| Research Article | ||
Open Vet. J.. 2023; 13(5): 620-628 Open Veterinary Journal, (2023), Vol. 13(5): 620–628 Original Research Prevalence of ocular findings and their association with glycemia in dogs with diabetes mellitus: A 10-year clinical study (2009–2019)Francisco Cantero1,2, Ángel Ortillés1,2, M. Teresa Peña1,2 and Marta Leiva1,2*1Servei d’Oftalmologia, Fundació Hospital Clínic Veterinari, Universitat Autònoma de Barcelona, Bellaterra, Spain 2Departament de Medicina i Cirurgia Animals, Facultat de Veterinària, Universitat Autònoma de Barcelona, Bellaterra, Spain *Corresponding Author: Marta Leiva. Servei d’Oftalmologia, Fundació Hospital Clínic Veterinari, Universitat Autònoma de Barcelona, Bellaterra, Spain. Email: marta.leiva [at] uab.cat Submitted: 13/10/2021 Accepted: 17/04/2023, Published: 16/05/2023 © 2023 Open Veterinary Journal
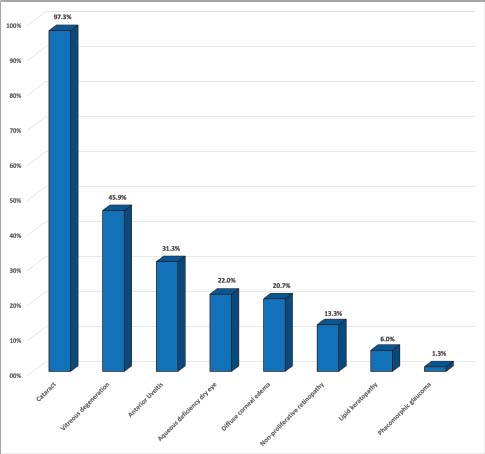
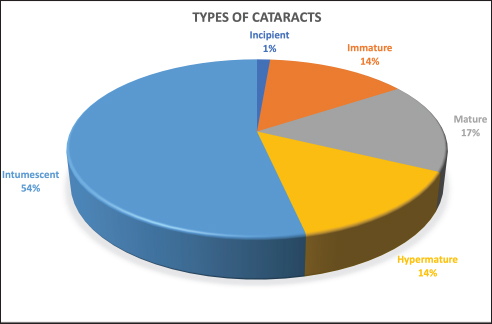
AbstractBackground: Although ocular findings in diabetic patients are well described, prevalence data for those is unknown. Aim: To describe the prevalence of ocular findings and their association with glycemia in dogs with diabetes mellitus. Methods: Medical records from diabetic dogs assessed by the ophthalmology and the internal medicine services at the Veterinary Teaching Hospital of the Autonomous University of Barcelona were reviewed (2009–2019). Results: Seventy-five dogs (150 eyes) of both genders (51/75 females; 68% and 24/75 males; 32%) and a mean age of 9.37 ± 2.43 years, were included. The most common ocular findings were cataracts (146/150; 97.3%), vitreous degeneration (45/98; 45.9%), anterior uveitis (47/150; 31.3%), aqueous deficiency dry eye (ADDE) (33/150; 22%), diffuse corneal edema (31/150; 20.7%), non-proliferative retinopathy (13/98; 13.3%), and lipid keratopathy (9/150; 6%). The most prevalent type of cataracts observed (78/146; 53.4%) was intumescent, which was commonly accompanied by non-proliferative retinopathy (p =0.003). Among the diabetic dogs, blood glucose levels were statistically higher in dogs with non-proliferative retinopathy or anterior uveitis (p < 0.005). Conclusions: Ocular complications of diabetes mellitus in dogs are numerous, being the most frequent intumescent cataracts, vitreous degeneration, anterior uveitis, ADDE, diffuse corneal edema, and non-proliferative retinopathy. This high prevalence warrants a more detailed ophthalmic evaluation in diabetic dogs especially for those undergoing cataract surgery. Furthermore, a predisposition for anterior segment inflammation and non-proliferative retinopathy is suggested when fasting plasma glucose is higher than 600 mg/dl.. Keywords: Cataracts, Glucose, Intumescent, Phacoemulsification, Retinopathy. IntroductionDiabetes mellitus (DM) is a common endocrine disorder characterized by chronic hyperglycemia resulting from a deficit in insulin production, action, or both (Fracassi, 2017). The prevalence of DM in dogs has been estimated from 0.3% in first-opinion practices to 1% in referral institutions (Guptill et al., 2003; Fracassi et al., 2004; Davison et al., 2005; Mattin et al., 2014). Two clinical forms are recognized in dogs: insulin-dependent and non-insulin-dependent. The former is the most common, being characterized by permanent hypoinsulinemia and, thereby, necessitating regular exogenous insulin to maintain glycemic regulation (Fracassi, 2017). Unfortunately, glycemic control can be difficult to attain in some insulin-dependent dogs, resulting in chronic exposure to high blood levels of glucose and ketoacidosis that can lead to systemic complications. Eyes are particularly vulnerable to the pathophysiological changes that occur because of chronic hyperglycemia, and consequently, ocular complications are a common finding in DM. Ocular findings being historically associated with DM in dogs are as follows: keratoconjunctivitis sicca (Cullen et al., 2005; Williams et al., 2007; Gemensky-Metzler et al., 2015), polymegathism and pleomorphism in corneal endothelial cells (Yee et al., 1985), corneal hypoesthesia (MacRae et al., 1982), non-healing corneal ulcers (Good et al., 2003), lipid keratopathy (Crispin, 2002), corneal xanthogranulomas (Harvey et al., 2020), corneal abscesses (Michau, 2020), intracorneal stromal hemorrhages (Matas et al., 2012; Violette and Ledbetter, 2017), lipid-laden aqueous humor (Violette and Ledbetter, 2019; Schechtmann et al., 2020), cataracts (Miller and Brines, 2018), lens-induced uveitis (Paulsen et al., 1985), phacomorphic glaucoma (Williams, 2004), vitreous degeneration (West et al., 2020), non-proliferative retinopathy (Landry et al., 2004), and peripheral neuropathies (Katherman and Braund, 1983). Unfortunately, until now, there are no studies reporting the prevalence of ocular findings in diabetic canine patients nor the association between those and glucose blood levels. Thus, the present study was conducted to describe the prevalence of the most common ocular findings in diabetic dogs, as well as to establish if there is a correlation between ophthalmic findings and blood glucose levels. Materials and MethodsInclusion criteriaMedical records of dogs evaluated by the ophthalmology service at the Veterinary Teaching Hospital of the Autonomous University of Barcelona between 2009 and 2019 were retrospectively reviewed. Dogs were included in the study if diagnosed with DM by a board-certified specialist in small animal internal medicine or a resident-in-training under direct supervision. DM was diagnosed by means of three findings as follows: appropriate clinical signs (polyuria, polydipsia, polyphagia, and weight loss), persistent fasting hyperglycemia (>200 mg/dl), and glycosuria. Animals with systemic hypertension were excluded from the study because of the important co-morbidity that those patients could have with the ocular findings. Blood pressure measurement was performed during internal medicine evaluation or during preanesthetic examination, and in all cases animals were excluded if systolic readings were over 160 mmHg. In all dogs, ophthalmic examination included neuro-ophthalmic evaluation, Schirmer tear test I (STT-1) (MSD Animal Health, Madison, NJ), slit-lamp biomicroscopy (Kowa SL-15 and -17®; Kowa Company Ltd., Tokyo, Japan), rebound tonometry (TonoVet® using “d” setting; Icare Finland Oy, Helsinki, Finland), and fluorescein stain (FluoroTouch®; Madhu Instruments, New Delhi, India). In addition, binocular indirect ophthalmoscopy (Heine Omega 500®, Heine, Herrsching, Germany), gonioscopy, and B-mode ultrasonography were performed when applicable. Animals were excluded if primary glaucoma, evidence of trauma, or infectious diseases were detected. Data retrievalData collected and reviewed from the medical records included signalment (breed, age, and gender), levels of fasting glycemia at the time of the ocular examination, eye/s affected, ophthalmic findings, whether or not cataract surgery was performed and post-phacoemulsification ocular fundus findings. Ophthalmic findings: diagnosis and grading systemAqueous deficiency dry eye (ADDE) was considered when STT-1 readings were under 15 mm/minute and no signs of corneal or conjunctival inflammation were observed. Cataract classification was established according to what was previously reported (Leiva and Peña, 2020): incipient, when lens opacity extended from 1% to 15%; immature when opacity covered between 16%–99% and fundic reflection was still able to be visualized; mature, when the whole lens was affected and no fundic reflection was visible after maximal mydriasis; hypermature, when cortex resorption, capsule wrinkling, capsule plaques, or deposition of pigment on the anterior capsule were present; and intumescent, when the size of the lens was bigger than normal, lens sutures were broken, and/or liquid was seen within the lens. Similarly, the degree of anterior uveitis was also graded semiquantitatively (on a scale from trace to +4) based on conjunctival congestion, aqueous flare (0 to +4), ocular hypotony, and miosis or resistance to mydriasis after tropicamide application. Non-exudative uveitis was considered when previous signs were present, but no visible aqueous flare was observed. Phacomorphic glaucoma was diagnosed when intraocular pressure (IOP) increased over 25 mmHg, the anterior chamber depth was diminished and no alterations were observed on gonioscopy. Vitreous degeneration was diagnosed by B-mode ultrasound examination and graded using a previously reported scheme whereby the vitreous degeneration is classified into four grades (0–III), depending on the number of echoes seen on ultrasonography (Labruyère et al., 2008). Non-proliferative diabetic retinopathy was considered if retinal petechia or subretinal hemorrhages were present and no systemic hypertension was detected. Statistical analysisQuantitative results were reported by the mean and standard deviation (SD) or median and range for normally or non-normally distributed data, respectively (as assessed by the Kolmogorov-Smirnov test). Qualitative results were described by absolute and relative frequencies. Fisher's exact test was used for qualitative variables, and unpaired t-test and Mann–Whitney U test for quantitative variables. For all statistical analyses, a commercial software (SPSS 25.0®; IBM, Chicago, IL) was used, and a significance threshold of p < 0.05 was set. Ethical approvalThis paper refers to a retrospective study with no animals involved in the experimental process, so no consent of ethics is needed for this study. ResultsMedical records of 75 diabetic dogs met the inclusion criteria (150 eyes). The mean (±SD) age of the study population at first ophthalmic examination was 9.37 years (±2.43 years). There were 12 castrated males (16%), 12 intact males (16%), 27 spayed females (36%), and 24 intact females (32%). Twenty-nine dog breeds were identified in the study population, including mix breed (27/75 dogs; 36%), Yorkshire Terrier (4/75; 9.3%), West Highland White Terrier (4/75; 5.3%), English Cocker Spaniel (4/75; 5.3%), Golden Retriever (3/75; 4%), and one or two dogs from 24 other different breeds. Ophthalmic findingsOcular lesions and their prevalence are summarized in Figures 1 and 2, and shown in Figure 3. All dogs had bilateral, although not always symmetrical, clinical findings. Cataract was the most common ocular finding (146/150 eyes; 97.3%), being bilateral in all the affected dogs. Intumescent cataracts were seen in 78 eyes (78/146; 53.4%), mature cataracts in 24 eyes (24/146; 16.4%), hypermature and immature in 21 eyes each (21/146; 14.4%), and incipient in 2 eyes (2/146; 1.4%) (Fig. 2). Vitreous degeneration was the second most common ocular finding (45/98 eyes; 45.9%), being bilateral in 21 dogs (21/24; 87.5%), and unilateral in 3 (3/24; 12.5%). According to Labruyere´s scoring system, grade I vitreous degeneration was recorded in 35/45 eyes (77.7%) and grade II in 10 eyes (22.3%) (Labruyère et al., 2008). Anterior uveitis was the next most common finding (47/150 eyes; 31.3%), being bilateral in 18 dogs (18/29; 62%) and unilateral in 11 dogs (11/29; 38%). In 6 of those eyes, uveitis was classified as non-exudative (6/47; 12.8%) and in 41 eyes as exudative (41/47; 87.2%). Of the latter, 31 eyes were classified as mild (31/41; 75.6%), 7 eyes as moderate (7/41; 17.1%), and 3 eyes as severe (3/41; 7.3%). ADDE was diagnosed in 33/150 eyes (22%), being bilateral in 10 dogs (10/13; 77%), and unilateral in 3 (3/13; 23%). The mean STT-1 value for the affected and unaffected animals was 10.9 ± 3.00 and 20 ± 3.4 mm/min, respectively. Despite these values, only 4 eyes showed overt corneal secondary changes (4/33; 12.1%). Diffuse corneal edema was encountered in 31/150 eyes (20.7%), with 12 dogs affected bilaterally (12/19; 63.1%) and 7 dogs unilaterally (7/19; 36.9%). Lipid keratopathy was detected in 9/150 eyes (6%) with 4 animals being affected bilaterally (4/5; 80%). Unilateral phacomorphic glaucoma was found in 2/150 eyes (1.3%). Gonioscopy was performed as part of the preoperative protocol in those cases in which phacoemulsification was elected. We used the actual European College of Veterinary Ophthalmologists (ECVO) approved gonioscopy scheme and all animals were classified as non-affected, but a narrow angle was detected in 10 eyes [(5 dogs); 10.2%], partially narrow in 18 eyes [(9 dogs); 18.4%], and fibra latae < 50% of circumference in 13 eyes [(7 dogs); 13.2%].
Fig. 1. Graph depicting the prevalence of ocular findings associated with DM in dogs with ocular signs.
Fig. 2. Sectorial graph showing results regarding type of cataract and its prevalence in diabetic dogs.
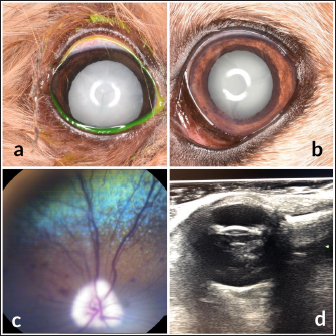
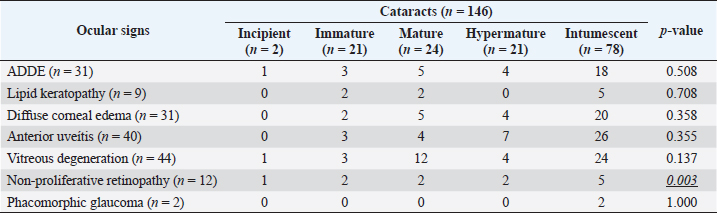
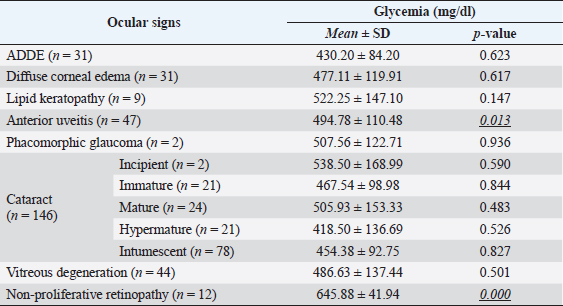
Fig. 3. Images of the main ocular lesions seen in diabetic dogs. (a): intumescent cataract; (b): phacolytic uveitis; (c): non-proliferative retinopathy; (d): vitreous degeneration grade II with hypermature cataract. Phacoemulsification was performed in 98 eyes (98/150 eyes; 65.3%), allowing for postoperative ocular fundus examination in all of them. Non-proliferative diabetic retinopathy was detected in 13/98 eyes (13.3%) examined within 7 days after phacoemulsification, being bilateral in 5 patients (5/8; 62.5%) and unilateral in 3 patients (3/8; 37.5%). Ocular fundus examination revealed intraretinal punctate hemorrhages in 13 eyes and preretinal hemorrhages in 6 eyes. The statistical analysis evaluating the correlation between the type of cataracts and the other ocular findings is displayed in Table 1. Interestingly, non-proliferative retinopathy was the only ocular finding directly associated wsith cataracts (p=0.003), specifically with intumescent cataracts (5/12; 42%). Table 2 shows the statistical association between fasting glycemia and each ocular finding, depicting a significant correlation between blood glucose levels, non-proliferative retinopathy (p =0.000), and anterior uveitis (p=0.013). These findings suggest a predisposition for anterior segment inflammation and non-proliferative retinopathy when fasting plasma glucose is higher than 600 mg/dl. DiscussionThe present work provides new information on the ophthalmic findings associated with DM in dogs that could be helpful in the prevention and management of diabetic ocular disease. Also, shows a direct association between blood glucose levels and some of the most commonly identified ocular findings concomitant with the disease. This suggests that 600 mg/dl may be established as a cut-off value from which some ocular clinical signs, such as non-proliferative retinopathy and anterior uveitis, may be more frequently seen. Different epidemiologic studies suggest that some canine breeds are at higher risk of developing DM (Guptill et al., 2003; Fracassi et al., 2004; Cullen et al., 2005). To the authors´ knowledge, there are no reports on whether there is a breed predisposition to develop secondary ocular signs. In the present study, mix breed dogs were clearly overrepresented, but this fact could indicate regional differences in breed distribution, rather than a true predisposition for ocular complications of DM. According to previous reports (Guptill et al., 2003; Fall et al., 2007), middle-aged to older females were the most commonly affected by DM, with a similar incidence of ocular findings. Although neutering status has been associated with better glycemic control (Guptill et al., 2003), the findings herein suggest that neutering status may not influence the prevalence of ocular complications associated with DM. Cataract was the most prevalent finding in this population of diabetic dogs with ophthalmic clinical signs (97.3%), intumescent cataract being the most common. This agrees with previous studies in which cataracts were consistently found as the main ophthalmic complaint in diabetic animals (Ling et al., 1977). In fact, Beam et al. (1999) stated that 75% of the canine population diagnosed with DM will develop cataracts by approximately 12 months after the first diagnosis of diabetes. It is well-known that intumescent cataracts develop due to an osmotic imbalance secondary to intralenticular sorbitol accumulation. The speed of development depends on sugar concentration and endogenous activity of aldose reductase, which very often ends with an acute onset cataract (Engerman et al., 1982). This acute onset is often the main complaint for requesting an ophthalmologic examination, which could explain why intumescent was the most common type of cataracts seen in the present study. Table 1. Statistical correlation between ophthalmic findings (in absolute frequencies) and type of cataracts in diabetic dogs. Statistically significant p-values are underlined and in italics. A significance threshold of p < 0.05.
Table 2. Glycemia levels are shown as mean ± SD in diabetic dogs with ocular findings. For each ocular finding, the statistical results of the evaluation between affected and non-affected eyes are shown, establishing a significance threshold of p < 0.05. Significant p-values are underlined and in italics.
Vitreous degeneration is an age-related ocular condition in dogs, with a higher prevalence in female dogs (Krishnan et al., 2020). In fact, it is described that dogs have 24 times more likelihood of developing vitreous degeneration per year of age (Krishnan et al., 2020). In the present study, due to the impossibility of fully evaluating and grading the condition by biomicroscopy, a B-mode ultrasound was performed for this purpose. This technique has been reported to have high sensitivity and specificity when diagnosing vitreous diseases (Labruyère et al., 2008). Vitreous degeneration was surprisingly the second most common ocular finding (45.9%) in the present study. Most of the affected eyes displayed a mild degree of the condition (77.7%), not showing a statistical correlation with the type of cataracts (p > 0.05). This agrees with Park et al. (2015) who found an increased degree of vitreous degeneration when associated with chronic cataracts (mature or hypermature), but not with acute, intumescent, incipient, or immature cataracts. Similarly, Krishnan et al. (2020) ruled out a possible correlation between vitreous degeneration and other ocular comorbidities, such as cataracts, lens luxation, glaucoma, and/or retinal detachment. Tear film dysfunction, both aqueous and evaporative, has been documented in dogs (Cullen et al., 2005; Williams et al., 2007) and humans (Manaviat et al., 2008) with DM. Human medicine studies have demonstrated that while basal rates of aqueous tear secretion were equivocal between diabetic and nondiabetic patients, STT values were lower in diabetics (Goebbels, 2000). In the present study, ADDE was found in 22% of the dogs. Surprisingly, many of these animals had no obvious clinical signs at diagnosis, making it possible to go undetected if no STT-1 had been performed. Duration of diabetic disease has been considered a risk factor for ADDE, as it negatively influences STT-1 values in diabetic dogs (Williams et al., 2007). Diabetic dogs undergoing phacoemulsification have been reported to be almost twice as likely to develop dry eye within the first 2-week postoperative period, compared to nondiabetic dogs. Tear supplementation rather than an institution of a stimulant tear product may be considered to protect the cornea during the anesthetic and surgery recovery period as tear values may improve after the 2-week postoperative period (Gemensky-Metzler et al., 2015). Furthermore, small diabetic dogs (<10 kg) have been reported as 1.7 times more likely to be diagnosed with dry eye postoperatively rather than small nondiabetic dogs (Gemensky-Metzler et al., 2015). It is unclear as to whether poor DM control is an important factor in the manifestation of dry eye diseases in diabetic canines as has been documented in humans (Ozdemir et al., 2003). Some canine studies suggest no significant correlation between poor DM control and low STT (Cullen et al., 2005; Williams et al., 2007); however, no complete cohort observational studies evaluating this factor have been published to date. Anterior uveitis was the third most common clinical finding in the study (31.3%). It has been reported that 71% of patients with cataracts will show lens-induced uveitis (LIU) elicited by an inflammatory response that occurs secondary to protein leakage from the lens capsule occurs (also known as phacolytic uveitis) (Paulsen et al., 1985). The degree of inflammation depends on the amount of soluble and insoluble proteins within the lens (Wilcock and Peiffer, 1987). Taking this into consideration, it is expected that LIU secondary to DM would be more severe in young patients than in older ones, as albuminoid proteins inside the lens increase with age (Ortwerth and Olesen, 1989). Young animals have a higher number of soluble proteins that can diffuse through intact lens capsules when an intumescent cataract develops quickly in diabetic patients. Although no correlation was found between the degree of uveitis and the type of cataract, it was more commonly diagnosed in dogs with intumescent cataracts. Another mechanism for LIU in diabetic dogs is associated with the rapid increase in the size of intumescent cataracts, thus leading to tears in the lens capsule at the equator (phacoclastic uveitis) (Wilkie et al., 2006). This type of uveitis tends to be more aggressive and to have a more acute onset. Wilkie et al. (2006) reported that dogs affected with spontaneous lens capsule rupture had, based on their histories, been diabetic for an average of 123 days and had cataracts for an average of 39 days. In the present study, no cases of phacoclastic uveitis were diagnosed. Based on the low amount of aqueous flare, the majority of the uveitis cases were classified as mild anterior phacolytic exudative uveitis (75.6%). Diabetic corneal endothelial changes have been widely described in the human literature, but in veterinary medicine, they are scarcely reported (Yee et al., 1985). Conversely to what has been described in human medicine (Shenoy et al., 2008; El-Agamy and Alsubaie, 2017; Liaboe et al., 2017), diabetic dogs exhibit pleomorphism and polymegathism but no changes in endothelial density (Yee et al., 1985). These changes appeared to be indirectly correlated with the level of diabetic control (Yee et al., 1985). This should be taken into consideration when assessing patients for phacoemulsification surgery, as it may predispose diabetic dogs to more severe postoperative corneal edema. Furthermore, preoperative specular microscopy could help in presurgical decision making, such as viscoelastic selection, phacoemulsification machine settings, or postoperative use of topical hyperosmotic drugs. Punctate retinal hemorrhages were found in 13.3% of the eyes examined (out of 98 eyes assessed by indirect ophthalmoscopy). This prevalence markedly differs from reports in human medicine, in which the prevalence is much higher (Herring et al., 2014). Conversely to the proliferative changes seen commonly in humans (Manaviat et al., 2008), canine diabetic retinopathy is characterized by microvascular changes including capillary microaneurysms and hemorrhages (Braga-Sá et al., 2018). The pathogenesis of diabetic retinopathy has been hypothesized to be the same as for cataract formation (Muñana, 1995). In one retrospective study, retinal hemorrhages following cataract surgery in dogs occurred in 21% of diabetic patients with only 0.6% of nondiabetics having these lesions (Landry et al., 2004). While the median time from diagnosis of diabetes to the onset of non-proliferative retinopathy in dogs has been reported as 1.4 years (Landry et al., 2004), the proliferative retinopathy commonly seen in humans has been associated with longer periods of time. The lower incidence of proliferative changes in dogs might be explained by the age of onset of DM and the shorter life span of dogs, yielding a shorter duration of disease during their lifetime (Miller and Brines, 2018). Furthermore, differences in the presence of vascular endothelial growth factor in the aqueous humor of diabetic patients with retinopathy (Funatsu et al., 2005) could explain these interspecies disparities. Dr. Williams found that diabetic cataracts had significantly increased axial thickness compared to non-diabetic cataracts as demonstrated by B-mode ultrasonography (Williams, 2004). This reinforces the recommendation for performing early phacoemulsification to reduce the risk of phacomorphic glaucoma. In the present study, a very low incidence of phacomorphic glaucoma was documented which can be explained by the short time between DM diagnosis and the ophthalmic examination. Phacoemulsification is the gold standard treatment for cataracts in dogs. Although the success rate in diabetic dogs is approximately the same as that for cataract extraction in nondiabetic patients (Bagley and Lavach, 1994), postoperative complications such as corneal ulcers, diffuse corneal edema, lipemic aqueous humor, and ocular neuropathies have been reported to be more likely to develop in diabetic dogs (MacRae et al., 1982; Ledbetter et al., 2006; Klein et al., 2011; Foote et al., 2019). Based on the prevalence of ocular signs observed in the present study, and the incidence of postoperative complications reported, a more complete ocular preoperative assessment is recommended in diabetic dogs undergoing cataract surgery. Specular microscopy, Tear Film Break-up Time (TFBUT), STT-1, gonioscopy, and corneal esthesiometry could be helpful for anticipating surgical and postoperative complications, and therefore, helping with their management. In the dog, hyperglycemia does not cause symptoms until glucose values are persistently elevated, usually above 180–220 mg/dl (Fracassi, 2017). Although ocular findings are a well-known complication for diabetic dogs, no glycemic threshold values have been previously established for any of the more common ophthalmic presentations. In the present study, a statistical association between fasting glycemia levels, non-proliferative retinopathy, and anterior uveitis was seen, suggesting that diabetic dogs with glycemic values higher than 600 mg/dl could be predisposed to anterior segment inflammation and non-proliferative retinopathy. Limitations of this study include those inherent to the retrospective nature, the lack of homogeneity in data assessment, incomplete medical records, and variations in clinical approach. ConclusionOcular complications of DM in dogs are numerous. This high prevalence warrants a more detailed preoperative evaluation in diabetic dogs undergoing cataract surgery. Furthermore, the positive correlation between cataracts and retinopathy suggests an early surgical treatment as the most appropriate approach. Moreover, a predisposition for anterior segment inflammation and non-proliferative retinopathy is suggested when fasting plasma glucose is higher than 600 mg/dl. Data from this study can help ophthalmologists to better understand the disease and provide more effective treatments and surgical approaches to canine patients. Conflict of interestThe authors declare that there is no conflict of interest. Authors contributionAll authors contributed to making the completion of this manuscript possible. Francisco Cantero, Marta Leiva, and Teresa Peña designed the study and had direct patient contact. Francisco Cantero obtained all data supervised by Marta Leiva and Teresa Peña. Angel Ortillés analyzed the data and gave statistical conclusions. Francisco Cantero, Angel Ortillés, and Marta Leiva wrote the paper. Teresa Peña, Marta Leiva, and Angel Ortillés critically reviewed the manuscript for important intellectual content. ReferencesBagley, L.H. and Lavach, J.D. 1994. Comparison of postoperative phacoemulsification results in dogs with and without diabetes mellitus: 153 cases (1991-1992). J Am Vet Med Assoc. 205(8), 1165–1169. Beam, S., Correa, M.T. and Davidson, M.G. 1999. A retrospective-cohort study on the development of cataracts in dogs with diabetes mellitus: 200 cases. Vet Ophthalmol. 2(3), 169–172. Braga-Sá, M.B.P., Barros, P.S.M., Jorge, J.S., Dongo, P., Finkensieper, P., Bolzan, A.A., Watanabe, S.S. and Safatle, A.M.V. 2018. Retina assessment by optical coherence tomography of diabetic dogs. Pesqui Vet Bras. 38, 1966–1971. Crispin, S. 2002. Ocular lipid deposition and hyperlipoproteinaemia. Prog Retin Eye Res. 21(2), 169–224. Cullen, C.L., Ihle, S.L., Webb, A.A. and McCarville, C. 2005. Keratoconjunctival effects of diabetes mellitus in dogs. Vet Ophthalmol. 8(4), 215–224. Davison, L.J., Herrtage, M.E. and Catchpole, B. 2005. Study of 253 dogs in the United Kingdom with diabetes mellitus. Vet Rec. 156(15), 467–471. El-Agamy, A. and Alsubaie, S. 2017. Corneal endothelium and central corneal thickness changes in type 2 diabetes mellitus. Clin Ophthalmol. 11, 481–486. Engerman, R., Finkelstein, D., Aguirre, G., Diddie, K.R., Fox, R.R., Frank, R.N. and Varma, S.D. 1982. Ocular complications. Diabetes 31, 82–88. Fall, T., Hamlin, H.H., Hedhammar, Å., Kämpe, O. and Egenvall, A. 2007. Diabetes Mellitus in a Population of 180,000 Insured Dogs: Incidence, Survival, and Breed Distribution. J Vet Intern Med. 21(6), 1209–1216. Foote, B.C., Michau, T.M., Welihozkiy, A. and Stine, J.M. 2019. Retrospective analysis of ocular neuropathies in diabetic dogs following cataract surgery. Vet Ophthalmol. 22(3), 284–293. Fracassi, F. 2017. Canine diabetes mellitus. In textbook of veterinary internal medicine: diseases of the dog and the cat. Eds., Ettinger S.J., Feldman E.C. and Côté, E. 8th ed. Amsterdam, NL: Elsevier, p: 4280. Fracassi, F., Pietra, M., Boari, A., Aste, G., Giunti, M. and Famigli-Bergamini, P. 2004. Breed distribution of canine diabetes mellitus in Italy. Vet Res Commun. 28, 339–342. Funatsu, H., Yamashita, H., Noma, H., Mimura, T., Nakamura, S., Sakata, K. and Hori, S. 2005. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 243, 3–8. Gemensky-Metzler, A.J., Sheahan, J.E., Rajala-Schultz, P.J., Wilkie, D.A. and Harrington, J. 2015. Retrospective study of the prevalence of keratoconjunctivitis sicca in diabetic and nondiabetic dogs after phacoemulsification. Vet Ophthalmol. 18(6), 472–480. Goebbels, M. 2000. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol. 84(1), 19–21. Good, K.L., Maggs, D.J., Hollingsworth, S.R., Scagliotti, R.H. and Nelson, R.W. 2003. Corneal sensitivity in dogs with diabetes mellitus. Am J Vet Res. 64(1), 71–11. Guptill, L., Glickman, L. and Glickman, N. 2003. Time trends and risk factors for diabetes mellitus in fogs: analysis of veterinary medical data base records (1970–1999). Vet J. 165(3), 240–247. Harvey, A.M., Teixeira, L.B.C. and Dubielzig, R.R. 2020. A clinicopathological study of 17 cases of ocular surface xanthogranuloma in dogs. Vet Ophthalmol. 23(1), 190–198. Herring, I.P., Panciera, D.L. and Werre, S.R. 2014. Longitudinal prevalence of hypertension, proteinuria, and retinopathy in dogs with spontaneous diabetes mellitus. J Vet Intern Med. 28, 488–495. Katherman, A.E. and Braund, K.G. 1983. Polyneuropathy associated with diabetes mellitus in a dog. J Am Vet Med Assoc. 182(5), 522–4. Klein, H.E., Krohne, S.G., Moore, G.E. and Stiles, J. 2011. Postoperative complications and visual outcomes of phacoemulsification in 103 dogs (179 eyes): 2006–2008. Vet Ophthalmol. 14(2), 114–120. Krishnan, H., Diehl, K., Stefanovski, D. and Aguirre, G.D. 2020. Vitreous degeneration and associated ocular abnormalities in the dog. Vet Ophthalmol. 23(2), 219–224. Krolewski, A.S., Warram, J.H., Rand, L.I., Christlieb, A.R., Busick, E.J. and Kahn, C.R. 1986. Risk of proliferative diabetic retinopathy in juvenile-onset type I diabetes: a 40-yr follow-up study. Diabetes Care. 9(5), 443–452. Labruyère, J.J., Hartley, C., Rogers, K., Wetherill, G., McConnell, J.F. and Dennis, R. 2008. Ultrasonographic evaluation of vitreous degeneration in normal dogs. Vet Radiol Ultrasound. 49(2), 165–171. Landry, M.P., Herring, I.P. and Panciera, D.L. 2004. Funduscopic findings following cataract extraction by means of phacoemulsification in diabetic dogs: 52 cases (1993-2003). J Am Vet Med Assoc. 225(5), 709–716. Ledbetter, E.C., Riis, R.C., Kern, T.J., Haley, N.J. and Schatzberg, S.J. 2006. Corneal ulceration associated with naturally occurring canine herpesvirus-1 infection in two adult dogs. J Am Vet Med Assoc. 229(3), 376–384. Leiva, M. and Peña, T. 2020. Diseases of the lens and cataract formation. In Veterinary Ophthalmology. Eds., Gelatt K.N., Ben-Shlomo G., Gilger B.C., Hendrix D.V., Kern T.J. and Plummer C.E. 6th ed. Hoboken NJ: Wiley-Blackwell, pp: 1317–1370. Liaboe, C.A., Aldrich, B.T., Carter, P.C., Skeie, J.M., Burckart, K.A., Schmidt, G.A., Reed, C.R., Zimmerman, M.B. and Greiner, M.A. 2017. Assessing the impact of diabetes mellitus on donor corneal endothelial cell density. Cornea 36(5), 561–566. Ling, G.V., Lowenstine, L.J., Pulley, L.T. and Kaneko, J.J. 1977. Diabetes mellitus in dogs: a review of initial evaluation, immediate and long-term management, and outcome. J AmVet Med Assoc. 170, 521–30. Macrae, S.M., Engerman, R.L., Hatchell, D.L. and Hyndiuk, R.A. 1982. Corneal sensitivity and control of diabetes. Cornea 1, 223–226. Manaviat, M.R., Rashidi, M., Afkhami-Ardekani, M. and Shoja, M.R. 2008. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 8, 10. Matas, M., Donaldson, D. and Newton, R.J. 2012. Intracorneal hemorrhage in 19 dogs (22 eyes) from 2000 to 2010: a retrospective study. Vet Ophthalmol. 15, 86–91. Mattin, M., O’Neill, D., Church, D., McGreevy, P.D., Thomson, P.C. and Brodbelt, D. 2014. An epidemiological study of diabetes mellitus in dogs attending first opinion practice in the UK. Vet Rec. 174, 349–355. Michau, T.M. 2020 Surgery of the lens. In Veterinary Ophthalmology. Eds., Gelatt K.N.,Ben-Shlomo G., Gilger B.C., HendrixD.V., Kern T.J. and Plummer C.E. 6th ed. Hoboken, NJ: John Wiley & Sons. pp. 1371–1458. Miller, E.J. and Brines, C.M. 2018. Canine diabetes mellitus associated ocular disease. Top Companion Anim Med. 33, 29–34. Misra, S.L., Patel, D.V., McGhee, C.N.J., Pradhan, M., Kilfoyle, D., Braatvedt, G.D. and Craig, J.P. 2014. Peripheral neuropathy and tear film dysfunction in type 1 diabetes mellitus. J Diabetes Res. 2014, 1–6. Muñana, K.R. 1995. Long-term complications of Diabetes Mellitus, part I: Retinopathy, nephropathy, neuropathy. Vet Clin North Am Small Anim Pract. 25(3), 715–730. Ortwerth, B.J. and Olesen, P.R. 1989. Studies on the nature of the water-insoluble fraction from aged bovine lenses. Exp Eye Res. 48(5), 605–619. Ozdemir, M., Buyukbese, M.A., Cetinkaya, A. and Ozdemir, G. 2003. Risk factors for ocular surface disorders in patients with diabetes mellitus. Diabetes Res Clin Prac. 59(3), 195–199. Park, Y.-W., kim, J.-Y., Jeong, M.-B., Kim, S.-H., Yoon, J. and Seo, K. 2015. A Retrospective study on the association between vitreous degeneration and cataract in dogs. Vet Ophthalmol. 18, 304–308. Paulsen, M.E., Lavach, J.D. and Severin, G.A. 1985. The effect of lens-induced uveitis on the success of extracapsular cataract extraction: a retrospective study of 65 lens removals in the dog. J Am Anim Hosp Assoc. 22, 49–56. Schechtmann, S.A., Stine, J.M., Miller, T.R., Welihozkiy, A. and Michau, T.M. 2020. A retrospective analysis of lipid-laden aqueous humor in dogs: thirty cases. Vet Ophthalmol. 23, 277–285. Shenoy, R., Khandekar, R., Bialasiewicz, A.A. and Muniri, A.A. 2008. Corneal endothelium in patients with diabetes mellitus: a historical cohort study. Eur J Ophthalmol. 19(3), 369–375. Violette, N.P. and Ledbetter, E.C. 2017. Intracorneal stromal hemorrhage in dogs and its associations with ocular and systemic disease: 39 cases. Vet Ophthalmol. 20(1), 27–33. Violette, N.P. and Ledbetter, E.C. 2019. Lipemic uveitis and its etiologies in dogs: 75 cases. Vet Ophthalmol. 22(5), 577–583. West, M.C., Sila, G.H., Aquino, S.M. and Rose, M.D. 2020. Evaluation of vitreous degeneration as a potential risk factor for retinal detachment after phacoemulsification in dogs. Vet Ophthalmol. 23(4), 721–729. Wilcock, B.P. and Peiffer, R.L. 1987. The pathology of lens-induced uveitis in dogs. Vet Pathol. 24(6), 549–553. Wilkie, D.A., Gemensky-Metzler, A.J., Colitz, C.M.H., Bras, I.D., Kuonen, V.J., Norris, K.N. and Basham, C.R. 2006. Canine cataracts, diabetes mellitus and spontaneous lens capsule rupture: a retrospective study of 18 dogs. Vet Ophthalmol. 9(5), 328–334. Williams, D.L. 2004. Lens morphometry determined by B-mode ultrasonography of the normal and cataractous canine lens. Vet Ophthalmol. 7(2), 91–95. Williams, D.L., Pierce, V., Mellor, P. and Heath, M.F. 2007. Reduced tear production in three canine endocrinopathies. J Small Anim Pract. 48(5), 252–256. Yee, R.W., Matsuda, M., Kern, T.S., Engerman, R.L. and Edelhauser, H.F. 1985. Corneal endothelial changes in diabetic dogs. Curr Eye Res. 4(7), 759–766. | ||
| How to Cite this Article |
| Pubmed Style Cantero F, Ortillés �, Teresa-peña M, Leiva M. Prevalence of ocular findings and their association with glycemia in dogs with diabetes mellitus: A 10-year clinical study (2009-2019). Open Vet. J.. 2023; 13(5): 620-628. doi:10.5455/OVJ.2023.v13.i5.15 Web Style Cantero F, Ortillés �, Teresa-peña M, Leiva M. Prevalence of ocular findings and their association with glycemia in dogs with diabetes mellitus: A 10-year clinical study (2009-2019). https://www.openveterinaryjournal.com/?mno=132606 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i5.15 AMA (American Medical Association) Style Cantero F, Ortillés �, Teresa-peña M, Leiva M. Prevalence of ocular findings and their association with glycemia in dogs with diabetes mellitus: A 10-year clinical study (2009-2019). Open Vet. J.. 2023; 13(5): 620-628. doi:10.5455/OVJ.2023.v13.i5.15 Vancouver/ICMJE Style Cantero F, Ortillés �, Teresa-peña M, Leiva M. Prevalence of ocular findings and their association with glycemia in dogs with diabetes mellitus: A 10-year clinical study (2009-2019). Open Vet. J.. (2023), [cited January 25, 2026]; 13(5): 620-628. doi:10.5455/OVJ.2023.v13.i5.15 Harvard Style Cantero, F., Ortillés, . �., Teresa-peña, . M. & Leiva, . M. (2023) Prevalence of ocular findings and their association with glycemia in dogs with diabetes mellitus: A 10-year clinical study (2009-2019). Open Vet. J., 13 (5), 620-628. doi:10.5455/OVJ.2023.v13.i5.15 Turabian Style Cantero, Francisco, Ángel Ortillés, M. Teresa-peña, and Marta Leiva. 2023. Prevalence of ocular findings and their association with glycemia in dogs with diabetes mellitus: A 10-year clinical study (2009-2019). Open Veterinary Journal, 13 (5), 620-628. doi:10.5455/OVJ.2023.v13.i5.15 Chicago Style Cantero, Francisco, Ángel Ortillés, M. Teresa-peña, and Marta Leiva. "Prevalence of ocular findings and their association with glycemia in dogs with diabetes mellitus: A 10-year clinical study (2009-2019)." Open Veterinary Journal 13 (2023), 620-628. doi:10.5455/OVJ.2023.v13.i5.15 MLA (The Modern Language Association) Style Cantero, Francisco, Ángel Ortillés, M. Teresa-peña, and Marta Leiva. "Prevalence of ocular findings and their association with glycemia in dogs with diabetes mellitus: A 10-year clinical study (2009-2019)." Open Veterinary Journal 13.5 (2023), 620-628. Print. doi:10.5455/OVJ.2023.v13.i5.15 APA (American Psychological Association) Style Cantero, F., Ortillés, . �., Teresa-peña, . M. & Leiva, . M. (2023) Prevalence of ocular findings and their association with glycemia in dogs with diabetes mellitus: A 10-year clinical study (2009-2019). Open Veterinary Journal, 13 (5), 620-628. doi:10.5455/OVJ.2023.v13.i5.15 |