| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 108-115 Original Research Fluctuations of testosterone and cortisol levels in male dromedary camels in response to behavioral and management situationsBassam A. Alhawas1, Mohamad Abdulmohsen1,2, Mohammed A. Abdelghani3,4, Faisal Almathen1,5, Ahmed I. El Sheikh1 and Sherief M. Abdel-Raheem1,6*1Department of Public Health, College of Veterinary Medicine, King Faisal University, Al-Hofuf, Saudi Arabia 2Department of Animal Behavior and Management, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt 3Department of Clinical Sciences, College of Veterinary Medicine, King Faisal University, Al-Hofuf, Saudi Arabia 4Department of Theriogenology, Faculty of Veterinary Medicine, Assuit University, Assuit, Egypt 5Camel Research Center, King Faisal University, Al-Hofuf, Saudi Arabia 6Department of Animal Nutrition and Clinical Nutrition, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt *Corresponding Author: Sherief M. Abdel-Raheem. Department of Public Health, College of Veterinary Medicine, King Faisal University, Al-Hofuf, Saudi Arabia. Email: sdiab [at] kfu.edu.sa Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
AbstractBackground: Biostimulation is a management practice that improves the reproductive parameters, potentiates the desire, and improves the reproductive efficiency during the short breeding season in camels. Aim: This study aims to investigate the concurrent changes in hormonal profiles in response to management and behavioral situations on camel farms in the Eastern region of Saudi Arabia. Methods: A total of 10 male and 50 female camels were used in this study. The hormonal profile of male camels was evaluated weekly starting from December to August. Results: The results show that both serum testosterone and cortisol levels increased (p < 0.01) from December to March compared to April to August. A strong negative correlation was observed between testosterone levels and temperature (r=−0.81, p < 0.05), and a similarly robust negative correlation was found between cortisol levels and temperature (r=−0.83, p < 0.05). The dominant rutting males showed higher levels of testosterone than the submissive males. Serum testosterone levels increased (p < 0.01) in males out of rutting after hearing the sounds of other couples before and during mating. Conclusion: Hearing sounds emitted during mating increases the testosterone output, and improves the libido of male camels during the nonrutting period. It is important to keep two individually housed males for mating purposes. One male joins the herd alone in winter (winter rutter), and the other joins in spring (spring rutter), this could keep the libido extended rutting and improve the reproductive performance in camel herds. Keywords: Camel, Cortisol, Testosterone, Dominant, Submissive. IntroductionThe male dromedary camel is a seasonal short-day breeder. During the breeding season (winter season), the hormonal profile showed alteration and the male camels became highly aggressive against other males and humans (Fatnassi et al., 2014). The short breeding season of dromedary camels, aggressiveness, difficulty handling, and weak libido are incriminated among the factors responsible for impaired reproduction, economic losses, and injuries (Fatnassi et al., 2014; Farh et al., 2018). Accordingly, improvement of the reproductive capacity of male camels is important for maximizing reproductive efficiency, which could be assisted by the extension of the breeding season. During rutting, some Bedouins keep a male for mating purposes in a neighbor’s yard or in the same yard of female camels. Other breeders keep two males not only to increase reproductive effectiveness throughout the brief breeding season and to intensify male desire. Biostimulation is a management practice that improves reproductive parameters (Villamayor et al., 2022). For efficient biostimulation, males should be kept in the female yard during breeding. The breeding season showed variations in its duration that ranged from 2 to 6 months. These variations were attributed to environmental, nutritional, hormonal, psychological, and physical factors (El-wishy, 1988). In UAE, the rutting season extended from October to April (Tibary and Anouassi, 1997), while it continued from December to March in Tunisia (Hammadi et al., 2008). The sexual desire of male camels intensifies in the coolest and rainy months and declines after that. Some male camels showed an intense desire for longer periods, while others lost their desire early for unknown causes (Deen et al., 2003). The testosterone hormone could act as a good indicator of sexual desire in several species including humans (Podlasek et al., 2016; Abdulla et al., 2020). Low testosterone levels in the blood of male camels were reported to be the cause of low desire, long reaction time, and frequent mounting per ejaculate (Al-Qarawi, 2005; Deen, 2008). Furthermore, previous studies have attributed variations in hormone concentrations during the breeding season to factors such as the geographic locations of the animals studied, the diverse populations of sampled animals, and exposure to various experimental conditions (Abdulla et al., 2020). As far as our current knowledge goes, no research has investigated the impact of dominance shifts on the hormonal profile of male camels. Hence, the primary goal of this study was to assess the dynamics of housing two male dromedary camels together in the female yard. In addition, we aimed to monitor alterations in testosterone and cortisol levels during both the breeding and nonbreeding phases of male camels. In addition, to assess whether the change of dominance could have an impact on alterations in hormone concentrations. This study also investigates the possibility of prolonging the breeding season by sexual Stimulation of males by voices of couples heard during previous mating incidents. Materials and MethodsAnimalsIn this investigation, a total of 10 male and 50 female camels were utilized, with ages ranging from 6 to 8 years and body weights between 700 and 800 kg. The study took place across five private camel farms located in Saudi Arabia’s Eastern region, each maintaining a sex ratio of 2 males to 10 females. The health and condition of the animals were carefully assessed, and only those without systemic issues or deformities were considered suitable for participation, while those displaying abnormalities or pathological conditions were excluded from the study. The enclosure provided a minimum of 28 m2 of floor space per adult camel, following the guidelines outlined by Higgins (1986). This space area (allowed/individual) enables the submissive males to avoid fighting and the audience effect of the dominant males. The adult males persisted in the same yard with females during the two consecutive breeding seasons. On each farm, the dominant male was identified as the one who held authority over the herd and exhibited a higher frequency of soft palate extrusion. The submissive male extruded the soft palate less frequently and avoided any conflict with the dominant. Furthermore, the dominance matrix, which recorded instances of one camel supplanting another in mating and feeding situations, was employed to ascertain the dominant male on each farm, as described in Abdulmohsen et al. (2018). Monthly averages of ambient temperature and relative humidity were also calculated from December to August. Blood sampling and hormonal assaysBlood samples were obtained from all animals participating in the experiments through jugular venipuncture. These samples were collected on a weekly basis over the course of 9 months. After collection, following the collection of samples, they were left at room temperature to allow clot formation, after which they were subjected to centrifugation, which was subsequently stored in Eppendorf tubes at −20℃. Circulating levels of testosterone and cortisol were quantified using RIA kits from (Pantex Company, Santa Monica, CA). These assays were conducted in accordance with the manufacturer’s instructions. For testosterone analysis, blood samples from the male camels were collected at 9 a.m. before hearing auditory stimuli and then 30 minutes. after hearing. Testosterone and cortisol levels were measured according to the following protocols:
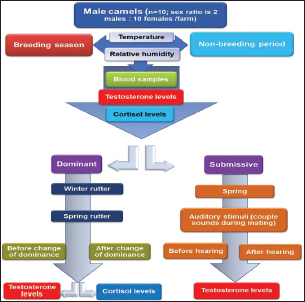
Experimental designA schematic representation of the experimental design is depicted in Figure 1. It is worth noting that rutting, which is the mating season for camels, occurs during the winter season, which is relatively short and crucial for achieving a higher conception rate. Trials were made by others to prolong the season by hormonal interference. Therefore, this experiment was applied in spring to investigate the possibility of prolonging the rutting season in natural ways on the three farms.
This experiment was done twice on the three farms. Statistical analysisThe data’s normal distribution was assessed through the application of the Kolmogorov-Smirnov test, and subsequent statistical analysis was performed utilizing JMP software, specifically version 11.0.0, developed by SAS Institute in Cary, NC 11. Independent sample t-tests were employed for comparing means. Figures were created using Graphpad Prism v5 software, which is developed by (Graphpad Software, Inc., San Diego, CA). To analyze correlation coefficients (r) between testosterone, relative humidity, temperature, and cortisol, a correlation coefficient matrix was employed. In addition, a linear regression relationship was used to examine the association between testosterone, relative humidity, temperature, and cortisol. The different means were determined to have statistical significance at a significance level of p < 0.05.
Fig. 1. Flow chart showing the experimental design.
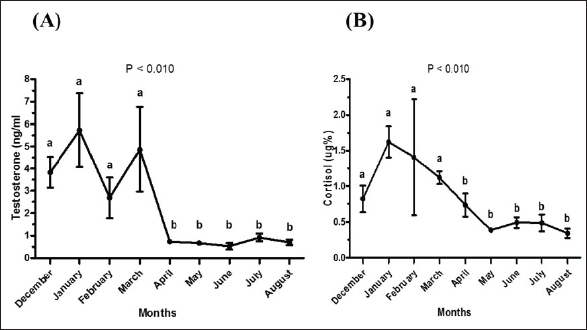
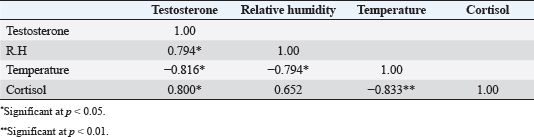
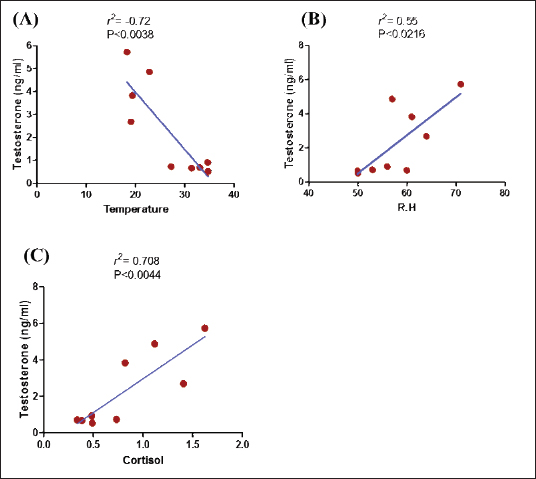
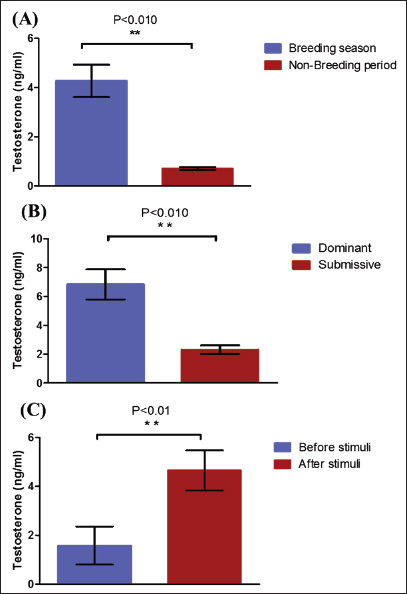
Fig. 2. Testosterone (ng/ml) (A) and cortisol (µg %) (B) concentrations monthly from December to August in male camels. (a,b): Different letters indicate a significant difference (p < 0.01). Ethical approvalThe experimental protocols employed in this study received approval in accordance with the Animal Ethics Committee guidelines at King Faisal University, Saudi Arabia (KFU-412). ResultsHormonal profile in male camelsThe average serum testosterone levels (measured in ng/ml) in male camels exhibited a significant increase (p < 0.01) from December to March. However, there was no statistically significant change observed from April to August, as illustrated in Figure 2A. Simultaneously, cortisol concentrations (measured in µg%) were significantly higher (p < 0.01) during the months from December to March in comparison to the months from April to August, as depicted in Figure 2B. As indicated in Table 1, the most pronounced correlations were found between testosterone and temperature (r=−0.81, p < 0.011), testosterone and cortisol (r=0.80, p < 0.014), and cortisol and temperature (r=−0.83, p < 0.008). Testosterone values exhibited a negative correlation with temperature (r=−0.72, p < 0.003), as depicted in Figure 3A. In addition, testosterone concentrations displayed positive correlations with both cortisol (r=0.70, p < 0.004) and relative humidity (r=0.55, p < 0.002), as shown in Figures 3B and C, respectively. Mean values of testosterone in male camels during the breeding season were higher (p < 0.05) than during the nonbreeding period (Fig. 4A). During the breeding season, the mean values of testosterone in dominant males were higher (p < 0.05) than those in submissive ones (submissive males are socially stressed males in the presence of dominant male); however, testosterone values did not differ (p > 0.05) between dominant and submissive males during the nonbreeding period (Fig. 4B). Effect of external stimuli on male camelsAt the end of rutting and after applying the inductive external stimuli (sounds of other couples heard during mating to arouse the male’s desire), the mean testosterone levels in male camels increased (p < 0.01) than before hearing (Fig. 4C). The increase in testosterone levels after hearing these stimuli coincided with the appearance of behavioral activities indicating libido improvements, such as erection, mounting, and extruding soft palate. Table 1. Spearman’s correlation matrix among testosterone (ng/ml), relative humidity, temperature, and cortisol (µg %) in male camels (n=10).
Fig. 3. Relationship between testosterone level (ng/ml) and temperature (A), testosterone level (ng/ml) and relative humidity (R.H) (B), and testosterone level (ng/ml) and cortisol (µg %) (C) in male camels.
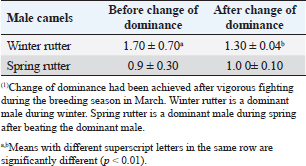
Fig. 4. Mean testosterone levels (ng/ml) during the breeding and nonbreeding season (A), means of testosterone levels (ng/ml) during breeding season (Submissive males are socially stressed males in presence of dominant male) (B), and means of testosterone levels (ng/ml) during the nonbreeding period before and after applying of an inductive external stimuli for their sexual drives (Hearing of couple sounds during copulation) (C). (*): Asterisk indicates a significant difference between the experimental groups (p < 0.05). Hormonal profile in winter rutter and spring rutterIn the winter season, values of testosterone in dominant males (winter rutter) were higher (p < 0.05) than in submissive males (Table 2). However, in May, the submissive male dominated the herd after beating the dominant (e.g., changing the dominance). Concurrently, testosterone levels in the submissive male in the spring rutter were higher (p < 0.05) than those in the winter rutter (Table 2). On the other hand, the cortisol concentrations in dominant males (winter rutter) were higher (p < 0.05) than those in submissive males (Table 3). Table 2. The means testosterone levels (ng/ml) with a change of dominance in male camels (n=10).
Table 3. The mean cortisol levels (ug%) with a change of dominance(1) in male camels (n=10).
DiscussionMonthly testosterone level tracking from December to August shows that mean serum testosterone levels reached rutting levels and increased significantly from December to March compared to the rest of the year. At the same time, the mean cortisol levels increased significantly from December to March. Consistently, research has demonstrated that testosterone is positively linked to risk-taking, dominance, and aggression in males (Mehta et al., 2015; Prasad et al., 2017; Grotzinger et al., 2018). On the contrary, cortisol, which is a glucocorticoid hormone produced by the hypothalamic-pituitary-adrenal (HPA) axis, is released in response to various physiological and psychological stressors. Increased levels of cortisol have been linked to anxiety and avoidance behaviors, as highlighted by Cooke et al. (2020). In the case of camels and other animals, cortisol has been used as an indicator of stress (El-Khasmi et al., 2015; Tajik et al., 2016). This may explain the increase in serum cortisol levels observed in this study. However, it is important to note that cortisol concentrations in dromedary camels can be influenced by stressors and physical stimuli, particularly during the rutting season when they are exposed to various stress-inducing factors. Certainly, there was a noticeable and significant inverse correlation observed between testosterone levels and temperature. Likewise, a significant negative correlation was identified between cortisol levels and relative humidity. Furthermore, a noteworthy positive correlation was detected between the levels of testosterone and cortisol in the serum of male camels. The coincident increase in both testosterone and cortisol levels during the period from December to March rather than the rest of the year could indicate that the rutting season in camels continued from December to the end of March. The breeding season in camels is a period of stress for the whole herd members; this could explain the rise of cortisol levels in the rutting males. This finding aligns partially with the research by Al-Hazmi (2000), who documented that the mating season of dromedary camels typically commenced during cooler ambient temperatures and extended from late October to early February. The mean testosterone levels exhibited an increase during the breeding season compared to the nonbreeding period. This rise in testosterone concentrations correlated with significant behavioral changes in male camels, such as increased aggression, frequent soft palate extrusion, melling the female genital organs, and heightened urination frequency, among other behaviors. These observations align with findings reported by Agarwal and Khanna (1990) and El-Hassanein (2003). In the present study, the dominant rutting male showed a higher level of testosterone than the submissive male. The significant decrease in testosterone levels in the submissive male may be attributed to the prolonged exposure to the social stress imposed by the dominant, which depresses not only the testosterone output but also the associated behavioral characteristics. On the other hand, the increased concentrations of testosterone in the dominant male were associated with aggressiveness, frequent dulla extrusion, recurrent urination, increased poll gland secretion, and so on. These findings are similar to those reported by Miller et al. (1987) and Farh et al. (2018). Our findings showed that serum testosterone levels increased significantly in males out of rutting after applying the inductive auditory stimuli (hearing the sounds of other couples before and during mating). The increase in testosterone levels may be due to the LH in response to the heard auditory stimuli, which resulted in higher testosterone secretion. This indicates that hearing such sounds could potentiate the male’s desire. In winter, testosterone levels increased in the dominant male (winter rutter) than after losing his rank with the change in dominance. However, in March, the submissive male became the president (spring rutter) after defeating the dominant and governing the herd. This could justify the significant increase in testosterone levels in the submissive (spring rutter) after the change of dominance compared to before. The increase in testosterone secretion was associated with radical changes in the behavior of male camels. Therefore, rut signs appeared on the spring rutter after domination and diminished in the winter rutter after submission. This may be attributed to the effect of psychological status and disposition on testosterone secretion. This agrees with Yagil and Etzion (1980), who reported that testosterone secretion in camels was dependent on the disposition of males from which specimens were obtained. The findings show that male camels after hearing the couple’s sounds (emitted during mating), could stimulate their desire, and such sounds play a role in the mediation of their reproductive behavior. Despite the desire for stimulation, the presence of two males in the herd is hostile to each other because of fighting that may lead to serious injuries and fractures. It is important to keep two individually housed males for a herd of camels during rutting to ensure reproductive efficiency. One of them is kept for mating purposes in the winter (winter rutter). The libido of the late male weakens in the spring after being sexually exhausted in the winter. As a result, the other male (spring rutter) takes the place of the first (winter rutter), who exhibits intense desire in the spring. In conclusion, hearing sounds emitted during mating increases the testosterone output and improves the libido of male camels during the nonrutting period. To ensure a strong libido and prolong the rutting season, it is important to keep two individually housed males for mating purposes. One male joins the herd alone in winter (winter rutter), and the other joins in spring (spring rutter), this could keep the libido and extend rutting. AcknowledgmentsThis work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Project No. GRANT5676). Conflict of interestThe authors affirm that there are no conflicts of interest that might be interpreted as potentially biasing the objectivity of the research presented. Authors contributionsBassam A. Alhawas: Conceptualization, methodology, validation, formal analysis, data curation, writing original draft, funding acquisition. Mohamad Abdulmohsen: Conceptualization, methodology, validation, formal analysis, writing—review & editing visualization of manuscript. Mohammed A. Abdelghani: Conceptualization, methodology, formal analysis, investigation, resources, data curation, Writing original draft, writing—Writing, review & editing, visualization. Faisal Almathen: Conceptualization, methodology, formal analysis, investigation, resources, writing, review & editing, supervision. Ahmed I. El Sheikh: Conceptualization, methodology, formal analysis, investigation, resources, data curation, Writing—original draft, writing—Writing—review & editing, visualization. Sherief M. Abdel-Raheem: Conceptualization, methodology, formal analysis, investigation, resources, data curation, original draft writing, review & editing, visualization. FundingThis work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Project No. GRANT5676). Data availabilityAll data are provided within the manuscript. Any extra data needed can be provided upon reasonable request from the corresponding author. ReferencesAbdulmohsen, M., Al-Shami, S.A., Al-Sultan, S. and Al-Ekna, M. 2018. Evaluation of mating and the causes of noises at night in small dromedary camel herds. Emirates J. Food Agric. 23, 709–714. Abdulla, A., Jaber ALbdeery, A.H. and Abed Ali, B.H. 2020. Effect of blood sampling method during a mating time in male camels (Dromedary Camels). Plant Arch. 20(1), 1681–1168. Agarwal, S.P., Khanna, N.D. Endocrine profiles of Indian camel under different phases of reproduction. Workshop: is it possible to improve the reproductive performance of the camel. Proceedings Unite de Coordination pour l’Elevage Camelin, Paris, France, pp: 77–100, 1990. Al-Hazmi, M.A. 2000. Mating behavioural aspects of one humped camel (Camelus dromedaries) in Jeddah Province, Saudi Arabia. Saudi J. Biol. Sci. 7, 113–123. Al-Qarawi, A.A. 2005. Infertility in the dromedary bull: a review of causes, relations and implications. Anim. Reprod. Sci. 87(1–2), 73–92. Cooke, E.M., Connolly, E.J., Boisvert, D.L., Armstrong, T.A., Lewis, R.H., Kavish, N., Woeckener, M., Wells, J. and Harper, J. 2020. Examining how testosterone and cortisol influence the relationship between strain, negative emotions, and antisocial behavior: a gendered analysis. Crim. Delinq. 66(10), 1470–1501. Deen, A. 2008. Testosterone profiles and their correlation with sexual libido in male camels. Res. Vet. Sci. 85, 220–226. Deen, A., Vyas, S. and Sahani, M.S. 2003. Semen collection, cryopreservation and artificial insemination in the dromedary camel. Anim. Reprod. Sci. 77(3–4), 223–233. El-Hassanein, E. 2003. An invention for easy semen collection from dromedary camels. In recent advances in camelid reproduction. Eds., L. Skidmore and G.P. Adams. Ithaca, NY: International Veterinary Information Service (www.ivis.org). El Khasmi, M., Chakir, Y., Bargaâ, R., Barka, K., Lektib, I., El Abbadi, N., Belhouari, A. and Faye, B. 2015. Impact of transport distance on stress biomarkers levels in dromedary camel (Camelus dromedarius). Emirates J. Food. Agric. 27, 507–512. Farh, I., Lektib, R., Bargaa, N., Elabbadi, F., Riad, E.H., Tahri, A. and Safwate, M.E. 2018. Hormonal profile in rutting and non-rutting periods in dromedary camel (Camelus dromedaries). Rev. Mar. Sci. Agron. Vet. 6, 168–177. Fatnassi, M., Padalino, B., Monaco, D., Khorchani, T., Lacalandra, G.M. and Hammadi, M. 2014. Effect of different management systems on rutting behaviour and behavioral repertoire of housed maghrebi male camels (Camelus dromedarius). Trop. Anim. Health. Prod. 46, 861–867. Grotzinger, A.D., Mann, F.D., Patterson, M.W., Tackett, J.L., Tucker-Drob, E.M. and Harden K.P. 2018. Hair and salivary testosterone, hair cortisol, and externalizing behaviors in adolescents. Psych. Sci. 29, 688–699. Hammadi, M., Zarrouk, O., Barmat, A., Trimeche, A., Khorchani, T. and Khaldi, G. 2008. Characterization and conservation of Maghrabi camel semen. Eds., Nagy, P., Huscenicza, G. and Juhasz, J. In WBC/ICAR Satellite Meeting on Camelid Reproduction, Budapest, Hungary, 2008, July 12–13, pp: 32–36. Higgins, A. 1986. The camel in health and disease, 1st ed. London, UK: Bailliere Tindall. JMP®. 2014. Version 11. Cary, NC: SAS Institute Inc. Miller, K.V., Marchinton, R.L, Forand, K.J. and Johansen, K.L. 1987. Dominance, testosterone levels, and scraping activity in a captive herd of white-tailed deer. J. Mammal. 168(4), 812–817. Mehta, P.H., Welker, K.M., Zilioli, S. and Carré, J.M. 2015. Testosterone and cortisol jointly modulate risk-taking. Psychoneuroendocrinology 56, 88–99. Podlasek, C.A., Mulhall, J., Davies, K., Wingard, C.J., Hannan, J.L., Bivalacqua, T.J., Musicki, B., Khera, M., González-Cadavid, N.F. and Burnett, A.L. 2016. Translational perspective on the role of testosterone in sexual function and dysfunction. J. Sex. Med. 13(8), 1183–1198. Prasad, S., Narayanan, J., Lim, V.K., Koh, G.C., Koh, D.S. and Mehta, P.H. 2017. Preliminary evidence that acute stress moderates basal testosterone’s association with retaliatory behavior. Horm. Behav. 92, 128–40. Tajik, J., Nazifi, S. and Eshtraki, R. 2016. The influence of transportation stress on serum cortisol, thyroid hormones, and some serum biochemical parameters in Iranian cashmere (Raini) goat. Vet. Arhiv. 86 (6), 795–804. Tibary, A. and Anouassi, A. 1997. Theriogenology in Camelidae: anatomy. Physiology, pathology and artificial breeding. Rabat, Morocco: Actes Editions. Villamayor, P.R., Gullón, J., Yáñez, U., Sánchez, M., Sánchez-Quinteiro, P., Martínez, P. and Quintela, L. 2022. Assessment of biostimulation methods based on chemical communication in female doe reproduction. Animals 12(3), 308. Yagil, R. and Etzion, Z. 1980. Hormonal and behavioural patterns in the male camel (Camelus dromedarius). J. Reprod. Fertil. 58, 61–65 . | ||
| How to Cite this Article |
| Pubmed Style Alhawas BA, Abdulmohsen M, Abdelghani MA, Almathen F, Sheikh AIE, Abdel-raheem SM. Fluctuations of testosterone and cortisol levels in male dromedary camels in response to behavioral and management situations. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 108 -115. doi:10.5455/OVJ.2024.v14.i1.10 Web Style Alhawas BA, Abdulmohsen M, Abdelghani MA, Almathen F, Sheikh AIE, Abdel-raheem SM. Fluctuations of testosterone and cortisol levels in male dromedary camels in response to behavioral and management situations. https://www.openveterinaryjournal.com/?mno=175118 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i1.10 AMA (American Medical Association) Style Alhawas BA, Abdulmohsen M, Abdelghani MA, Almathen F, Sheikh AIE, Abdel-raheem SM. Fluctuations of testosterone and cortisol levels in male dromedary camels in response to behavioral and management situations. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 108 -115. doi:10.5455/OVJ.2024.v14.i1.10 Vancouver/ICMJE Style Alhawas BA, Abdulmohsen M, Abdelghani MA, Almathen F, Sheikh AIE, Abdel-raheem SM. Fluctuations of testosterone and cortisol levels in male dromedary camels in response to behavioral and management situations. Open Vet. J.. (2024), [cited January 25, 2026]; 14((1) (Zagazig Veterinary Conference)): 108 -115. doi:10.5455/OVJ.2024.v14.i1.10 Harvard Style Alhawas, B. A., Abdulmohsen, . M., Abdelghani, . M. A., Almathen, . F., Sheikh, . A. I. E. & Abdel-raheem, . S. M. (2024) Fluctuations of testosterone and cortisol levels in male dromedary camels in response to behavioral and management situations. Open Vet. J., 14 ((1) (Zagazig Veterinary Conference)), 108 -115. doi:10.5455/OVJ.2024.v14.i1.10 Turabian Style Alhawas, Bassam A., Mohamad Abdulmohsen, Mohammed A. Abdelghani, Faisal Almathen, Ahmed I. El Sheikh, and Sherief M. Abdel-raheem. 2024. Fluctuations of testosterone and cortisol levels in male dromedary camels in response to behavioral and management situations. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 108 -115. doi:10.5455/OVJ.2024.v14.i1.10 Chicago Style Alhawas, Bassam A., Mohamad Abdulmohsen, Mohammed A. Abdelghani, Faisal Almathen, Ahmed I. El Sheikh, and Sherief M. Abdel-raheem. "Fluctuations of testosterone and cortisol levels in male dromedary camels in response to behavioral and management situations." Open Veterinary Journal 14 (2024), 108 -115. doi:10.5455/OVJ.2024.v14.i1.10 MLA (The Modern Language Association) Style Alhawas, Bassam A., Mohamad Abdulmohsen, Mohammed A. Abdelghani, Faisal Almathen, Ahmed I. El Sheikh, and Sherief M. Abdel-raheem. "Fluctuations of testosterone and cortisol levels in male dromedary camels in response to behavioral and management situations." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 108 -115. Print. doi:10.5455/OVJ.2024.v14.i1.10 APA (American Psychological Association) Style Alhawas, B. A., Abdulmohsen, . M., Abdelghani, . M. A., Almathen, . F., Sheikh, . A. I. E. & Abdel-raheem, . S. M. (2024) Fluctuations of testosterone and cortisol levels in male dromedary camels in response to behavioral and management situations. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 108 -115. doi:10.5455/OVJ.2024.v14.i1.10 |