| Research Article | ||
Open Vet. J.. 2024; 14(2): 674-682 Open Veterinary Journal, (2024), Vol. 14(2): 674-682 Original Research Efficiency of C-type natriuretic peptide on improvement of Iraqi local ram’s epididymal spermsMassar Saeb Khadim* and Nazih Wayes ZaidDepartment of Surgery and Obstetrics, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq *Corresponding Author: Massar Saeb Khadim. Department of Surgery and Obstetrics, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq. Email: massar.saeb1102d [at] covm.uobaghdad.edu.iq Submitted: 29/10/2023 Accepted: 21/01/2023 Published: 29/02/2024 © 2024 Open Veterinary Journal
AbstractBackground: Fertility plays a great role in animal reproduction since high-quality semen improves sheep industry reproduction. The current worldwide data revealed the close relation of C-type natriuretic peptide (CNP) to the reproductive function of rams. Aims: Evaluation of the effect of CNP on cooled sperms using traditional and molecular assays. Methods: Totally, of 20 testicular samples were collected, processed to obtain the semen samples, and divided into two parts; one was treated with a suitable dose of CNP, and the other served as a control. Sperm samples of both groups were cooled for 3 days and tested at 0, 24, 48, and 72 hours. Results: The findings revealed that the suitable dose of CNP-treated sperms was 0.01 × 10−13. Values of individual motility, live sperms, and sperm concentration were reduced significantly in CNP-24h, CNP-48h, and CNP-72h when compared to control; however, abnormal sperms were increased in both control and CNP groups at 24, 48, and 72 hours when compared to values of 0 hour. Concerning turbidmetric analysis, a significant reduction in values of lag time was observed in CNP when compared to control at all times of cooling intervals. In both CNP and control groups, motility index was decreased at 24, 48, and 72 hours when compared to 0 hour. For velocity, significant increases were shown in CNP compared with control at all cooling intervals. However, values of both groups were increased significantly at 24, 48, and 72 hours times when compared to 0 hour. Fraction of rapidly moving sperm of CNP was elevated at 0 hour and decreased at 24, 48, and 72 hours when compared to control. Expression of the hNPR-B gene was reduced gradually in sperms of CNP and control groups at times of cooling intervals. Conclusion: To the best of our knowledge, this first Iraqi study targets the effect of CNP on epididymal sperms of rams. However, changes that occur after excessive CNP exposure remain unclear, and the toxicological profile of CNP requires furthermore supplements. Keywords: Sheep fertility, Turbidimetric analysis, MTT assay, CNP, Iraq. IntroductionSheep are one of the most common livestock animals in the world, which are raised under different climates, topographic, and management conditions, and served in many countries as a main source of wool, milk, and meat (Shinde and Naqvi, 2015). The fertility of rams was detected to have great economic roles in animal reproduction because the high-quality semen is favorable for the sheep industry and the production of a large number of preferred lambs (Mohapatra and Shinde, 2018; Redden and Thorne, 2020). Although rams can breed at any time of the year, loss of libido and reduced sperm quantity can affect their performance throughout the season of breeding (Maquivar et al., 2021). In combination with estrus and ovulation induction/synchronization, artificial insemination is an assisted reproductive technology that was used as a device to inject an appropriate amount of sperm into the female reproductive system to increase the genetic value of animals (Hafez, 2015; Mochida, 2020; Al-Chaabawi et al., 2020). Worldwide, artificial insemination in sheep farming should consider the creation of appropriate knowledge suitable for the breed such as ram age, ram fertility, mass motility, and the type of sperm or secretions (Madrigali et al., 2021). Natriuretic peptide (NP) is a family of three hormones that correlate structurally and act distinctly and uniquely in the body; atrial NP (ANP) and brain NP (BNP) in the heart, and C-type NP (CNP) (Goetze et al., 2020). ANP and BNP are endocrine hormones that help with the regulation of heart structure as well as blood volume and pressure (Nakagawa et al., 2019). Comparatively, the mechanism of action of CNP is diverse and researchers in the last decade showed that CNP affects coronary blood flow regulation electrophysiology, vascular integrity, endothelial cell and smooth muscle proliferation, angiogenesis, leukocyte activation, and vascular tone (Perez-Ternero et al., 2019). In the male reproductive system, several studies showed that testicular function and fertility and regulation of erectile tissue can be regulated by CNP (Hannema et al., 2013; Shahin et al., 2018; Ückert et al., 2019). In farm animals particularly ram, studies conducted for elucidating the effect of CNP on sperm functions are scarce and need to be supported. Hence, this study aims to estimate the level of CNP toxicity to prepare an effective dose of sperm and detect the influence of CNP on sperm samples cooled for 3 days by the traditional microscopic examination and using the turbidimetric analysis. Real-time polymerase chain reaction (RT-PCR) was applied to detect the genetic expression of the hNPR-B gene in sperm samples at different cooling intervals. Materials and MethodsSamplesTotally, 20 testicular samples were collected from the adult slaughtered rams at the official abattoir of Al-Shoula city (Baghdad, Iraq), and transported as soon as possible using the plastic icebox to the laboratory (Fig. 1). The testicle samples were first washed in distilled water, then in normal saline supplemented with 0.1 mg/ml streptomycin and 100 IU/ml penicillin. Small scissors were used to dissect and separate the epididymis from the whole testicle, and the caudae were injected with 5–8 ml of TCM199 medium supplemented with 100 IU/ml Nystatin and 100 IU/ml penicillin/streptomycin using gauge 18-needle attached to a 5-ml syringe and then was aspirated. CNPAccording to manufacturer instructions (Sigma Aldrich, Germany), the vial of CNP was prepared at room temperature (22°C–25°C), dissolved in distilled water, shacked vigorously, and diluted to obtain different concentrations. Initially, CNP was diluted serially to detect the lowered lethal concentration (LC) of CNP on sperm samples; and then, the LC1, LC50, and LC95 of CNP were re-evaluated and statistically compared. Post selection of the recommended concentration of CNP, the CNP-treated sperms in addition to untreated sperms (control) was subjected to cooling at 3 days and tested at 0 hour (0h), 24 hours (24h), 48 hours (48h), and 72 hours (72h). Traditional examinationBased on different protocols of sperm examination; morphology, individual motility, viability, sperm concentration, and turbidimetric analysis [lag time, motility index, velocity, and fraction of rapidly moving sperm (FRMS)] were analyzed (AL-Ebady et al., 2012; Esteves and Varghese, 2012). Molecular assayAs described by the manufacturer’s instruction of the total RNA Extraction Mini Kit (Favorgen, Taiwan) and the cDNA Synthesis SuperMix kit (Transgen, China), the control and CNP-treated sperms samples preserved in the TRIzolTM reagent were subjected to RNA extraction and cDNA synthesis. Targeting hNPR-B gene [(F: 5'-GAA CAC TGC TTC TCG AAT GGA G-3') and (R: 5'-CCA GGT ACC AGC AGA AGA-3')] and housekeeping gene [(F: 5'-CCG CAT CTT CTT GTG CAG TG-3') and (R: 5'-ACC AGC TTC CCA TTC TCA GC-3')], the qPCR SYBR Green MasterMix Kit (Promega, USA) was served to preparing the mastermix tubes at a final volume of 20 μl. The thermocycler conditions involved 1 cycle of initial denaturation (95°C, 2 minutes), 50 cycles of denaturation (95°C, 15 seconds) as well as annealing and extension (95°C, 1 minute), and 100 cycles of melting curve analysis (95°C, 15 seconds). The final gene expression was calculated with 2-DDCT.
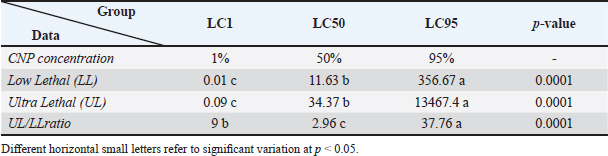
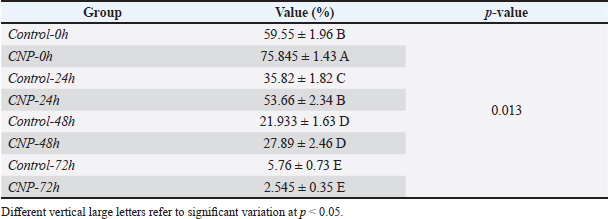
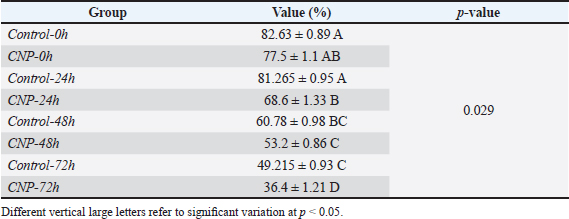
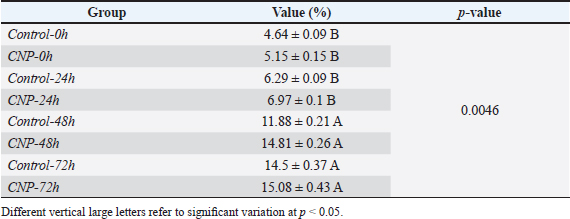
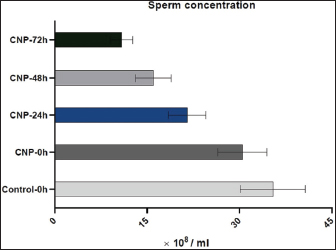
Fig. 1. Collection and maturation of sperm samples from epididymis. Statistical analysisThe t-test, One- and Two-Ways ANOVA in the GraphPad Prism Software (version 6.0.1) were served to detect significant variation between the values of different study groups at p < 0.05. The study values appeared as Mean ± Standard Errors (M±SE) (Gharban, 2023). Ethical approvalThis study was licensed and supervised by the Scientific Committee of the Department of Surgery and Obstetrics in the College of Veterinary Medicine (University of Baghdad, Baghdad, Iraq). ResultsCNP-toxicity and effective dose of spermSignificant variation ( p < 0.0001) between values of LC1 (0.03 pg/ml), LC50 (19.997 pg/ml), and LC95 (2191.964 pg/ml) was seen in the current study; however, the lowered CNP toxicity and suitable dose of sperms suspension treated with CNP was LC1 (0.01 x 10−13) (Table 1). Sperms qualityIndividual motility of sperm suspensions was increased significantly in CNP-0h (75.845 ± 1.43); and reduced significantly in CNP-48h (27.89 ± 2.46) and CNP-72h (2.545 ± 0.35) but not in CNP-24 hours (53.66 ± 2.34) when compared to control groups (Table 2). Value of live sperms in CNP-0h group (77.5 ± 1.1) was differed insignificantly (p > 0.05) when compared to control-0h (82.63 ± 0.89) and control-24h (81.265 ± 0.95) groups; however, gradual significant decreases (p < 0.05) were observed in values of CNP-24h (68.6 ± 1.33), CNP-48h (60.78 ± 0.98), and CNP-72h (36.4 ± 1.21) in comparison with those of control-48h (60.78 ± 0.98) and control-72h (49.215 ± 0.93), (Table 3). Values of abnormal sperms were increased significantly (p < 0.05) in both control and CNP groups at the time of 24 (6.29 ± 0.09 and 6.97 ± 0.1, respectively), 48 (11.88 ± 0.21 and 14.81 ± 0.26, respectively), and 72 hours (14.5 ± 0.37 and 15.08 ± 0.43, respectively) when compared to values of CNP-0h (5.15 ± 0.15) and control-0h (4.64 ± 0.09) groups (Table 4, Fig. 2). Significant decreases (p < 0.05) in sperm concentration were started gradually in CNP-24h (21.51 ± 3.05), CNP-48h (10.06 ± 2.87), and CNP-72h (10.89 ± 1.77) when compared to values of CNP-0h (30.49 ± 3.98) and control-0h (35.41 ± 5.29), (Fig. 3). Table 1. Toxicity study of CNP addition and determine the effective dose of LC1, LC50, and LC95 on ram sperms.
Table 2. CNP addition effect on sperms individual motility percentage of ram sperms during three days of cooling preservation.
Table 3. CNP addition effect on sperms live percentage of ram sperms during three days of cooling preservation.
Table 4. CNP addition effect on sperms abnormal percentage of rams sperms during three days of cooling preservation.
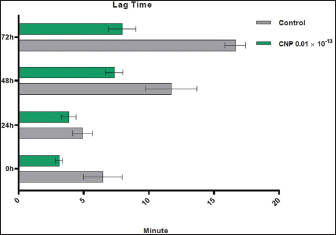
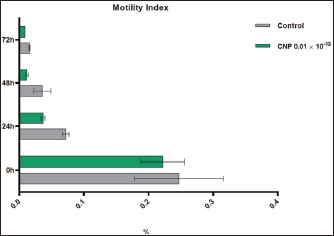
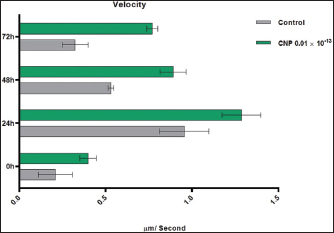
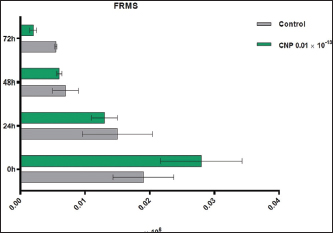
Turbidimetric analysisRegarding the lag time, significant reduction (p < 0.05) was observed in CNP-treated sperms when compared to those of control-0 (3.11 ± 0.26 and 6.46 ± 1.48, respectively), 24 (3.86 ± 0.56 and 4.92 ± 0.75, respectively), 48 (7.35±0.68 and 11.74 ± 1.95, respectively), and 72 hours (7.95±1.06 and 16.66 ± 0.79, respectively). However, values of control were decreased at a time of 0 hour but increased at times of 48 and 72 hours; while in CNP 0.01 ´ 10-13 group, values of 24, 48, and 72 hours were elevated significantly when compared to 0 hour (Fig. 4). Although, the percentages of motility index in CNP-treated sperms and control groups varied insignificantly (p > 0.05) at 0 hour (0.222 ± 0.034 and 0.247 ± 0.069, respectively); there was a significant reduction (p < 0.05) at 24 (0.037 ± 0.003 and 0.072 ± 0.005, respectively), 48 (0.012 ± 0.002 and 0.036 ± 0.0132, respectively), and 72 hour (0.0089 ± 0.0001 and 0.016 ± 0.0011, respectively). In both CNP-treated sperms and control groups, there were significant decreases (p < 0.05) in values of motility index at 24, 48, and 72 hours when compared to 0h (Fig. 5). The velocity of CNP-treated sperms was increased significantly (p < 0.05) when compared to control at all cooling intervals; 0 (0.399 ± 0.048 and 0.208 ± 0.098, respectively), 24 (1.286 ± 0.112 and 0.954 ± 0.143, respectively), 48 (0.89 ± 0.075 and 0.53 ± 0.016, respectively), and 72 hours (0.77 ± 0.031 and 0.324 ± 0.073, respectively). However, values of both CNP-treated sperms and control groups were increased significantly at 24, 48, and 72 hours when compared to 0h (Fig. 6). Although, FRMS was elevated significantly (p < 0.05) in CNP-treated sperms at the time of 0 hour (0.028 ± 0.0063) when compared to control (0.019 ± 0.0047), significant decreases (p < 0.05) were reported at 24 (0.013 ± 0.002), 48 (0.006 ± 0.0004), and 72 hour (0.002± 0.0005) when compared to control (0.015 ± 0.0054, 0.007 ± 0.0002, and 0.0055± 0.00014, respectively). However, values of both CNP 0.01´10-13 and control groups were reduced significantly at 24, 48, and 72 hours when compared to 0h (Fig. 7).
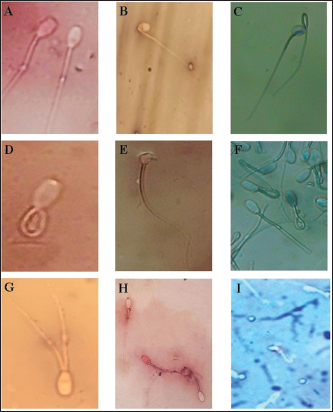
Fig. 2. Microscopic detection of abnormal sperms shows (A): Acrosome defect, (B): Bent neck defect, (C): Double tail, (D): Coiled tail, (E): Irregular head defect, (F): Piriform, (G): Double tail, (H): Plasia sperm, and (I): Tape head.
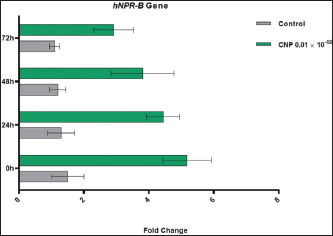
Fig. 3. CNP effect on the concentration of ram sperms during three days of cooling preservation. Genetic expression of hNPR-BDuring 3 days of cooling, the findings of the hNPR-B gene were elevated significantly (p < 0.05) in CNP-treated sperms at 0 (5.18 ± 0.75), 24 (4.45 ± 0.51), 48 (3.81 ± 0.97), and 72 hours (2.92 ± 0.62) when compared to control groups (1.50 ± 0.50, 1.30 ± 0.42, 1.20 ± 0.25, and 1.10 ± 0.15, respectively). Although significant decreases (p < 0.05) in the expression of the hNPR-B gene were detected in CNP-treated sperms with advancing days of cooling, the values of this group remain higher than those of control at all times of cooling (Fig. 8).
Fig. 4. CNP effect of Turbidimetric analysis of Lag time of ram sperms during three days of cooling preservation.
Fig. 5. CNP effect of Turbidimetric analysis of motility index of ram sperms during three days of cooling preservation.
Fig. 6. CNP effect of Turbidimetric analysis of the velocity of ram sperms during three days of cooling preservation.
Fig. 7. CNP effect of Turbidimetric analysis of FRMS of ram sperms during three days of cooling preservation.
Fig. 8. Expression of hNPR-B gene in control and CNP-sperm samples during three days of cooling preservation. DiscussionFor years, semen cryopreservation that comprises different cooling and freezing steps has been a valuable procedure for the application of reproductive technologies such as artificial insemination, in vitro embryo production, and embryo transfer since it eliminates the limitations of time and space (Dias et al., 2018). In Iraq, the field studies have focused on the significant influence of the quality of thawed semen of bulls with a consequence of variable success rates (Al-Daraji et al., 2002; AL-Badry, 2016; Hussain et al., 2016; Saleh, 2019; Alhelal and Abdulkareem, 2023); whereas globally, the effect of storage or cooling rates on fertility of semen suspension was studied in different domestic and wild animals such as buck (Ahmad et al., 2015), stallion (Cuervo-Arango et al., 2015), cattle (Dias et al., 2018), donkey (Zhang et al., 2018), llama (Zampini et al., 2020), and dog (Colombo et al., 2022). Therefore, different additives have been used to improve the physical quality of sperms such as arginine (AL-Ebady et al., 2012), caffeine (Shahad et al., 2020), and silymarin (Ali et al., 2022). In this study, the effect of CNP on cooled sperms of rams was studied for the first time in Iraq, and the results revealed that the lowered concentration of CNP revealed the suitable dose of sperms suspension could be used for artificial insemination of ewes. Worldwide, a number of studies have been performed to detect the effect of CNP on normal human sperm (Xia et al., 2016), sperm attraction for fertilization (Kong et al., 2017), regulation of sperm capacitation in the genital tract of female rats (Wu et al., 2019), anti-inflammatory role in rat epididymitis (Mei et al., 2021), sperm motility and reproduction function of azthenozoospermia in mice (Li et al., 2022), and spermatozoa maturation in rats (Zhao et al., 2023); however, no data were available concerned with the toxicological effect of CNP on sperms. Microscopic examination of sperms suspension treated with the suitable dose of CNP (0.01 ´ 10-13) showed that there were significant decreases in levels of individual motility, live sperms, and sperm concentration with advancing the periods of cooling when compared to control, but abnormal sperms were increased more significantly in control compared to CNP. For the turbidimetric analysis, values of lag time, motility index, velocity, and FRMS showed that the impact of cooling on sperms suspension treated with CNP was lowered in comparison with that detected in sperms samples of control. Various studies suggested that mouse NPs, including CNP, can exhibit chemoattractant features with the ability to attract mammalian spermatozoa and increase sperm motility (Zamir et al., 1993; Bian et al., 2012). Xia et al. (2016) showed that CNP can induce a significant dose-dependent increase in spermatozoa motility and acrosome reaction, thus, regulating the reproductive function of males. Kong et al. (2017) reported that CNP can increase the levels of intracellular cGMP and Ca+2 of spermatozoa and induce sperm accumulation by attraction. Özbek et al. (2019) observed the CNP expression in the epithelial and smooth muscle cells of epididymis, suggesting that CNP can increase the motility of spermatozoa in epididymis via cGMP. Wu et al. (2019) concluded that CNP secreted by the female genital tract might bind to spermatozoa and stimulate successively the intracellular cGMP/PKG signaling by increasing the Ca+2 and tyrosine-phosphorylated proteins, promoting hyperactivation and inducing the acrosome reaction which ultimately facilitated sperm capacitation in the genital tract of female rat. Mei et al. (2021) showed that the semen samples of asthenospermia patients have a lower concentration of CNP than normal people, with an observable activity in improving the motility of sperm and alleviation of cyclophosphamide damage to sperm motility and reproductive system. Similar findings were recorded by Li et al. (2022) who found that CNP alleviates the acute epididymitis injury and decreases invasion and inflammatory reaction of macrophages; suggesting the possibility of using CNP as a potential treatment for epididymitis. Zhao et al. (2023) found that CNP plays a role in epididymal sperm maturation as it promotes the acquisition of epididymal sperm fluidity. Based on PCR analyses at different times of cooling intervals, the findings of the current study showed that the expression of the hNPR-B gene was elevated significantly in CNP samples in comparison with controls. Our findings are in agreement with those demonstrated by other researchers (Ueda et al., 2020; Yu et al., 2021; Nakagawa and Nishikimi, 2022). NPR-B is a guanylate cyclase-linked receptor that appears to be activated CNP (Pagel-Langenickel et al., 2007). Lu and Pan (2017) concluded that CNP can elicit exercise preconditioning-induced protection against tissue injury via the up-regulation of NPR-B receptors. Zhao et al. (2023) recorded a significant increase in the expression of AKAP4, Bin1b, CD52, Dnah17, and LDCH genes in semen samples incubated with CNP; suggesting that, CNP could initiate epididymal motility and regulate the expression of sperm motility and mature-related genes. ConclusionThis study, done for the first time in Iraq, highlights the important role of CNP in improving the physical properties of ram epididymal sperms while increasing the expression of the hNPR-B gene that might play a role in treating growth failure. However, changes that occur after excessive exogenous CNP exposure remain to be clarified, and the toxicological profile of the CNP and its derivatives has required furthermore supplements. AcknowledgementsThe authors thank the Department of Surgery and Obstetrics in the College of Veterinary Medicine (University of Baghdad) for providing all facilities and support throughout carrying out this study. Conflict of interestAll authors have no conflict of interest to disclose. Authors’ contributionMSK: Collection and processing of testicular tissues and maturation of sperms. NWZ: Preparation of CNP detection of the level of toxicity at different concentrations. Both authors participate in microscopic examination, turbidimetric analysis, and molecular assaying of gene expression. The final copy of the manuscript was read and approved carefully by both authors. FundingThere is no external fund (private funding). Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAhmad, M., Nasrullah, R. and Ahmad, N. 2015. Effect of cooling rate and equilibration time on pre-freeze and post-thaw survival of buck sperm. Cryobiol. 70(3), 233–238. AL-Badry, K.I. 2016. Effect of magnetically treated water on enzymes and total protein in seminal plasma of Holstein bulls born in Iraq. Iraqi J. Vet. Med. 40(2), 82–88. Al-Chaabawi, J.J.A., Hasan, H.A.K., Alazawe, K.J.Y. and Mohammed, F.F.I. 2020. Seminal and testosterone hormone evaluation in Iraq-born Holstein Friesian bulls used for artificial insemination. Plant Arch. 20(2), 8953–8962. Al-Daraji, H.J., Hassan, K.H., Ibrahim, F.F. and AJ-Timimi, B.A. 2002. Comparison of the effect of various cryopreservative agents on biochemical traits of Iraqi roosters semen. Iraqi J. Agricult. Sci. 33(2), 215–222. AL-Ebady, A.S., Hussain, S.O., AL-Badry, K.I. and Rajab, B.A. 2012. Effect of adding arginine in different concentrations on some physical properties of poor motile bull sperms during different months. JVMAH, 4(9), 130–135. Alhelal, A.M. and Abdulkareem, T.A. 2023. Effect of adding resveratrol to soybean-lecithin extender semen attributes of buffalo bulls. Iraqi J. Agricult. Sci. 54(4), 1074–1083. Ali, J.S., Hussain, S.O. and George, R. 2022. Influence of different concentrations of silymarin and steps of freezing on frozen semen properties of holstein bulls born in Iraq. Int. J. Health Sci. 6(S3), 6767–6780. Bian, F., Mao, G., Guo, M., Mao, G., Wang, J., Li, J. and Xia, G. 2012. Gradients of natriuretic peptide precursor A (NPPA) in oviduct and of natriuretic peptide receptor 1 (NPR1) in spermatozoon are involved in mouse sperm chemotaxis and fertilization. J. Cell. Physiol. 227(5), 2230–2239. Colombo, M., Morselli, M.G., Franchi, G., Schäfer-Somi, S. and Luvoni, G.C. 2022. Freezability of dog semen after collection in field conditions and cooled transport. Animals 12(7), 816. Cuervo-Arango, J., Nivola, K., Väihkönen, L. and Katila, T. 2015. The effect of storage temperature of stallion semen on pregnancy rates. J. Equine Vet. Sci. 35(7), 611–616. Dias, E.A.R., Campanholi, S.P., Rossi, G.F., Dell’Aqua, C.D.P.F., Junior, J.A.D.A., Papa, F.O. and Monteiro, F.M. 2018. Evaluation of cooling and freezing systems of bovine semen. Anim. Reprod. Sci. 195, 102–111. Esteves, S.C. and Varghese, A.C. 2012. Laboratory handling of epididymal and testicular spermatozoa: What can be done to improve sperm injections outcome. J. Hum. Reprod. Sci. 5(3), 233. Gharban, H.A. 2023. Molecular prevalence and phylogenetic confirmation of bovine trichomoniasis in aborted cows in Iraq. Vet. World 16(3), 580–587. Goetze, J.P., Bruneau, B.G., Ramos, H.R., Ogawa, T., de Bold, M.K. and de Bold, A.J. 2020. Cardiac natriuretic peptides. Nature Rev. Cardiol. 17(11), 698–717. Hafez, Y.M. 2015. Assisted reproductive technologies in farm animals. ICMALPS, Alexandria University, Egypt. Hannema, S.E., Van Duyvenvoorde, H.A., Premsler, T., Yang, R.B., Mueller, T.D., Gassner, B. and Wit, J.M. 2013. An activating mutation in the kinase homology domain of the natriuretic peptide receptor-2 causes extremely tall stature without skeletal deformities. J. Clin. Endocrinol. Metab. 98(12), E1988–E1998. Hussain, S.O., Shahad, H.K. and Al-Badry, K.I. 2016. Effect of dilution, cooling and freezing on physical and biochemical properties of semen for holstein bull born in Iraq. Adv. Anim. Vet. Sci. 4(11), 575–579. Kong, N., Xu, X., Zhang, Y., Wang, Y., Hao, X., Zhao, Y. and Zhang, M. 2017. Natriuretic peptide type C induces sperm attraction for fertilization in mouse. Sci. Rep. 7(1), 39711. Li, N., Dong, X., Fu, S., Wang, X., Li, H., Song, G. and Huang, D. 2022. C-type natriuretic peptide (CNP) could improve sperm motility and reproductive function of asthenozoospermia. Int. J. Mol. Sci. 23(18), 10370. Lu, J. and Pan, S.S. 2017. Elevated C-type natriuretic peptide elicits exercise preconditioning-induced cardioprotection against myocardial injury probably via the up-regulation of NPR-B. J. Physiol. Sci. 67(4), 475–487. Madrigali, A., Rota, A., Panzani, D., Castellani, S., Shawahina, M., Hassan, A. and Camillo, F. 2021. Artificial insemination in sheep with fresh diluted Semen: comparison between two different semen extenders and management protocols. Trop. Anim. Sci. J. 44(3), 255–260. Maquivar, M.G., Smith, S.M. and Busboom, J.R. 2021. Reproductive management of rams and ram lambs during the pre-breeding season in US sheep farms. Animals 11(9), 2503. Mei, C., Kang, Y., Zhang, C., He, C., Liao, A. and Huang, D. 2021. C-type natriuretic peptide plays an anti-inflammatory role in rat epididymitis induced by UPEC. Front. Cell. Infect. Microbiol. 11, 711842. Mochida, K. 2020. Development of assisted reproductive technologies in small animal species for their efficient preservation and production. J. Reprod. Develop. 66(4), 299–306. Mohapatra, A. and Shinde, A.K. 2018. Fat-tailed sheep-an important sheep genetic resource for meat production in tropical countries: an overview. Indian J. Small Rumin. 24(1), 1–17. Nakagawa, Y. and Nishikimi, T. 2022. CNP, the third natriuretic peptide: its biology and significance to the cardiovascular system. Biology, 11(7), 986. Nakagawa, Y., Nishikimi, T. and Kuwahara, K. 2019. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides, 111, 18–25. Özbek, M., Hitit, M., Öztop, M., Beyaz, F., Ergün, E. and Ergün, L. 2019. Spatiotemporal expression patterns of natriuretic peptides in rat testis and epididymis during postnatal development. Andrologia, 51(10), e13387. Pagel-Langenickel, I., Buttgereit, J., Bader, M. and Langenickel, T.H. 2007. Natriuretic peptide receptor B signaling in the cardiovascular system: protection from cardiac hypertrophy. J. Mol. Med. 85, 797–810. Perez-Ternero, C., Aubdool, A.A., Moyes, A.J. and Hobbs, A.J. 2019. C-type natriuretic peptide (CNP) regulates adipogenesis, thermogenesis and glucose sensitivity via natriuretic peptide receptor (NPR)-C signalling. Circulation 140(Suppl. 1), A13284–A13284. Redden, R. and Thorne, J.W. 2020. Reproductive management of sheep and goats. In Animal Agriculture. Cambridge, MA: Academic Press, pp: 211–230. Saleh, W.M. 2019. Evaluation of in vitro fertilization index by caudal spermatozoa capacitation with different heparin concentration in Iraqi Sheep: Wafir Mahdi Saleh and Ali Abdul-Ameer. Iraqi J. Vet. Med. 43(1), 171–182. Shahad, H.K., Arrak, J.K. and Hussain, S.O. 2020. Caffeine as semen additive to improve poor motility of sperms cryopreserved in liquid nitrogen for holstein bulls born in Iraq. Biochem. Cell. Arch. 21, 510–514. Shahin, P., Rahardjo, H.E., Bannowsky, A., Tsikas, D., Sohn, M., Kuczyk, M.A. and Ückert, S. 2018. Endopeptidase inhibition attenuates the contraction induced by big endothelin‐1 of isolated human penile erectile tissue. Andrologia, 50(5), e13008. Shinde, A.K. and Naqvi, S.M.K. 2015. Prospects of dairy sheep farming in India: an overview. Indian J. Small Rumin. 21(2), 180–195. Ückert, S., Rahardjo, H., Bannowsky, A., Tsikas, D. and Kuczyk, M. 2019. HP-01-003 Expression of genes encoding for endopeptidase enzymes (EnPEs) and peptide binding receptors in the human vagina# Comparison to penile erectile tissue. J. Sex. Med. 16(Suppl. 2), S30–S31. Ueda, Y., Hirota, K., Yamauchi, I., Hakata, T., Yamashita, T., Fujii, T. and Inagaki, N. 2020. Is C-type natriuretic peptide regulated by a feedback loop? A study on systemic and local autoregulatory effect. PLoS One, 15(10), e0240023. Wu, K., Mei, C., Chen, Y., Guo, L., Yu, Y. and Huang, D. 2019. C-type natriuretic peptide regulates sperm capacitation by the cGMP/PKG signalling pathway via Ca2+ influx and tyrosine phosphorylation. Reprod. BioMed. Online, 38(3), 289–299. Xia, H., Chen, Y., Wu, K.J., Zhao, H., Xiong, C.L. and Huang, D.H. 2016. Role of C-type natriuretic peptide in the function of normal human sperm. Asian J. Androl. 18(1), 80. Yu, Y., Chen, Y., Mei, C., Li, N., Wu, K. and Huang, D. 2021. C-type natriuretic peptide stimulates function of the murine Sertoli cells via activation of the NPR-B/cGMP/PKG signaling pathway. Acta Biochimica Polonica, 68(4), 603–609. Zamir, N., Rivenkreitman, R., Manor, M., Makler, A., Blumberg, S., Ralt, D. and Eisenbach, M. 1993. Atrial natriuretic peptide attracts human spermatozoa in vitro. Biochem. Biophys. Res. Commun. 197(1), 116–122. Zampini, R., Castro-González, X.A., Sari, L.M., Martin, A., Diaz, A.V., Argañaraz, M.E. and Apichela, S.A. 2020. Effect of cooling and freezing on llama (Lama glama) sperm ultrastructure. Front. Vet. Sci. 7, 587596. Zhang, H., Ye, H., Shao, Y., Wu, S., Yu, J., Ji, C. and Zeng, S. 2018. The effects of egg yolk concentration and particle size on donkey semen preservation. J. Equine Vet. Sci. 65, 19–24. Zhao, H., Yu, Y., Mei, C., Zhang, T., Kang, Y., Li, N. and Huang, D. 2023. Effect of C-type natriuretic peptide (CNP) on spermatozoa maturation in adult rat epididymis. Current Issues Mole Biol, 45(2), 1681–1892. | ||
| How to Cite this Article |
| Pubmed Style Khadim MS, Zaid NW. Efficiency of C-type natriuretic peptide on improvement of Iraqi local ram’s epididymal sperms. Open Vet. J.. 2024; 14(2): 674-682. doi:10.5455/OVJ.2024.v14.i2.7 Web Style Khadim MS, Zaid NW. Efficiency of C-type natriuretic peptide on improvement of Iraqi local ram’s epididymal sperms. https://www.openveterinaryjournal.com/?mno=174833 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i2.7 AMA (American Medical Association) Style Khadim MS, Zaid NW. Efficiency of C-type natriuretic peptide on improvement of Iraqi local ram’s epididymal sperms. Open Vet. J.. 2024; 14(2): 674-682. doi:10.5455/OVJ.2024.v14.i2.7 Vancouver/ICMJE Style Khadim MS, Zaid NW. Efficiency of C-type natriuretic peptide on improvement of Iraqi local ram’s epididymal sperms. Open Vet. J.. (2024), [cited January 25, 2026]; 14(2): 674-682. doi:10.5455/OVJ.2024.v14.i2.7 Harvard Style Khadim, M. S. & Zaid, . N. W. (2024) Efficiency of C-type natriuretic peptide on improvement of Iraqi local ram’s epididymal sperms. Open Vet. J., 14 (2), 674-682. doi:10.5455/OVJ.2024.v14.i2.7 Turabian Style Khadim, Massar Saeb, and Nazih Wayes Zaid. 2024. Efficiency of C-type natriuretic peptide on improvement of Iraqi local ram’s epididymal sperms. Open Veterinary Journal, 14 (2), 674-682. doi:10.5455/OVJ.2024.v14.i2.7 Chicago Style Khadim, Massar Saeb, and Nazih Wayes Zaid. "Efficiency of C-type natriuretic peptide on improvement of Iraqi local ram’s epididymal sperms." Open Veterinary Journal 14 (2024), 674-682. doi:10.5455/OVJ.2024.v14.i2.7 MLA (The Modern Language Association) Style Khadim, Massar Saeb, and Nazih Wayes Zaid. "Efficiency of C-type natriuretic peptide on improvement of Iraqi local ram’s epididymal sperms." Open Veterinary Journal 14.2 (2024), 674-682. Print. doi:10.5455/OVJ.2024.v14.i2.7 APA (American Psychological Association) Style Khadim, M. S. & Zaid, . N. W. (2024) Efficiency of C-type natriuretic peptide on improvement of Iraqi local ram’s epididymal sperms. Open Veterinary Journal, 14 (2), 674-682. doi:10.5455/OVJ.2024.v14.i2.7 |