| Research Article | ||
Open Vet. J.. 2024; 14(5): 1146-1153 Open Veterinary Journal, (2024), Vol. 14(5): 1146–1153 Research Article Changes in the hematobiochemical, acid–base and blood gas elements as well as biomarkers of inflammation and bone metabolism in donkeys (Equus asinus) with acute bleedingMohamed Tharwat1,2*, Fahd Al-Sobayil1 and Haytham Ali3,41Department of Clinical Sciences, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia 2Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 3Department of Animal and Veterinary Sciences, College of Agricultural and Marine Sciences, Sultan Qaboos University, Muscat, Sultanate of Oman 4Department of Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Mohamed Tharwat. Department of Clinical Sciences, College of Veterinary Medicine, Qassim University, P.O. Box 6622, Buraidah, 51452, Saudi Arabia. Email: atieh [at] qu.edu.sa Submitted: 25/01/2024 Accepted: 21/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
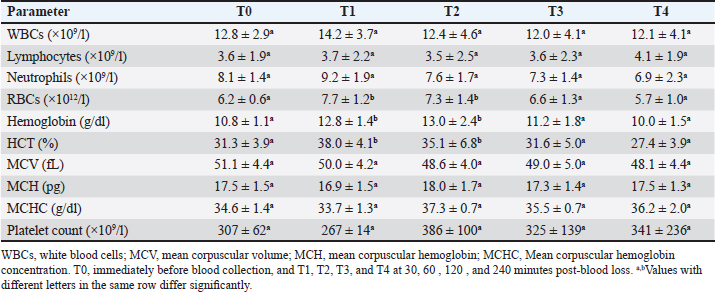
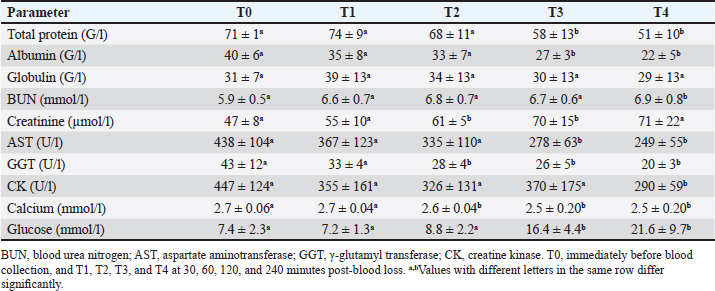
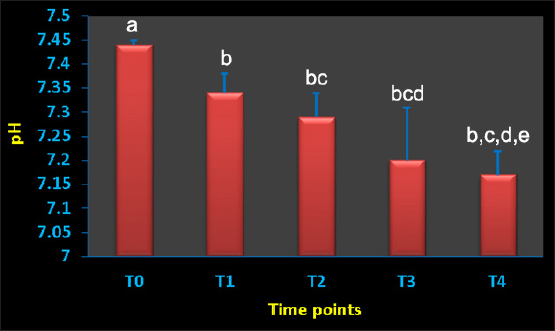
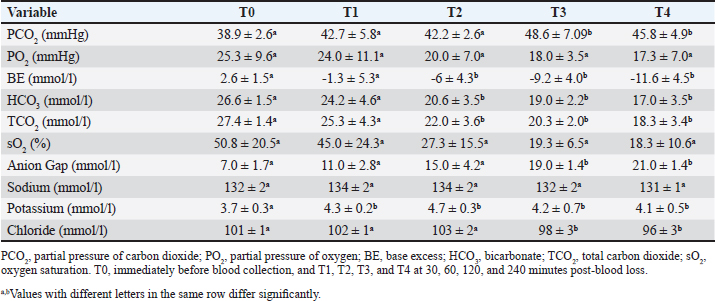
AbstractBackground: Acute hemorrhage is fatal in equines with a complication of severe hypovolemic shock that causes a sudden death in such cases. Aim: This study was designed to report the influences of acute bleeding in conscious non-sedated donkeys (Equus asinus) on the hematobiochemical variables, acid-base, blood gas elements, and markers of inflammation and bone metabolism. Methods: Eight healthy donkeys were used where a total of 900 ml of whole blood was collected. Five blood samples were collected from each animal: just before collection of blood (T0); (2) 30 (T1), 60 (T2), 120 (T3), and 240 minutes (T4) later. The blood panels including total white blood cells, lymphocytes, neutrophils, red blood cell counts (RBCs), HCT, hemoglobin (Hg), and RBCs indices were measured. Biochemical parameters and electrolytes were evaluated. The activity of aspartate aminotransferase (AST), creatine kinase (CK), and γ-glutamyl transferase (GGT) were also determined. Complete acid-base and blood gas panels were assessed. Serum amyloid A (SAA), haptoglobin (Hp), osteocalcin (OC), bone alkaline phosphatase (b-ALP), and pyridinoline cross-links (PYD) were measured. Results: The RBCs, Hg, and HCT increased significantly at points T1, T2, and T3 compared to T0. The concentrations of total proteins and albumin decreased significantly at points T3 and T4. The blood urea nitrogen concentrations increased significantly at T4. Creatinine concentrations increased significantly at T2 and T3. The AST, GGT, and CK decreased significantly. On the other hand, glucose increased significantly at T3 and T4. The pH decreased significantly at points T1, T2, T3, and T4. The PCO2 increased significantly at T3 and T4. The BE, HCO3, and TCO2 values decreased significantly at T2, T3, and T4. Contrary, the AG increased significantly at points T3 and T4. The potassium increased significantly at T1-T4 and chloride decreased significantly at T3 and T4. Lactate showed significant increases at T1-T4. The SAA, Hp, OC, b-ALP, and PYD did not differ significantly at T1-T4. Conclusion: In conscious non-sedated donkeys, induced bleeding resulted in significant changes in the hematobiochemical elements, the acid-base status, and blood gas and electrolyte parameters. However, it did not change the markers of inflammation and bone metabolism. Keywords: Biomarkers, Blood, Inflammation, Physiology, Shock. IntroductionBlood is composed primarily from erythrocytes hanging in extracellular plasma, electrolytes, and proteins (Farley et al., 2012). These ingredients play in part a vital part in tissue oxygenation, blood clotting, immunological tasks, conservation of blood osmotic and hydrostatic pressure (Ramsey, 2015; Cap et al., 2018). Transfusion of blood is therefore crucial in equine animals suffering from anemia, bleeding, and intravascular hemolysis (Jamieson et al., 2022). Manifestations point to the need for blood transfusion include, but are not limited to, increased heart and respiratory rates, diminished pulse quality, hypothermia paler mucosae, low hematocrit (HCT), and increased lactic acid in the blood (Mudge, 2014). The judgment of blood transfusion in horses depends on the volume and speed of blood loss (Williams, 2020). Acute bleeding may terminate the life of a horse when not managed as quickly as possible (Mudge, 2014). Acute-phase proteins or biomarkers of infection and inflammation are proteins increased or decreased in the blood owing to infection or inflammation or trauma (Murata et al., 2004; Tharwat et al., 2014a; Tharwat and Al-Sobayil, 2015a,b, 2018a, 2022a; Tharwat, 2020a, 2023). Biomarkers of bone metabolism are also used for assessing the bone reaction to either invasive or non-invasive interventions and for the evaluation of musculoskeletal diseases (Frisbie et al., 2008; Tharwat and Al-Sobayil, 2018b, 2022a; Tharwat, 2020b, 2023; Al-Sobayil and Tharwat, 2021). Recently, we have reported the influence of acute bleeding in the sedated donkey on the markers of inflammation and on those of bone metabolism, acid–base status, blood gas parameters, and hematobiochemical variables (Tharwat and Al-Sobayil, 2022a). However, the levels of the same measurements in the natural non-sedated donkeys suffering from acute bleeding have not been documented. To mimic cases of spontaneous hemorrhage in equines, this study was designed. Material and MethodsAnimals and blood collectionEight donkeys (six males and two non-pregnant females); aged 8.5 ± 1.6 y and weighed 85 ± 25 kg were enrolled in this investigation. Clinical and laboratory tests proved they were free from diseases. Special cardiac examinations including echocardiography and electrocardiography confirmed that the cardiovascular system is non-diseased. A total of 900 ml of whole blood was collected from each animal while standing by the aid of a 14G × 45 mm cannula (Mais Co, Riyadh, Saudi Arabia) inserted to the common carotid artery. No sedatives were injected for any of the donkeys. The collected blood was preserved in sterile 500 ml-blood collecting bags that contained 50 ml sodium citrate (concentration 3.8%). Five blood samples, 10 ml each, were collected from each animal; (1) T0, just before collection of blood (T0); (2) 30 minutes after collection (T1); (3) 60 minutes after collection (T2); (4) 120 minutes after collection (T3); and the final (5) 240 minutes after collection (T4). The 10 ml blood samples were divided as follows: (1) 2 ml in EDTA tubes for determination of complete blood count; (2) 2 ml in tubes containing heparin for acid-base balance, blood gases, and electrolyte measurements (2) 6 ml in tubes without anticoagulant for collection of serum for estimating inflammation and bone metabolism biomarkers. Determination of the hematobiochemical and blood gas variablesThe complete blood count panels including total white blood cells (WBCs), lymphocytes, neutrophils, red blood cell counts (RBCs), HCT, hemoglobin (Hg), and RBCs indices were measured in EDTA samples by a veterinary analyzer (VetScan HM5, Abaxis, USA). Different biochemical parameters and electrolytes including albumin, total protein, globulin, creatinine, blood urea nitrogen (BUN), glucose, calcium, potassium, sodium, and chloride were also evaluated using a veterinary analyzer (VetScan VS2, Abaxis, USA). The activity of aspartate aminotransferase (AST), creatine kinase (CK), and γ-glutamyl transferase (GGT) were also determined. Complete acid-base and blood gas panel was measured immediately after the heparinized collected sample collection by a portable veterinary analyzer (I-STAT®, Abaxis, USA). This panel included (1) pH, (2) oxygen partial pressure (PO2), (3) carbon dioxide partial pressure (PCO2), (4) total carbon dioxide (TCO2), (5) excess of base (BE), (6) oxygen saturation (So2), (7) bicarbonate (HCO3), (8) anion gap (AG), and (9) lactate (Tharwat et al., 2014b, 2024a,b; Tharwat and Al-Sobayil, 2014a,b,c; Tharwat, 2015, 2023; Tharwat and Al-Hawas, 2024). Measurements of inflammation and bone metabolism biomarkersThe inflammation biomarkers serum amyloid A (SAA) and haptoglobin (Hp) were tested in serum samples by commercial kits (Multispecies SAA ELISA kit and Hp colorimetric kit, Tridelta Ltd., Ireland) as reported (Tharwat et al., 2014a; Tharwat and Al-Sobayil, 2015a,b, 2018a; Tharwat, 2020a, 2022a, 2023; Al-Sobayil and Tharwat, 2021). In addition, the serum concentrations of the bone metabolism biomarkers osteocalcin (OC), bone alkaline phosphatase (b-ALP), and pyridinoline cross-links (PYD) were evaluated in serum samples by an immunoassay kits (Metra Biosystems Inc., a division of Quidel Corp.) as published (Tharwat et al., 2014a; Tharwat and Al-Sobayil, 2015b, 2018a,b, 2022b; Tharwat 2020b, 2023; Al-Sobayil and Tharwat 2021). Statistical analysisResults were presented as means±standard deviation, and it was evaluated by a statistical package (SPSS, 2017). A repeated measure analysis of variance (ANOVA) was used as the primary pattern to assess the changes by time (T0–T4 points) and then by Dunnett’s multiple comparisons, with a significance level of p < 0.05. Ethical approvalThe experimental animal committee regulations of Qassim University have approved our experimental protocol. These regulations are correlated well with the recommendations of laboratory animals of the national institutes of animal health (number 86–23). ResultsVariables of hematology differences are tabulated (Table 1). Concerning the total leukocytic count, lymphocytes, and neutrophils, no significant changes were recorded among T0 before bleeding and other points (T1–T4) post-bleeding (p > 0.05). The RBCs, Hg, and HCT increased significantly at points T1, T2, and T3 compared to T0 time point (p < 0.05). The RBCs indices mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and Mean corpuscular hemoglobin concentration (MCHC), and platelets counts showed no significant differences at points T1–T4 compared to pre-bleeding values (p > 0.05). Changes in the blood biochemistry are shown in Table 2. The concentrations of total proteins and albumin decreased significantly at points T3 and T4 compared to T0 point (p < 0.05). However, globulin did not change in a significant manner (p > 0.05). The BUN concentrations increased significantly at T4 compared to T0 (p=0.04). Creatinine concentrations increased significantly at T2 and T3 compared to T0 (p=0.006 and p=0.02, respectively). The AST enzyme activity decreased significantly at T3 and T4 (p=0.01 and p=0.003, respectively). Parallel, the GGT activity decreased significantly at points T2, T3, and T4 (p=0.01, 0.005, and 0.0009, respectively) and the CK decreased significantly at T4 (p=0.01). Decreased calcium concentrations were measured significantly at T2, T3, and T4 (p=0.003, 0.04, and 0.01, respectively). On the other hand, glucose concentrations increased significantly at T3 and T4 points (p=0.04 and 0.01, respectively). Figure 1 displays the blood pH at the points T0–T4. It decreased significantly towards acidity at points T1, T2, T3, and T4 compared to pre-bleeding values (T0) ( p < 0.0001). The changes in acid-base balance, blood gases, and electrolytes are tabulated (Table 3). The PCO2 increased significantly at T3 and T4 (p=0.007 and 0.01, respectively). The BE, HCO3 and TCO2 values decreased significantly at T2, T3, and T4 (p < 0.05). Contrarily, the anion gap increased significantly at points T3 and T4 (p=0.004 and 0.003, respectively). The PO2 and sO2 did not differ significantly at points T1–T4 (p > 0.05). Concerning electrolytes, the potassium increased significantly at T1–T4 (p < 0.05), chloride decreased significantly at T3 and T4 (p < 0.03) and sodium did not change significantly. Table 1. Means ± SD of hematological parameters in non-sedated donkeys with acute blood loss.
Table 2. Means ± SD of biochemical parameters in non-sedated donkeys with acute blood loss.
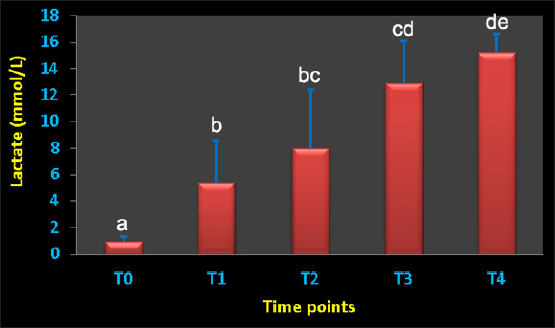
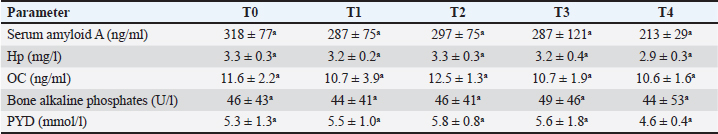
Figure 2 shows the serum concentration of lactate at the points T0–T4. It showed a dramatic significant increase from T1, T2, T3, and T4 (p=0.01, 0.008, 0.0001 and p < 0.0001, respectively). None of the inflammation biomarkers SAA and Hp nor the bone metabolism biomarkers OC, b-ALP, and PYD differed significantly throughout the points T1–T4 compared to pre-bleeding T0 point (Table 4). DiscussionAcute spontaneous hemorrhage is fatal in equines, and is reported in veterinary literature as either spontaneous (Varegg et al., 2019; Camacho-Rozo et al., 2020; Calero Rodriguez and deGrauw, 2022; Hurcombe et al., 2022), exercise-induced (Lyle et al., 2011; Hinchcliff et al., 2015; Poole and Erickson, 2016) or due to invasive procedures causing serious trauma to the nasal conchae, or lung biopsy (Relave et al., 2010; Hinchcliff et al., 2015; Varegg et al., 2019). Severe hypovolemic shock is usually the cause of sudden death in such cases (Mudge 2014). In order to mimic spontaneous hemorrhage in equines, this investigation has been performed. To the best of our knowledge, this report is the first documenting influences of acute bleeding on acid–base status, blood gas, and hematobiochemical variables as well as on the markers of inflammation and bone metabolism in non-sedated donkeys (Equus asinus).
Fig. 1. Blood pH value in conscious and non-sedated donkeys suffering from acute bleeding. T0=immediately before blood collection; T1, 30 minutes; T2, 60 minutes; T3, 120 minutes; and T4, 240 minutes after blood collection. a,b,c,d,eDifferent letters differ significantly (p < 0.05). Table 3. Means ± SD of acid-base balance, blood gases, electrolytes, and lactic acid concentration in non-sedated donkeys with acute blood loss.
Regarding the hematobiochemical parameters tested in this study, the RBCs, Hg, and HCT increased significantly within the first hour of induced bleeding. This may be simply explained on the basis of a splenic blood reservoir. However, the HCT percent was reported to be decreased in sedated equines with acute hemorrhage (Wilson et al., 2003; Tharwat and Al-Sobayil, 2022a). Most measured biochemical parameters decreased as a result of acute blood loss. However, serum concentrations of BUN and creatinine increased pointing to adverse effects on renal functions. The most important biochemical finding was that profound hyperglycemia appeared one hour of the induced bleeding. It is reported that surgical trauma can elevate cortisol levels together with catecholamines, and thus induce insulin resistance resulting in elevated blood sugar levels (Duggan et al., 2007; Peacock, 2019; Tharwat and Al-Sobayil, 2022a). Concerning the acid-base status and blood gas parameters, the blood pH sharply decreased indicating metabolic acidosis at the tested points T1–T4 when compared to baseline values. Remarkable significant decreases were detected in the BE, HCO3, and TCO2 following the induced bleeding starting from 30 minutes after bleeding to the end of sampling at 240 minutes. In a published report with acute bleeding in anesthetized horses, the pH, HCO3, and BE did not change in a significant manner and anesthesia may be the cause (Wilson et al., 2003). On the other side, the AG increased significantly 60–240 minutes after bleeding. Similar findings were shown in sedated donkeys (Tharwat and Al-Sobayil, 2022a). Hyperkalemia was measured significantly at the tested points 30–240 minutes when compared to pre-bleeding values. Dramatic significant increases from point 30 minutes to point 240 minutes in the levels of lactate were detected. Similar results were reported in sedated donkeys (Tharwat and Al-Sobayil, 2022a). The increase in lactate level post-bleeding was reported to be a signal of reduced volume of blood in horses (Magdesian et al., 2006). Our results agree well with the increased lactate levels in horses with severe hemorrhage (Magdesian et al., 2006).
Fig. 2. Lactate levels in conscious and non-sedated donkeys suffering from acute bleeding. T0=immediately before blood collection; T1, 30 minutes; T2, 60 minutes; T3, 120 minutes; and T4, 240 minutes after blood collection. a,b,c,d,eDifferent letters differ significantly (p < 0.05). Table 4. Means±SD of inflammation and bone biomarkers in non-sedated donkeys with acute blood loss.
Neither the inflammation biomarkers SAA and Hp nor the bone biomarkers OC, b-ALP, and PYD showed any significant changes in non-sedated donkeys with acute bleeding from 30 to 240 minutes sampling times when compared base line values. The short 240 minutes sampling period in this study may be the etiology beyond whey the SAA, Hp, OC, b-LP, and PYD did not differ significantly. Our results agree well with results obtained from sedated donkeys with induced acute bleeding (Tharwat and Al-Sobayil, 2022a). The serum level of SAA usually increases in serum about 6h of inflammation (Nunokawa et al., 1993; Jacobsen et al., 2006) and declines within 12 hours when infection subsides (Coutinho et al., 2013). This clearly explains why in the current experiment, SAA levels remained unchanged during the 4 hours sampling time. Hp was also reported to be less sensitive and negative acute phase protein in horses suffering from acute abdominal disorders, and compared to healthy horses, the diseased ones has significantly lower levels (Pihl et al., 2013; Dondi et al., 2015). ConclusionIn conscious non-sedated donkeys, induced bleeding resulted in significant increases in the hematological parameters RBCs, Hg, and HCT. It also decreased the biochemical elements including total proteins, albumin, globulin, AST, GGT, CK, and calcium. Contrary, it increased the BUN, creatinine, and blood sugar levels. Testing the acid-base status, blood gases, and electrolytes showed metabolic acidosis, decreases in HCO3, BE, TCO2, and chloride, and increases PCO2, AG, potassium, and lactate. The markers SAA and Hp nor the bone markers OC, b-ALP, and PYD differed significantly throughout the short-term 240min sampling time; the long-term responses of acute bleeding are therefore warranted in a future study. AcknowledgmentsResearchers would like to thank the Deanship of Scientific Research, Qassim University, for funding the publication of this project. Author ContributionsConceptualization and design: MT and FA; Practical work: MT and FA; formal analysis and interpretation of data: MT and HA; writing-original draft preparation: MT; review and editing: FA and HA. All authors revised and approved the final manuscript for publication. Conflict of interestThe authors declare that there is no conflict of interest. FundingPublication of this research was funded by the Deanship of Scientific Research, Qassim University, Saudi Arabia. Data availabilityAll data supporting the findings of this study are available within the manuscript and no additional data sources are required. ReferencesAl-Sobayil, F. and Tharwat, M. 2021. Effects of acute synovitis experimentally induced by Mphotericin-B on the biomarkers of camel joint structures. J. Camel Pract. Res. 28, 169–174. Calero Rodriguez, A. and deGrauw, J. 2022. Spontaneous pulmonary haemorrhage in a standing sedated horse. Vet. Rec. Case Rep.10, e319. Camacho-Rozo, C.A., Santos, G.O., Wenzen, D.P., Cousseau, S.B., Wronski, J.G., Argenta, F.F., Winter, G.H.Z., Pavarini, S.P. and Mattos, R.C. 2020. Sudden death by ovarian hemorrhage and hemoperitoneum in a pregnant miniature mare. J. Equine Vet. Sci. 90, 102996. Cap, A.P., Beckett, A., Benov, A., Borgman, M., Chen, J., Corley, J.B., Doughty, H., Fisher, A., Glassberg, E., Gonzales, R., Kane, S.F., Malloy, W.W., Nessen, S., Perkins, J.G., Prat, N., Quesada, J., Reade, M., Sailliol, A., Spinella, P.C., Stockinger, Z., Strandenes, G., Taylor, A., Yazer, M., Bryant, B. and Gurney, J. 2018. Whole blood transfusion. Mil. Med. 183, 44–451. Coutinho da Silva, M.A., Canisso, I.F., MacPherson, M.L., Johnson, A.E. and Divers, T.J. 2013. Serum amyloid A concentration in healthy periparturient mares and mares with ascending placentitis. Equine Vet. J. 45, 619–624. Dondi, F., Lukacs, R.M., Gentilini, F., Rinnovati, R., Spadari, A. and Romagnoli, N. 2015. Serum amyloid A, haptoglobin, and ferritin in horses with colic: association with common clinicopathological variables and short-term outcome. Vet. J. 205, 50–55. Duggan, E.W., Carlson, K. and Umpierrez, G. E. 2017. Perioperative hyperglycemia management: an update. Anesthesiology 126, 547–560. Farley, A., Hendry, C. and McLafferty, E. 2012. Blood components. Nurs. Stand. 27, 35–42. Frisbie, D.D., Al-Sobayil, F. and Billighurst, R.C. 2008. Changes in synovial fluid and serum biomarkers with exercise and early osteoarthritis in horses. Osteoarthritis Cartilage 16, 1196–1204. Hinchcliff, K.W., Couetil, L.L., Knight, P.K., Morley, P.S., Robinson, N.E., Sweeney, C.R. and van Erck, E. 2015. Exercise induced pulmonary hemorrhage in horses: American College of Veterinary Internal Medicine consensus statement. J. Vet. Intern. Med. 29, 743–758. Hurcombe, S.D.A., Radcliffe, R.M., Cook, V.L. and Divers, T.J. 2022. The pathophysiology of uncontrolled hemorrhage in horses. J. Vet. Emerg. Crit. Care 32(S1), 63–71. Jacobsen, S., Kjelgaard-Hansen, M., Petersen, H.H. and Jensen, A.L. 2006. Evaluation of a commercially available human serum amyloid A (SAA) turbidometric immunoassay for determination of equine SAA concentrations. Vet. J. 172, 315–319. Jamieson, C.A., Baillie, S.L. and Johnson, J.P. 2022. Blood transfusion in equids—a practical approach and review. Animals 12, 2162. Lyle, C.H., Uzal, F.A., McGorum, B.C., Aida, H., Blissitt, K.J., Case, J.T., Charles, J.T., Gardner, I., Horadagoda, N., Kusano, K., Lam, K., Pack, J.D., Parkin, T.D., Slocombe, R.F., Stewart, B.D. and Boden, L.A. 2011. Sudden death in racing Thoroughbred horses: an international multicentre study of post mortem findings. Equine Vet. J. 43, 324–331. Magdesian, K.G., Fielding, C.L., Rhodes, D.M. and Ruby, R.E. 2006. Changes in central venous pressure and blood lactate concentration in response to acute blood loss in horses. J. Am. Vet. Med. Assoc. 229, 1458–1462. Mudge, M.C. 2014. Acute hemorrhage and blood transfusions in horses. Vet. Clin. N. Am., Equine Pract. 30, 427–436. Murata, H., Shimada, N. and Yoshioka, M. 2004. Current research on acute phase proteins in veterinary diagnosis: an overview. Vet. J. 168, 28–40. Nunokawa, Y., Fujinaga, T., Taira, T., Okumura, M., Yamashita, K., Tsunoda, N. and Hagio, M. 1993. Evaluation of serum amyloid A protein as an acute-phase reactive protein in horses. J. Vet. Med. Sci. 55, 1011–1016. Peacock, T.S. 2019. Perioperative hyperglycemia: a literature review. AORN J. 109, 80–86. Pihl, T.H., Andersen, P.H., Kjelgaard-Hansen, M., Mørck, N.B. and Jacobsen, S. 2013. Serum amyloid A and haptoglobin concentrations in serum and peritoneal fluid of healthy horses and horses with acute abdominal pain. Vet. Clin. Pathol. 42, 177–183. Poole, D.C. and Erickson, H.H. 2016. Exercise-induced pulmonary hemorrhage: Where are we now? Vet. Med. Res. Rep. 7, 133–148. Ramsey, G. 2016. Hemostatic efficacy of pathogen-inactivated blood components. Semin. Thromb. Hemost. 42, 172–182. Relave, F., David, F., Leclère, M., Alexander, K., Hélie, P., Meulyzer, M., Lavoie, J.P. and Marcoux, M. 2010. Thoracoscopic lung biopsies in heaves-affected horses using a bipolar tissue sealing system. Vet. Surg. 39, 839–846. SPSS, 2017. Statistical Package for Social Sciences. Chicago, IL: SPSS, Inc. Copyright for Windows, version 25. Tharwat, M. 2015. Haematology, biochemistry and blood gas analysis in healthy female dromedary camels, their calves and umbilical cord blood at spontaneous parturition. J. Camel Pract. Res. 22, 239–245. Tharwat, M. 2020a. Hyalomma dromedarii ticks induce a distinct acute phase reaction in dromedary camels. J. Camel Pract. Res. 27, 329–332. Tharwat, M. 2020b. Serum concentration of bone metabolism biomarkers in goats during the transition period. Vet. Med. Intern. 2020, 4064209. Tharwat, M. 2021a. Acid-base balance, blood gases and haematobiochemical profiles in camels (Camelus dromedarius) with trypanosomiasis. J. Camel Pract. Res. 28, 143–147. Tharwat, M. 2021b. Alterations in acid-base balance, blood gases and hemato-biochemical profiles of whole blood and thoracic fluid in goats with contagious caprine pleuropneumonia. Vet. World 14, 1874–1878. Tharwat, M. 2023. Advanced biomarkers and its usage in Arabian camel medicine—a review. J. App. Anim. Res. 51, 350–357. Tharwat, M. and Al-Sobayil, F. 2014a. Cord and jugular blood acid–base and electrolyte status and haematobiochemical profiles in goats with naturally occurring pregnancy toxaemia. Small Rumin. Res. 117, 73–77. Tharwat, M. and Al-Sobayil, F. 2014b. The effect of tick infestation on the serum concentrations of the cardiac biomarker troponin I, acid–base balance and haematobiochemical profiles in camels (Camelus dromedarius). Trop. Anim. Health Prod. 46, 139–144. Tharwat, M. and Al-Sobayil, F. 2014c. Influence of the cardiac glycoside digoxin on cardiac troponin I, acid–base and electrolyte balance, and haematobiochemical profiles in healthy donkeys (Equus asinus). BVC Vet. Res. 10, 64. Tharwat, M. and Al-Sobayil, F. 2015a. The impact of racing on serum concentrations of acute-phase proteins in racing dromedary camels. Comp. Clin. Pathol. 24, 575–579. Tharwat, M. and Al-Sobayil, F. 2015b. Serum concentrations of acute phase proteins and bone biomarkers in female dromedary camels during the periparturient period. J. Camel Pract. Res. 22, 271–278. Tharwat, M. and Al-Sobayil, F. 2018a. Influence of electro-ejaculator on serum concentrations of acute phase proteins and bone metabolism biomarkers in male dromedary camels (Camelus dromedarius). J. App. Anim. Res. 46, 1226–1232. Tharwat, M. and Al-Sobayil, F. 2018b. The impact of racing on serum concentrations of bone metabolism biomarkers in racing Arabian camels. J. Camel Pract. Res. 25, 59–63. Tharwat, M. and Al-Sobayil, F. 2022a. The Effects of acute blood loss on inflammatory and bone biomarkers, acid base balance, blood gases and hemato-biochemical profiles in sedated donkeys (Equus asinus). Int. J. Vet. Sci. 11, 479–485. Tharwat, M. and Al-Sobayil, F. 2022b. Effect of a long road transport journey on serum biomarkers of bone formation and resorption in athletic horses. Int. J. Vet. Sci. 11, 268–271. Tharwat, M. and Al-Hawas, A. 2024. Suppurative pyelonephritis in a caprine buck: clinical, laboratory, ultrasonographic and pathologic findings. Int. J. Vet. Sci. 13, 479–483. Tharwat, M., Al-Sobayil, F. and Buczinski, S. 2014a. Influence of racing on the serum concentrations of acute phase proteins and bone metabolism biomarkers in racing greyhounds. Vet. J. 202, 372–377. Tharwat, M., Ali, A., Al-Sobayil, F., Derar, R. and Al-Hawas, A. 2014b. Influence of stimulation by electroejaculation on myocardial function, acid-base and electrolyte status and haematobiochemical profiles in male dromedary camels. Theriogenology 82, 800–806. Tharwat, M., Ali, A., Derar, D., Oikawa, S. and Almundarij, T. I. 2024a. Effects of dystocia on the cardiac biomarker troponin I, acid-base balance and blood gases alongside the hematobiochemical profiles in female dromedary camels. Int. J. Vet. Sci. 13, 115–119. Tharwat, M., El-Ghareeb, W.R. and Alkheraif, A.A. 2024b. Acute phase proteins, hematobiochemical profiles, acid–base balance and blood gas alterations in camel calves infested with ticks. Int. J. Vet. Sci. In press. https://doi.org/10.47278/journal.ijvs/2024.146. Varegg, M.S., Kløverød, K.M., Austnes, M.K., Siwinska, N., Słowikowska, M., Zak, A., Madej, J.A., Kandefer-Gola, M., Ciaputa, R., Nowak, M. and Niedzwiedz, A. 2019. Fatal pulmonary hemorrhage in a horse during bronchoalveolar lavage - single case report. BMC Vet. Res. 15, 169. Williams, A. 2020. Approaching acute haemorrhage in horses. Veterinary Practice. Available via https://veterinary-practice.com/article/approaching-acute-haemorrhage Wilson, D.V., Rondenay, Y. and Shance, P.U. 2003. The cardiopulmonary effects of severe blood loss in anesthetized horses. Vet. Anaesth. Analg. 30, 81–87. | ||
| How to Cite this Article |
| Pubmed Style Tharwat M, Al-sobayil F, Ali H. Changes in the hematobiochemical, acid–base and blood gas elements as well as biomarkers of inflammation and bone metabolism in donkeys (Equus asinus) with acute bleeding. Open Vet. J.. 2024; 14(5): 1146-1153. doi:10.5455/OVJ.2024.v14.i5.8 Web Style Tharwat M, Al-sobayil F, Ali H. Changes in the hematobiochemical, acid–base and blood gas elements as well as biomarkers of inflammation and bone metabolism in donkeys (Equus asinus) with acute bleeding. https://www.openveterinaryjournal.com/?mno=186029 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i5.8 AMA (American Medical Association) Style Tharwat M, Al-sobayil F, Ali H. Changes in the hematobiochemical, acid–base and blood gas elements as well as biomarkers of inflammation and bone metabolism in donkeys (Equus asinus) with acute bleeding. Open Vet. J.. 2024; 14(5): 1146-1153. doi:10.5455/OVJ.2024.v14.i5.8 Vancouver/ICMJE Style Tharwat M, Al-sobayil F, Ali H. Changes in the hematobiochemical, acid–base and blood gas elements as well as biomarkers of inflammation and bone metabolism in donkeys (Equus asinus) with acute bleeding. Open Vet. J.. (2024), [cited January 25, 2026]; 14(5): 1146-1153. doi:10.5455/OVJ.2024.v14.i5.8 Harvard Style Tharwat, M., Al-sobayil, . F. & Ali, . H. (2024) Changes in the hematobiochemical, acid–base and blood gas elements as well as biomarkers of inflammation and bone metabolism in donkeys (Equus asinus) with acute bleeding. Open Vet. J., 14 (5), 1146-1153. doi:10.5455/OVJ.2024.v14.i5.8 Turabian Style Tharwat, Mohamed, Fahd Al-sobayil, and Haytham Ali. 2024. Changes in the hematobiochemical, acid–base and blood gas elements as well as biomarkers of inflammation and bone metabolism in donkeys (Equus asinus) with acute bleeding. Open Veterinary Journal, 14 (5), 1146-1153. doi:10.5455/OVJ.2024.v14.i5.8 Chicago Style Tharwat, Mohamed, Fahd Al-sobayil, and Haytham Ali. "Changes in the hematobiochemical, acid–base and blood gas elements as well as biomarkers of inflammation and bone metabolism in donkeys (Equus asinus) with acute bleeding." Open Veterinary Journal 14 (2024), 1146-1153. doi:10.5455/OVJ.2024.v14.i5.8 MLA (The Modern Language Association) Style Tharwat, Mohamed, Fahd Al-sobayil, and Haytham Ali. "Changes in the hematobiochemical, acid–base and blood gas elements as well as biomarkers of inflammation and bone metabolism in donkeys (Equus asinus) with acute bleeding." Open Veterinary Journal 14.5 (2024), 1146-1153. Print. doi:10.5455/OVJ.2024.v14.i5.8 APA (American Psychological Association) Style Tharwat, M., Al-sobayil, . F. & Ali, . H. (2024) Changes in the hematobiochemical, acid–base and blood gas elements as well as biomarkers of inflammation and bone metabolism in donkeys (Equus asinus) with acute bleeding. Open Veterinary Journal, 14 (5), 1146-1153. doi:10.5455/OVJ.2024.v14.i5.8 |