| Original Article | ||
Open Vet. J.. 2022; 12(5): 709-717 Open Veterinary Journal, (2022), Vol. 12(5): 709–717 Original Research Vascular endothelial growth factor: A novel marker for torsion-induced incomplete cervical dilatation in Egyptian buffaloesAhmed Ibrahim1, Atef Badr1, Hussein Ahmed Amer1, Abdel-Salam Eidaroos1, Najmi Mariol2 and Ayman Mesalam1*1Department of Theriogenology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Department of Surgery and Theriogenology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya *Corresponding Author: Ayman Mesalam. Department of Theriogenology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: aymanmesalam [at] gmail.com Submitted: 27/06/2022 Accepted: 22/08/2022 Published: 19/09/2022 © 2022 Open Veterinary Journal
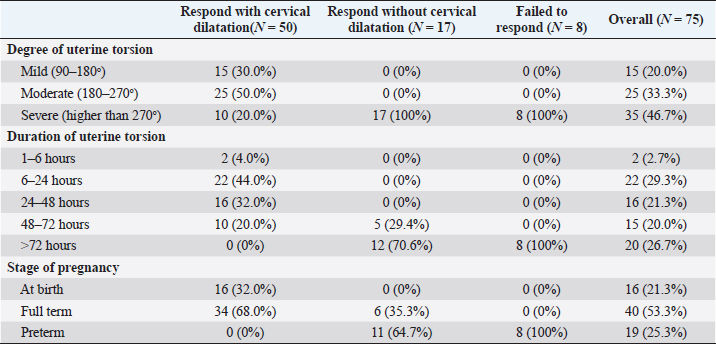
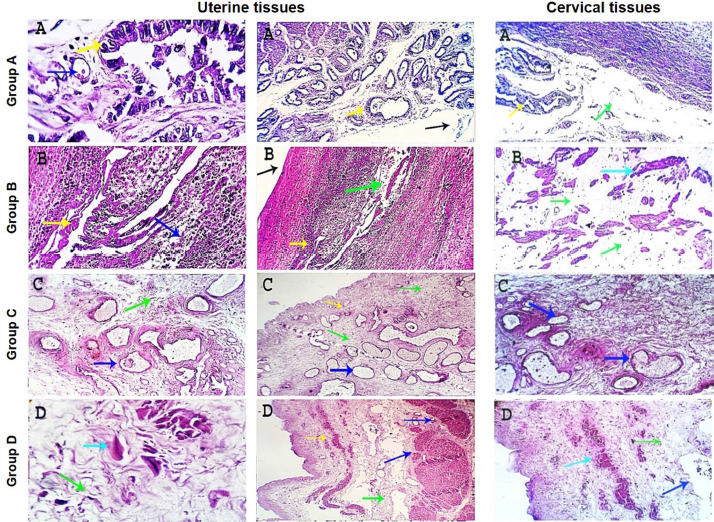
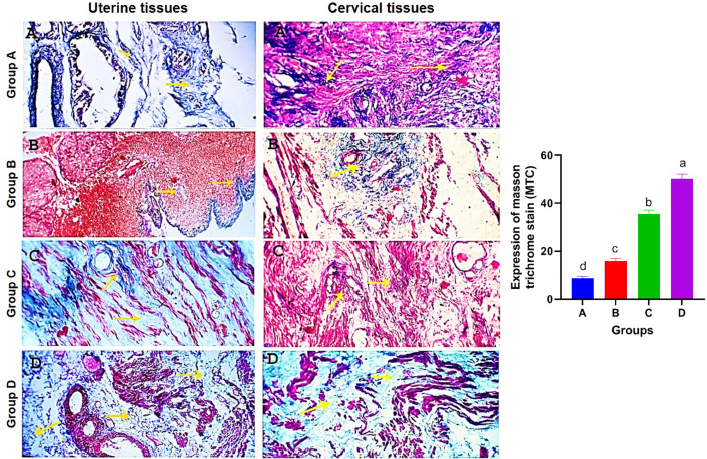
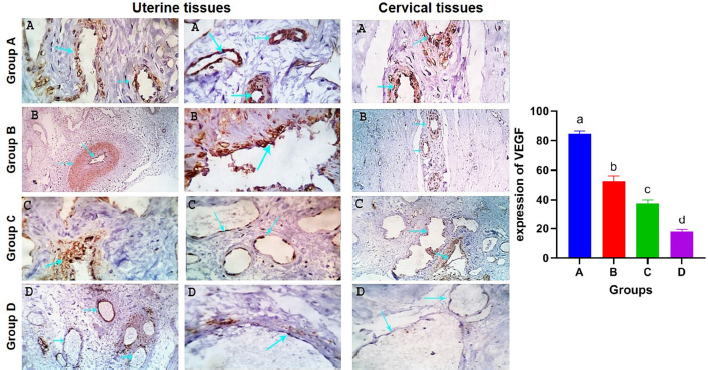
AbstractBackground: Uterine torsion, the most complicated cause of maternal dystocia recorded in bovine, usually followed by incomplete cervical dilatation after successful detorsion, which interfere with vaginal fetal delivery. Aim: This study aimed to evaluate the histopathological changes, variable collagen concentrations, and vascular endothelial growth factor expressions in uterine and cervical tissues following successful detorsion. Methods: Animals were classified into four groups; normally calved cases (group A), cases that respond successfully to detorsion and followed by vaginal fetal delivery without (group B) or with (group C) cervical laceration, and cases that suffered from failure of complete cervical dilatation after successful detorsion (group D). Results: Histopathological findings revealed variable changes in all uterine torsion affected groups, which were characteristic and marked in animals that suffered from failure of complete cervical dilatation following successful detorsion. Moreover, failure of cervical dilatation was associated with the highest collagen concentrations as shown by Masson trichrome stain. On the other hand, immunohistochemical findings showed that the normally calved cases have the highest vascular endothelial growth factor expression compared with animals that suffered from failure of complete cervical dilatation. Conclusion: Our results showed that the vascular endothelial growth factor is essential for cervical dilatation and its lower expression is accompanied by incomplete cervical dilatation following successful detorsion. Keywords: Uterine torsion, Buffaloes, Histopathological, Collagen, Vascular endothelial growth factor. IntroductionUterine torsion is the rotation of the pregnant uterine horn along its longitudinal axis, which leads to stenosis of the birth canal, resulting in dystocia (Balasopoulou et al., 2022; de Carvalho et al., 2022). It is the most common cause of dystocia in buffaloes (Nanda et al., 2003) and is commonly observed in pluriparous animals at the time of delivery or during the last month of gestation (Dhami et al., 2019). Surprisingly, the exact cause of a higher incidence of uterine torsion in buffaloes continues to be poorly understood, but the most logical explanation for the twisting of a pregnant uterus on its axis appears to be the unbalance of the uterus during unicornual pregnancy and excessive fetal or dam movements (Purohit et al., 2011). Uterine torsion is commonly followed by incomplete cervical dilatation and uterine inertia, so it should be handled as an emergency case in order to increase the survivability of the dam and livability of the fetus (Purohit and Gaur, 2014). It is well recognized that the incomplete cervical dilatation constitutes a major problem facing vaginal fetal delivery after successful correction of uterine torsion (Sahu et al., 2019). Collagen is the most myriad protein in the cervix and fibrillar collagen is the essential structural protein that influences the tensile characteristics of the cervix (Leppert, 1995; Ouellette et al., 2022). An essential requirement for cervical dilatation is softening of the cervix caused by decreased cervical tensile strength and change of collagen structure with the approach of parturition by decreasing collagen crosslinking and increasing its solubility (Timmons et al., 2010). Cervical ripening is a complex process that requires the association of hormonal actions, inflammatory reactions, and enzymatic breakdown of collagen (Balamurugan et al., 2018). Interestingly, the viscoelastic characteristics of the cervix, which are responsible for the dilatation of the cervix, are disturbed following the uterine torsion, producing a variable amount of cervical ischemia, leading to hypoxic degeneration of the cervical epithelium, obvious damage of elastic fibers, and incurable coagulative necrosis of smooth cells in the cervical tissue (Breeveld-Dwarkasing et al., 2003a). It has been reported that the uterine torsion has detrimental effects on cervical and uterine tissue, which is highly variable according to the degree and duration from occurrence till its handling. The limited arterial supply and venous return in the twisted uterus lead to hypoxia, ischemia, and necrosis, causing incurable damage to the endometrium, myometrium, and finally death of the fetus (Pearson and Denny, 1975; Frazer et al., 1996). Moreover, continued failure of blood supply results in loss of uterine wall elasticity and viability, and thus the uterine wall becomes necrosed, fragile, brittle, and susceptible to rupture (Ghuman, 2010). One of the efficient mechanisms used to enhance the oxygenation of hypoxic tissue to repay for these damaging effects is the induction of angiogenesis (Marti et al., 2000). Angiogenesis is defined as the formation of new blood vessels by the outgrowth of endothelial cells from pre-existing vessels (Holmes and Sleeman, 2000). Among the factors that have the ability to modify angiogenesis is vascular endothelial growth factor (VEGF), also known as vascular permeability factor, which is the best marker for a specific regulator of endothelial cell growth and differentiation (Gifre-Renom et al., 2022). Expression of VEGF is induced in cells suffered from ischemia or hypoxia (Hu et al., 2022). To the best of authors’ knowledge, VEGF has not been measured in cases of torsion-induced incomplete cervical dilatation. We hypothesized that the VEGF expression in uterine and cervical tissues may be altered in cases suffering from uterine torsion, and that may be the reason behind the lower possibility of complete cervical dilatation. We also investigated whether elevated collagen concentrations in uterine and cervical tissues are correlated with the degree of cervical dilatation after successful detorsion. Such correlation would suggest new approaches to both successful treatment and improving the survivability of the dam and livability of the fetus. Materials and methodsAnimalsA total of seventy-five Egyptian pregnant buffaloes were brought to our clinic, at Theriogenology department, Faculty of veterinary medicine, Zagazig University, from September 2020 to March 2022, with a history of anorexia, depression, and straining with colicy pain accompanied by frequent getting up and down. After rectal and vaginal examinations, uterine torsion was confirmed and these cases were handled by rolling procedure according to (Roberts and Hillman, 1973). Following successful detorsion, animals were therapeutically handled for induction of parturition and enhancement of cervical dilatation by using one or more of the following drugs according to the degree of cervical dilatation just after detorsion, such as PGF2α (cloprostenol) 500 µg i/m, carbetocin (oxytocin) 50 IU i/m, estradiol benzoate (folone) 100 mg i/m, denaverine hydrochloride (sensiblex) 400 mg, and calcium-magnesium borogluconate 500 ml slowly i/v. In addition, intracervical application of 1 mg PGE1 analogues (misoprostol) and cervical massage with warm sodium carboxy methyl cellulose gel for 15 minute, three times with 1 hour interval. Study designsIn order to identify the molecular markers and mechanisms behind failure of complete cervical dilatation following successful detorsion, we used normally calved cases as control group (Group A) and compared with uterine torsion affected cases that had different degrees of cervical dilatation following successful detorsion and therapeutic intervention as following; group B included animals that respond to rolling procedure and followed by complete cervical dilatation and vaginal fetal delivery without cervical laceration, group C included animals that respond to rolling procedure and followed by complete cervical dilatation and vaginal fetal delivery with cervical laceration due to excessive cervical edema, and group D included animals that respond to rolling procedure and followed by failure of complete cervical dilatation and subjected to caesarean section. Uterine and cervical biopsyUterine and cervical biopsies (n=5 per group) were collected after fetal delivery and placental separation or during caesarean section according to (Breeveld-Dwarkasing et al., 2003b). Briefly, the external genitalia and perineum were cleaned, and the sterilized biopsy instrument was introduced into the vagina. The instrument was guided into the cervix or the uterine horn beyond the uterine bifurcation by manipulation per rectum. With due care, cervical and uterine biopsies were obtained and fixed in 10% buffered formalin till further processing. Histopathological examinationThe fixed tissues were processed for histopathological studies through a routine alcohol benzene schedule for dehydration, then clearing of specimens in xylene, infiltrated and embedded in molten paraffin wax. The sections (5–7 μm) were cut and stained with Haematoxylin and Eosine and examined for evaluation of histopathological changes according to Slaoui and Fiette (2011). Evaluation of collagen fiber concentrationsTissue sections were histochemically stained with masson trichrome stain for evaluation of collagen fiber concentrations according to (Suvik and Effendy, 2012) with some modification. Briefly, fixed paraffin embedded tissue sections (5–7 μm) were deparafinze through xylene then rehydrated through a graded series of alcohol and rinsed in tape water. Tissue sections were stained in Weigert’s iron hematoxylin solution for 10 min, rinsed in running tap water. Then tissue sections were stained in Biebrich’s scarlet-acid fuchsine solution for 15 minutes and washed in distilled water. After that, sections were placed in phosphomolybdic–phosphotungstic acid solution for 10 minutes and directly transferred to aniline blue solution for 15 minutes. Next, tissues were washed in running tap water and then immersed in a 1% acetic acid solution for 3 minutes. The tissues were dehydrated with alcohol, cleared in xylene, and finally mounted under a cover-slip using permanent mounting media. Collagen deposition was marked as a dark blue fibril in the affected tissue and the scoring of such deposition was calculated morphometrically per five medium power fields. Evaluation of VEGF expressionsAn immunohistochemical examination of biopsies was conducted to determine expressions of VEGF in endothelial cells of blood vessels according to (Sojo et al., 2005). In brief, after deparaffinization and hydration, endogenous peroxidase was blocked with 1% hydrogen peroxide for 5 minutes. After blocking with 1% goat serum in PBS for 30 min, the sections were incubated with commercially available monoclonal anti-VEGF antibodies (SantaCruz Biotechnologies, Inc., USA) for 2 hours. The sections were rinsed with PBS and were subsequently reacted with biotinylated goat anti-mouse IgG for 30 min. They were incubated in a 3, 3-diaminobenzidine tetrachloride solution for visualization of sections. Finally, the sections were counterstained with hematoxylin and mounted under a cover-slip and examined under an Olympus microscope (Olympus BX-50, Tokyo, Japan), using a 40× objective. The result images were analyzed using Video Test Morphology 5.2 software (Russia) with a specific built-in routine for immunohistostaining analysis and stain quantification. The system measured the area percentage of VEGF expression according to Hashish and Kamal (2015). Statistical analysisStatistical analysis was performed using SPSS software v.18.0 (IBM Corp., Armonk, NY). The data were presented as the mean ± standard error of mean. The level of statistical significance was set at p < 0.05. Ethical approvalThe study was approved by the ethical committee of Zagazig University in accordance with the guidelines of the National Institutes of Health for the Care and Use of Animals. ResultsResponse of uterine torsion affected cases to rolling proceduresOut of the 75 uterine torsion affected cases, fifty buffaloes (66.67%) responded successfully to the rolling procedure, and followed by complete cervical dilatation and vaginal fetal delivery (Table 1), while 17 buffaloes (22.67%) responded successfully to the rolling procedure and followed by incomplete cervical dilatation. And only eight buffaloes (10.67%) failed to respond to the rolling procedure due to the presence of uterine adhesion with adjacent organs, so these animals were culled due to extensive parametritis and peritonitis after ultrasonographic examination (Fig. 1). The Effect of the degree of torsion on the response to rolling proceduresIt was found that all animals which suffered from mild (90–180o) to moderate (180–270o) degrees of uterine torsion responded successfully to the rolling procedure and followed by complete cervical dilatation. However, all animals which failed to respond to rolling procedure or followed by incomplete cervical dilatation were suffering from severe degree (higher than 270o) of uterine torsion (Table 1). Effect of duration of torsion on response to rolling proceduresMajority of animals (44.0%) that responded successfully to rolling procedure and followed by complete cervical dilatation have 6–24 hours duration of torsion. But, the majority of cases (70.6%) that responded to the rolling procedure and followed by incomplete cervical dilatation, and all animals that failed to respond to the rolling procedure had more than 72 hours duration of torsion (Table 1). Effect of stage of gestation on response to rolling proceduresAnimals that responded successfully to the rolling procedure and followed by complete cervical dilatation were presented at full term or at birth (68.0% and 32.0%, respectively). On the other hand, all animals that failed to respond to the rolling procedure were preterm (Table 1). Histopathological findingsExamined sections from control uterine and cervical tissues revealed apparently normal micro-morphological structures (Fig. 2A). However, sections from uterine and cervical tissues of uterine torsion affected cases revealed endometrial lining epithelial and glandular degenerative and necrotic changes in variable intensities (Fig. 2B–D). The subendometrial tissue was moderately to markedly edematous with tissue disintegration and mild inflammatory infiltration. The muscular coat showed moderate to severe tissue damage of congestive-hypoxic vascular origin as represented by muscle fiber degeneration, disintegration, and early gangrenous changes. The vascular changes were represented by moderate to marked venous dilatation, blood engorgement, vascular wall degeneration, and extravascular hemorrhages. The intermuscular tissue suffered from variable degrees of noninflammatory edematous reactions which led to tissue segregation with pressure atrophic and degradative changes, occasionally with early gangrenous changes. In all of the above-mentioned lesions, the changes were moderate in group B, moderate to marked in group C, and characteristic and marked in group D (Fig. 2B–D). Histochemical findings of collagen fiber concentrationsThe control uterine and cervical tissues showed normally presented collagen fibers as a supportive framework in a variable limited ratio according to many reasonable factors, which represent about 5%–10% of staining reactivity (Fig. 3A). On the other hand, uterine torsion affected groups showed significantly higher (p < 0.05) amounts of collagen deposition, with the highest collagen deposition seen in group D, as about 50%–55% of the affected tissues were replaced by fibroblasts and collagen. The lowest values were observed in group B, with 15%–20% staining reactivity, followed by group C, with 35%–40% staining reactivity (Fig. 3). Table 1. Relationship of degree and duration of torsion, with the stage of gestation in uterine torsion affected buffaloes (n=75).
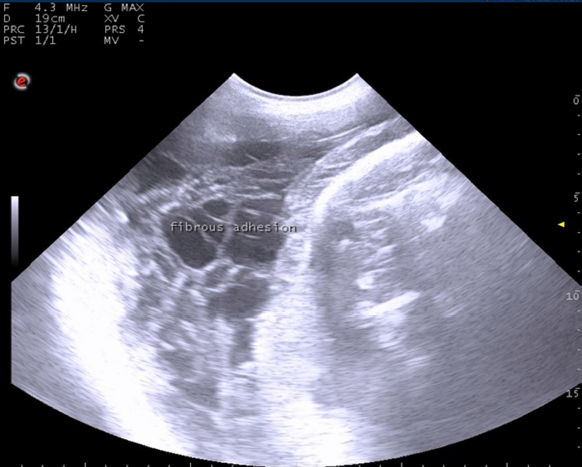
Fig. 1. Ultrasonographic examination with trans-abdominal probe at frequency 3.5–5 MHZ showing long standing uterine torsion case with severe degree at full term suffered from failure of detorsion due to presence of uterine adhesion with fibrin threads.
Fig. 2. Photomicrographs of uterine and cervical specimens stained with eosine and hematoxylin. Control uterine and cervical tissues revealed apparently normal micro-morphological structures (A). Uterine and cervical tissues of uterine torsion affected cases revealed extensive histopathological changes in variable intensities (B–D). Endometrial lining (black arrow), endometrial and endocervical glands (yellow arrows), blood vessels (dark blue arrows), muscular coat (light blue arrows) and intermuscular tissue (green arrows). X=100, 200, and 400. Immunohistochemical findings of VEGFThe most prominent VEGF expression was detected in the control group (85%–90%) with dark brownish nuclear and cytoplasmic stainabilities in almost all vascular endothelial and subendothelial tissues (Fig. 4A). The degrees of nuclear-cytoplasmic staining expression were significantly lower (p < 0.05) in uterine torsion cases, where it was moderately expressed (55%–60%) in group B, mildly to moderately expressed (35%–45%) in group C, and mildly expressed (15%–25%) in group D (Fig. 4B–D). DiscussionUterine torsion is termed as twisting of the gravid uterine horn around its longitudinal axis, which results in narrowing the birth canal and is usually followed by incomplete cervical dilatation (Kumar et al., 2014). It adversely affects the profitability as well as survivability of affected cases as it leads to the death of the dam, fetus or both , and impaired lactation if not treated early (Jeengar et al., 2015; Prakash et al., 2018). Incomplete cervical dilatation is a common cause of maternal dystocia in farm animals, which can occur alone or in association with other forms of dystocia such as torsion (Balamurugan et al., 2018). Our initial data revealed that after successful detorsion, the cervix showed variable degrees of incomplete cervical dilatation. In line with our finding, most cases were accompanied by a closed or incompletely dilated cervix rather than an opened or fully dilated cervix after detorsion (Jeengar et al., 2015). Our study reported that the degree and duration of uterine torsion from its occurrence till its presentation can influence the prognosis of uterine torsion affected animals. This result is consistent with the early findings which showed that the prognosis of uterine torsion cases is highly influenced by the degree and duration of uterine torsion and the presence of signs of approaching of parturition (Prabhakar et al., 1995; Schonfelder et al., 2005). We also reported that cases suffered from severe degree and prolonged duration of uterine torsion developed extensive uterine adhesion with adjacent abdominal organs with massive parametritis and peritonitis and did not respond to the detorsion procedure. Thus, these cases were culled because caesarean section was not useful in these cases. Because incision of the uterus during cesarean operation in prolonged cases of uterine torsion leads to drain of uterine content into abdominal cavity resulting into severe peritonitis and eventually death of dam (Purohit et al., 2013).
Fig. 3. Photomicrographs of uterine and cervical specimens stained with masson trichrome stain. Control tissues showed a normally presented collagen fibers (A). Collagen deposition appears marked as dark blue fibrils in uterine torsion affected cases (B–D). Fibroblasts and collagen are indicated by yellow arrows. X=200. Mean values of masson trichrome stain expression. Columns with different letters indicates a significant difference (p < 0.05).
Fig. 4. Photomicrographs of uterine and cervical specimens showing expression of VEGF in tissues of control group (A) and uterine torsion affected groups (B–D). Mean values of vascular endothelial growth factor expression. Columns with different letters indicates a significant difference (p < 0.05). Histopathological examination of uterine and cervical specimens with eosine and hematoxylin stain was conducted to evaluate histopathological alterations in uterine and cervical tissues and their relation to dilatation capacity of the cervix in normally calved and uterine torsion affected buffaloes. Results showed extensive histopathological changes in uterine torsion affected cases compared to normally calved cases as a result of the damaging effect of uterine torsion. In line with our finding, uterine torsion has been reported to be associated with a variable degree of histopathological changes in uterine and cervical tissue (Malik et al., 1990; Singh et al., 2010). Furthermore, histochemical examination of uterine and cervical specimens with masson trichrome stain was conducted to evaluate collagen concentrations, which revealed a characteristic tissue injury with consequent fibroplasia and collagen deposition, particularly in the sub-endometrial and in the superficial and middle myometrial interstitial tissues. Moreover, the results of the present study clearly indicated a marked collagen deposition in cases that suffer from incomplete cervical dilatation after detorsion, which hinders the dilatation capacity of the cervix, even after the therapeutic approach. These results are in accordance with previous studies reported that an extensive collagen deposition in the cervical matrix in uterine torsion cases with a severe degree and prolonged duration (Honparkhe et al., 2009; Singh et al., 2010). VEGF, a hypoxia–inducible representative marker, is a multifunctional cytokine that plays a key role in uterine vascularity, stimulates angiogenesis, and increases microvascular permeability (Görük and Deveci, 2018). Recent investigations reported a reduced VEGF expression in pathological placentas, pregnancies compromised by preeclampsia and fetal growth restriction (Babischkin et al., 2019; Gualdoni et al., 2021). Interestingly, the immuno-histochemical investigation of VEGF revealed different vascular endothelial and subendothelial tissue expression responses depending upon the degrees of vascular dynamic blood hemostasis and micro-structural tissue configurations. In this study, expression was evaluated in both uterine and cervical tissues of normally calved and uterine torsion affected buffaloes. We also demonstrated for the first time that the expression of VEGF was higher in normally calved cases than uterine torsion affected cases due to extensive vascular damage produced by uterine torsion, which leads to destruction of VEGF receptors in endothelial cells of blood vessels and more tissue damage due to failure of the angiogenesis process. Suggesting that VEGF can be used as an indicator for torsion-induced incomplete cervical dilatation. ConclusionIncomplete cervical dilatation, the major obstacle facing vaginal fetal delivery following successful detorsion, is significantly linked with elevated collagen concentrations in uterine and cervical tissues, and lower expressions of VEGF caused by tissue damage due to failure of the angiogenesis process can serve as a novel marker for torsion-induced incomplete cervical dilatation in Egyptian buffaloes. Prospective studies are required to evaluate the potential clinical applications of serum or urine VEGF levels in predicting the respond of uterine torsion affected cases to rolling procedures and the cervix to dilatation following successful detorsion. Authors’ contributionAB designed the experiments, AI performed the experiments and wrote the first draft of the manuscript, HAA, ASE, and NM provided assistance and AM analyzed the data, revised and edited the manuscript. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesBabischkin, J.S., Aberdeen, G.W., Lindner, J.R., Bonagura, T.W., Pepe, G.J. and Albrecht, E.D. 2019. Vascular endothelial growth factor delivery to placental basal plate promotes uterine artery remodeling in the primate. Endocrinology 160, 1492–1505. Balamurugan, B., Kharayat, N., Lavanya, M., Kumar, A., Ramamoorthy, M., Sushobhit, K., Deepesh Gautam, D.J. and Katiyar, R. 2018. Dystocia due to incomplete cervical dilation concurrent with cervico-vaginal prolapse in cattle: a case report. J. Entomol. Zool. Stud. 6, 544–545. Balasopoulou, V., Zablotski, Y., Zerbe, H. and Voigt, K. 2022. Retrospective analysis of 302 ovine dystocia cases presented to a veterinary hospital with particular attention to uterine torsion. Vet. Med. Sci. 8, 1683–1693. Breeveld-Dwarkasing, V., Struijk, P., Lotgering, F., Eijskoot, F., Kindahl, H., Van Der Weijden, G. and Taverne, M. 2003a. Cervical dilatation related to uterine electromyographic activity and endocrinological changes during prostaglandin F2α-induced parturition in cows. Biol. Reprod. 68, 536–542. Breeveld-Dwarkasing, V., Te Koppele, J., Bank, R., Van Der Weijden, G., Taverne, M. and van Dissel-Emiliani, F. 2003b. Changes in water content, collagen degradation, collagen content, and concentration in repeated biopsies of the cervix of pregnant cows. Biol. Reprod. 69, 1608–1614. de Carvalho, J.C., Lainetti, P.d.F., Alonso, J.d.M., Souza, F.F. and Ferreira, J.C.P. 2022. Ultrasonographic and haematology findings as an indicator of uterine torsion prognosis in cattle. Vet. Rec. Case Rep. e456. Dhami, A., Pateliya, C.S., Patel, J., Patel, S. and Makwana, H. 2019. Incidence of uterine torsion and related factors in buffaloes of amul milk shed area. Indian J. Vet. Sci. Biotechnol. 15, 17–20. Frazer, G.S., Perkins, N.R. and Constable, P.D. 1996. Bovine uterine torsion: 164 hospital referral cases. Theriogenology 46, 739–758. Ghuman, S. 2010. Uterine torsion in bovines: a review. Indian J. Anim. Sci. 80, 289–305. Gifre-Renom, L., Daems, M., Luttun, A. and Jones, E.A.V. 2022. Organ-specific endothelial cell differentiation and impact of microenvironmental cues on endothelial heterogeneity. Int. J. Mol. Sci. 23, 1477. Görük, N.Y. and Deveci, E. 2018. Immunoexpression of vascular endothelial growth factor and B-cell lymphoma 2 in the uterine tissue of rats treated with melatonin in the estrus phase1. Acta Cir. Bras. 33, 629–640. Gualdoni, G.S., Jacobo, P.V., Barril, C., Ventureira, M.R. and Cebral, E. 2021. Early abnormal placentation and evidence of vascular endothelial growth factor system dysregulation at the feto-maternal interface after periconceptional alcohol consumption. Front. Physiol. 12, 815760. Hashish, H. and Kamal, R. 2015. Effect of curcumin on the expression of Caspase-3 and Bcl-2 in the spleen of diabetic rats. J. Exp. Clin. Anat. 14, 18–23. Holmes, M.J. and Sleeman, B.D. 2000. A mathematical model of tumour angiogenesis incorporating cellular traction and viscoelastic effects. J. Theor. Biol. 202, 95-112. Honparkhe, M., Ghuman, S., Kumar, A., Gupta, K., Sood, N. and Ahuja, C. 2009. Cervical massage with sodium carboxy methyl cellulose for achieving complete cervical dilatation in successfully detorted uterine torsion affected buffaloes. Indian J. Anim. Sci. 79, 26–29. Hu, Y., Zheng, Y., Wang, T., Jiao, L. and Luo, Y. 2022. VEGF, a key factor for blood brain barrier injury after cerebral ischemic stroke. Aging Dis. 13, 647–654. Jeengar, K., Choudhary, V., Maharia, S. and Vivekanand, P.G. 2015. A retrospective study on type and extent of uterine torsion in buffaloes. Res. J. Vet. Pract 3, 25–28. Kumar, R., Singh, B., Srivastava, S. and Singh, K. 2014. Management of pre-cervical uterine torsion in a buffalo. Intas. Polivet. 15, 239–240. Leppert, P.C. 1995. Anatomy and physiology of cervical ripening. Clin. Obst. Gynecol. 38, 267–279. Malik, J., Verma, S. and Sharma, D. 1990. Histopathological studies on uterine torsion in buffaloes. Indian Vet. J. 67, 603–606. Marti, H.J., Bernaudin, M., Bellail, A., Schoch, H., Euler, M., Petit, E. and Risau, W. 2000. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am. J. Pathol. 156, 965–976. Nanda, A.S., Brar, P.S. and Prabhakar, S. 2003. Enhancing reproductive performance in dairy buffalo: major constraints and achievements. Reprod. Suppl. 61, 27–36. Ouellette, A., Mahendroo, M. and Nallasamy, S. 2022. Collagen and elastic fiber remodeling in the pregnant mouse myometrium. Biol. Reprod., ioac102. Pearson, H. and Denny, H. 1975. Spontaneous uterine rupture in cattle; a review of 26 cases. Vet. Rec. 97, 240–244. Prabhakar, S., Dhaliwal, G., Sharma, R. and Nanda, A. 1995. Success of treatment in relation to milk letdown and pelvic relaxation in bovines with torsion of uterus. Indian J. Dairy Sci. 48, 323–325. Prakash, S., Selvaraju, M., Ravikumar, K., Palanisamy, M., Manokaran, S. and Napoleon, R.E. 2018. Surgical management of right post cervical uterine torsion during 75 days of gestation in a graded Murrah buffalo. Buffalo Bull. 37, 93–96. Purohit, G., Barolia, Y., Shekhar, C. and Kumar, P. 2011. Diagnosis and correction of uterine torsion in cattle and buffaloes. Raksha. Techn. Rev. 1, 11–17. Purohit, G. and Gaur, M. 2014. Uterine torsion in buffaloes: a critical analysis. Buffalo Bull. 33, 363–378. Purohit, G.N., Mehta, J.S., Sharma, S., Shekher, C., Kumar, P., Kumar, A., Raiya, S., Ruhil, S. and Gaur, M. 2013. Uterine torsion in buffaloes: a retrospective analysis of 52 cases. Ruminant Sci. 2, 219–222. Roberts, S.J. and Hillman, R.B. 1973. An improved technique for the relief of bovine uterine torsion. Cornell Vet. 63, 111–116. Sahu, M., Gupta, T., Yadav, D.K. and Singh, C. 2019. Treatment of simultaneous occurrence of uterine torsion and incomplete cervical dilatation in surti buffalo. J. Entomol. Zool. Stud. 7, 89–90. Schonfelder, A., Richter, A. and Sobiraj, A. 2005. Stages of surgically incorrectable uterine torsion of cows: associations with clinical progress. Tierarztl. Umsch. 60, 199–205. Singh, A., Prabhakar, S., Banga, H., Brar, P. and Gandotra, V. 2010. Histopathological observations in cervix of dystocia affected vis-a-vis normally calved buffaloes. Indian J. Anim. Sci. 80, 842–846. Slaoui, M. and Fiette, L. 2011. Histopathology procedures: from tissue sampling to histopathological evaluation. Methods Mol. Biol. 691, 69–82. Sojo, K., Sawaki, Y., Hattori, H., Mizutani, H. and Ueda, M. 2005. Immunohistochemical study of vascular endothelial growth factor (VEGF) and bone morphogenetic protein-2,-4 (BMP-2,-4) on lengthened rat femurs. J. Cranio-Maxillofacial Surg. 33, 238–245. Suvik, A. and Effendy, A. 2012. The use of modified Masson’s trichrome staining in collagen evaluation in wound healing study. Mal. J. Vet. Res. 3, 39–47. Timmons, B., Akins, M. and Mahendroo, M. 2010. Cervical remodeling during pregnancy and parturition. Trends Endocrinol. Metab. 21, 353–361. | ||
| How to Cite this Article |
| Pubmed Style Ibrahim A, Badr A, Amer HA, Eidaroos A, Mariol N, Mesalam A. Vascular endothelial growth factor: A novel marker for torsion-induced incomplete cervical dilatation in Egyptian buffaloes. Open Vet. J.. 2022; 12(5): 709-717. doi:10.5455/OVJ.2022.v12.i5.16 Web Style Ibrahim A, Badr A, Amer HA, Eidaroos A, Mariol N, Mesalam A. Vascular endothelial growth factor: A novel marker for torsion-induced incomplete cervical dilatation in Egyptian buffaloes. https://www.openveterinaryjournal.com/?mno=69976 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i5.16 AMA (American Medical Association) Style Ibrahim A, Badr A, Amer HA, Eidaroos A, Mariol N, Mesalam A. Vascular endothelial growth factor: A novel marker for torsion-induced incomplete cervical dilatation in Egyptian buffaloes. Open Vet. J.. 2022; 12(5): 709-717. doi:10.5455/OVJ.2022.v12.i5.16 Vancouver/ICMJE Style Ibrahim A, Badr A, Amer HA, Eidaroos A, Mariol N, Mesalam A. Vascular endothelial growth factor: A novel marker for torsion-induced incomplete cervical dilatation in Egyptian buffaloes. Open Vet. J.. (2022), [cited January 25, 2026]; 12(5): 709-717. doi:10.5455/OVJ.2022.v12.i5.16 Harvard Style Ibrahim, A., Badr, . A., Amer, . H. A., Eidaroos, . A., Mariol, . N. & Mesalam, . A. (2022) Vascular endothelial growth factor: A novel marker for torsion-induced incomplete cervical dilatation in Egyptian buffaloes. Open Vet. J., 12 (5), 709-717. doi:10.5455/OVJ.2022.v12.i5.16 Turabian Style Ibrahim, Ahmed, Atef Badr, Hussein Ahmed Amer, Abdel-salam Eidaroos, Najmi Mariol, and Ayman Mesalam. 2022. Vascular endothelial growth factor: A novel marker for torsion-induced incomplete cervical dilatation in Egyptian buffaloes. Open Veterinary Journal, 12 (5), 709-717. doi:10.5455/OVJ.2022.v12.i5.16 Chicago Style Ibrahim, Ahmed, Atef Badr, Hussein Ahmed Amer, Abdel-salam Eidaroos, Najmi Mariol, and Ayman Mesalam. "Vascular endothelial growth factor: A novel marker for torsion-induced incomplete cervical dilatation in Egyptian buffaloes." Open Veterinary Journal 12 (2022), 709-717. doi:10.5455/OVJ.2022.v12.i5.16 MLA (The Modern Language Association) Style Ibrahim, Ahmed, Atef Badr, Hussein Ahmed Amer, Abdel-salam Eidaroos, Najmi Mariol, and Ayman Mesalam. "Vascular endothelial growth factor: A novel marker for torsion-induced incomplete cervical dilatation in Egyptian buffaloes." Open Veterinary Journal 12.5 (2022), 709-717. Print. doi:10.5455/OVJ.2022.v12.i5.16 APA (American Psychological Association) Style Ibrahim, A., Badr, . A., Amer, . H. A., Eidaroos, . A., Mariol, . N. & Mesalam, . A. (2022) Vascular endothelial growth factor: A novel marker for torsion-induced incomplete cervical dilatation in Egyptian buffaloes. Open Veterinary Journal, 12 (5), 709-717. doi:10.5455/OVJ.2022.v12.i5.16 |