito, A.A., Minguijón, E., Ortín, A. and De las Heras, M. 2008. Colostrum and milk can transmit jaagsiekte retrovirus to lambs. Vet. Microbiol. 130(3–4), 247–257. He, Y., Zhang, Q., Wang, J., Zhou, M., Fu, M. and Xu, X. 2017. Full-length genome sequence analysis of enzootic nasal tumor virus isolated from goats in China. Virol. J. 14(1), 1–11. Jassim, A., Al-Husseiny, S.H., Mansour, K.A. and Kshash, Q.H. 2017. First molecular diagnosis of ovine pulmonary adenocarcinoma in Awassi sheep in Iraq. Al-Qadisiyah J. Vet. Med. Sci. 16(1), 112-117. Jörger, A., Acevedo, C., Busley, D., Ganter, M., Schmiedl, A. and Humann-Ziehank, E. 2017. Stereological and biophysical characteristics of the ovine surfactant system and its changes caused by ovine pulmonary adenocarcinoma. Res. Vet. Sci. 114, 332–340. Karagianni, A.E., Vasoya, D., Finlayson, J., Martineau, H.M., Wood, A.R., Cousens, C. and Griffiths, D.J. 2019. Transcriptional response of ovine lung to infection with jaagsiekte sheep retrovirus. J. Virol. 93(21), e00876-19. Lee, A.M., Wolfe, A., Cassidy, J.P., Moriarty, J., O’Neill, R., Fahy, C. and Messam, L.L.M. 2019. An approach to diagnosis of Jaagsiekte sheep retrovirus infection in sheep based on assessment of agreement between macroscopic examination, histopathologic examination and reverse-transcriptase polymerase chain reaction. J. Anim. Sci. 181, 29–33. Luna, L.G. 1968. Manual of histologic staining methods of the Armed Forces Institute of Pathology, 3rd ed. New York: McGraw-Hill. Noordzij, M., Tripepi, G., Dekker, F.W., Zoccali, C., Tanck, M.W. and Jager, K.J. 2010. Sample size calculations: basic principles and common pitfalls. Nephrol. Dialy. Transplant. 25(5), 1388–1393. Mansour, K.A., Al-Husseiny, S.H., Kshash, Q.H. and Jassim, A. 2019. Clinical-histopathological and molecular study of ovine pulmonary adenocarcinoma in Awassi sheep in Al-Qadisiyah Province, Iraq. Vet. World 12(3), 454–458. Mekibib, B., Mikir, T., Fekadu, A. and Abebe, R. 2019. Prevalence of pneumonia in sheep and goats slaughtered at Elfora Bishoftu export abattoir, Ethiopia: A pathological investigation. J. Vet. Med. 2019, 5169040; doi: 10.1155/2019/5169040. Monot, M., Archer, F., Gomes, M., Mornex, J.F. and Leroux, C. 2015. Advances in the study of transmissible respiratory tumours in small ruminants. Vet. Microbiol. 181(1–2), 170–177. Mosa, A.H. and Zenad, M.M. 2020. First Molecular Detection of Maedi-Visna Virus in Awassi Sheep of Middle Iraq Regions. Bulg. J. Vet. Med. http://tru.uni-sz.bg/bjvm/2020-0069%20OnFirst.pdf Mustafa, E.S., Al-Jameel, W.H. and Al-Mahmood, S.S. 2021. Immunohistochemical detection of P53 and MDM2 and its correlation with histological grading system in ovine pulmonary adenocarcinoma. IJVS 35(4), 687–692. Ortín, A., De las Heras, M., Borobia, M., Ramo, M.A., Ortega, M. and de Arcaute, M.R. 2019. Ovine pulmonary adenocarcinoma: A transmissible lung cancer of sheep, difficult to control. J. Small Rum. 176, 37–41. Palmarini, M. and Fan, H. 2001. Retrovirus-induced ovine pulmonary adenocarcinoma, an animal model for lung cancer. J. National Cancer Institute 93(21), 1603-1614. Quintas, H., Pires, I., Garcês, A., Prada, J., Silva, F. and Alegria, N. 2021. The Diagnostic Challenges of Ovine Pulmonary Adenocarcinoma. Ruminants 1(1), 58–71. Radad, K. and Khalil, S. 2014. Natural ovine pulmonary adenocarcinoma in an Egyptian sheep farm. J. Vet. Sci. 30(1), 39–43. Radostits, O.M., Gay, C.C., Hinchcliff, K.W. and Constable, P.D. 2007. Veterinary medicine, 10th ed. Philadelphia, PA: Saunders Elsevier, pp: 1366–1368. Rezazadeh, F., Zarrini, G.H., Cousens, C.H. and Attarilar, N. 2012. Prevalence of Jaagsiekte sheep retrovirus infection in North-West Iran. Glob. Vet. 9(5), 535–540. Sharp, J.M., de las Heras, M., Spencer, T.E. and Palmarini, M. 2008. Jaagsiekte sheep retrovirus. In Encyclopedia of virology. Eds., Mahy, B. and van Regenmortel, M., 3rd ed. Oxford, UK: Elsevier Ltd., pp: 175–182. Shi, W., Jia, S., Guan, X., Yao, X., Pan, R., Huang, X. and Xu, Y. 2021. A survey of jaagsiekte sheep retrovirus (JSRV) infection in sheep in the three northeastern provinces of China. Arch. Virol. 166(3), 831–840. Singh, R., Singh, S., Singh, R., Varshney, R., Dhama, K., Kumari, S. and Singh, V. 2020. Patho-Epidemiological study of jaagsiekte sheep retrovirus infection in the sheep and goats’ population, India. Biol. Rhy. Res. 51(8), 1182–1196. Sonawane, G.G., Tripathi, B.N., Kumar, R. and Kumar, J. 2016. Diagnosis and prevalence of ovine pulmonary adenocarcinoma in lung tissues of naturally infected farm sheep. Vet. World 9(4), 365–370. Toma, C., Bâlteanu, V.A., Tripon, S., Trifa, A., Rema, A., Amorim, I. and Taulescu, M. 2020. Exogenous Jaagsiekte Sheep Retrovirus type 2 (exJSRV2) related to ovine pulmonary adenocarcinoma (OPA) in Romania: prevalence, anatomical forms, pathological description, immunophenotyping and virus identification. BMC Vet. Res. 16(1), 1–15. Voigt, K., Brügmann, M., Huber, K., Dewar, P., Cousens, C., Hall, M. and Ganter, M. 2007. PCR examination of bronchoalveolar lavage samples is a useful tool in pre-clinical diagnosis of ovine pulmonary adenocarcinoma (Jaagsiekte). Res. Vet. Sci. 83(3), 419–427. | ||
| How to Cite this Article |
| Pubmed Style Abass FA, Khudhair YI. Clinical, Molecular and Pathological Investigations of Ovine Pulmonary Adenocarcinoma (OPA) in the Middle of Iraq. Open Vet. J.. 2022; 12(2): 264-272. doi:10.5455/OVJ.2022.v12.i2.15 Web Style Abass FA, Khudhair YI. Clinical, Molecular and Pathological Investigations of Ovine Pulmonary Adenocarcinoma (OPA) in the Middle of Iraq. https://www.openveterinaryjournal.com/?mno=33198 [Access: November 23, 2025]. doi:10.5455/OVJ.2022.v12.i2.15 AMA (American Medical Association) Style Abass FA, Khudhair YI. Clinical, Molecular and Pathological Investigations of Ovine Pulmonary Adenocarcinoma (OPA) in the Middle of Iraq. Open Vet. J.. 2022; 12(2): 264-272. doi:10.5455/OVJ.2022.v12.i2.15 Vancouver/ICMJE Style Abass FA, Khudhair YI. Clinical, Molecular and Pathological Investigations of Ovine Pulmonary Adenocarcinoma (OPA) in the Middle of Iraq. Open Vet. J.. (2022), [cited November 23, 2025]; 12(2): 264-272. doi:10.5455/OVJ.2022.v12.i2.15 Harvard Style Abass, F. A. & Khudhair, . Y. I. (2022) Clinical, Molecular and Pathological Investigations of Ovine Pulmonary Adenocarcinoma (OPA) in the Middle of Iraq. Open Vet. J., 12 (2), 264-272. doi:10.5455/OVJ.2022.v12.i2.15 Turabian Style Abass, Falah Abd, and Yahia Ismail Khudhair. 2022. Clinical, Molecular and Pathological Investigations of Ovine Pulmonary Adenocarcinoma (OPA) in the Middle of Iraq. Open Veterinary Journal, 12 (2), 264-272. doi:10.5455/OVJ.2022.v12.i2.15 Chicago Style Abass, Falah Abd, and Yahia Ismail Khudhair. "Clinical, Molecular and Pathological Investigations of Ovine Pulmonary Adenocarcinoma (OPA) in the Middle of Iraq." Open Veterinary Journal 12 (2022), 264-272. doi:10.5455/OVJ.2022.v12.i2.15 MLA (The Modern Language Association) Style Abass, Falah Abd, and Yahia Ismail Khudhair. "Clinical, Molecular and Pathological Investigations of Ovine Pulmonary Adenocarcinoma (OPA) in the Middle of Iraq." Open Veterinary Journal 12.2 (2022), 264-272. Print. doi:10.5455/OVJ.2022.v12.i2.15 APA (American Psychological Association) Style Abass, F. A. & Khudhair, . Y. I. (2022) Clinical, Molecular and Pathological Investigations of Ovine Pulmonary Adenocarcinoma (OPA) in the Middle of Iraq. Open Veterinary Journal, 12 (2), 264-272. doi:10.5455/OVJ.2022.v12.i2.15 |