| Original Article | ||
Open Vet J. 2022; 12(6): 985-994 Open Veterinary Journal, (2022), Vol. 12(6): 985–994 Original Research Morphological and molecular studies on tick species in Ismailia Governorate in Egypt and Al Gabal Al Akhdar in LibyaMohamed S. Eljadar1, Nahla H. Sallam2, Mohamed A. Soltan3, Hamdy M. El Gawady2 and Eman M. Abouelhassan2*1Department of Parasitology, Faculty of Veterinary Medicine, Omar Al-Mukhtar University, Al Bayda, Libya 2Department of Parasitology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt 3Department of Veterinary Medicine, Infectious Diseases Division, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt Submitted: 23/07/2022 Accepted: 18/11/2022 Published: 17/12/2022 *Corresponding Author: Eman M. Abouelhassan. Department of Parasitology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt. Email: eman_abouelhassan [at] vet.suez.edu.eg © 2022 Open Veterinary Journal
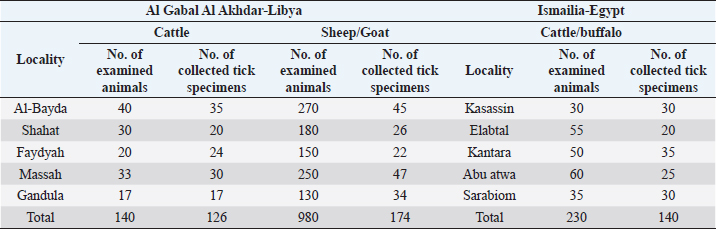
AbstractBackground: Ticks are obligate blood-sucking ectoparasites of vertebrates that have an impact on both domestic and wild animals, especially in tropical and subtropical areas. Aim: The objective of this study is to compare the prevalence and the tick species in both Al Gabal Al Akhder regions in northeastern Libya and Ismailia, Egypt. Methods: Tick specimens collected from predilection sites on the hosts were identified by morphological (light microscopy) and molecular methods. Results: In Ismailia, Egypt, 23.9% of the 230 (examined cattle and buffaloes) were infested with one species of hard ticks, Rhipicephalus annulatus. In the Libyan province of Al Gabal Al Akhdar, the prevalence of tick infestation in cattle, sheep, and goats, was 47% and 59%, respectively. R. annulatus is the identified tick species for cattle, Hyalomma marginatum, and Rhipicephalus bursa, are the identified tick species of the infested sheep and goats. Conclusion: The 16S rDNA gene sequencing and phylogenetic analysis of sample species from Egypt and Libya proved instrumental in overcoming the difficulties associated with morphological identification techniques. Keywords: Molecular, Microscopic, Rhipicephalus annulatus, Hyalomma marginatum, Rhipicephalus bursa. IntroductionTicks are obligate blood-sucking ectoparasites of vertebrates and have an impact on both domestic and wild animals, especially in tropical and subtropical areas (Abouelhassan et al., 2019). Currently, more than 900 tick species have been described worldwide and have been divided into four important families such as Argasidae, Ixodidae, Nuttalliellidae, and Deinocrotonidae. Of these, around 700 tick species belong to the family Ixodidae, having several genera, for instance, Dermacentor, Haemaphysalis, Hyalomma, Ixodes, and Rhipicephalus, which are responsible for the transmission of several pathogens (Dantas-Torres, 2018). Ticks can cause nonspecific symptoms like anemia, dermatosis, toxicosis, and paralysis, besides being important vectors for diseases such as theileriosis, anaplasmosis, babesiosis, and rickettsiosis in domestic animals (Gebre et al., 2001). Haemoprotozoans, especially Babesia, Theileria, and Trypanosomes, have major effects on the health and production of bovines (Rajput et al., 2005). These protozoa can cause substantial losses to the livestock industry throughout the world because of mortality, decreased productivity, lowered working efficiency (Uilenberg, 1995), and increased costs for control measures (Makala et al., 2003). In South Africa, the genera Hyalomma, Boophilus, and Rhipicephalus comprise the most important ixodid ticks infesting animals, specifically Hy. anatolicum excavatum, Hy. dromedarii, Hy. impeltatum, Hy. marginatum marginatum, B. annulatus, and Rhipicephalus sanguineus (Marufu, 2008). Morphological identification of tick species is not sufficient and molecular identification is needed for a correct species determination (Abouelhassan et al., 2019). The objective of this study is to compare the prevalence of tick species in both the Al Gabal Al Akhder region in northeastern Libya and the Ismailia governorate. Material and MethodsAnimals and study areaA total of 440 individual tick specimens were collected in this study. Samples were collected randomly from 1,350 apparently healthy animals (cattle, buffaloes, sheep, and goats) from different localities in Egypt and Libya (Fig. 1) during the period extending from January 2020 to 2021 (Table 1).
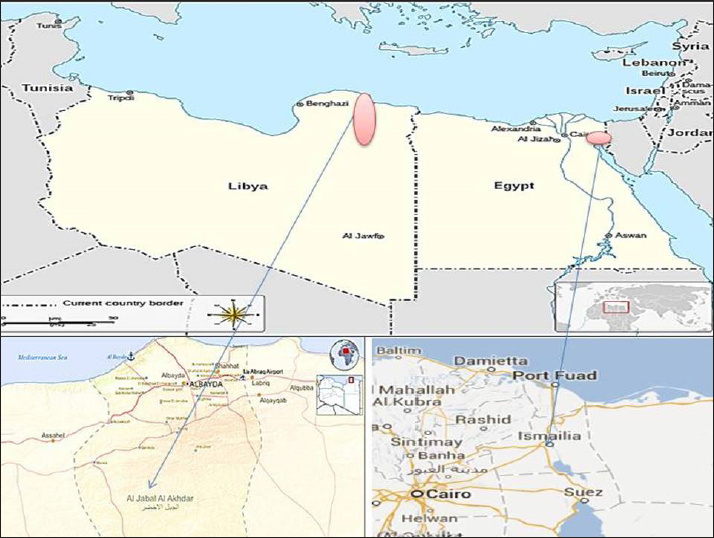
Fig. 1. Geographical map for Ismailia governorate, Egypt, and Al Gabal Al Akhdar in Libya. Table 1. Tick specimens collected from examined animals.
Tick collectionBefore the collection of tick samples, animals were restrained properly and their whole body was thoroughly inspected visually for the presence of ticks. Tick specimens were taken from predilection sites of the hosts, such as the ears, udder, and around the anus. The ticks collected from these sites with the help of forceps, and were transferred to bottles (containing either ethyl alcohol 70% and 10% glycerin or 70% alcohol only) and shifted to the laboratory for permanent slide preparation and identification based on the characters of the basis-capitulum, pedipalps, presence or absence of festoons, eyes, anal groove, adenal shields, coxa-1, coxa-IV, accessory adenal shields, and designs of colors present on scutum (Walker, 2003). Table 2. Prevalence of tick infestation on cattle and buffaloes at different localities in Ismailia, Egypt.
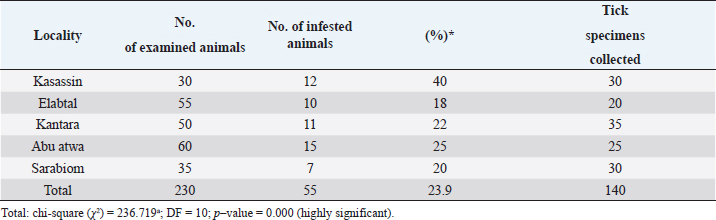
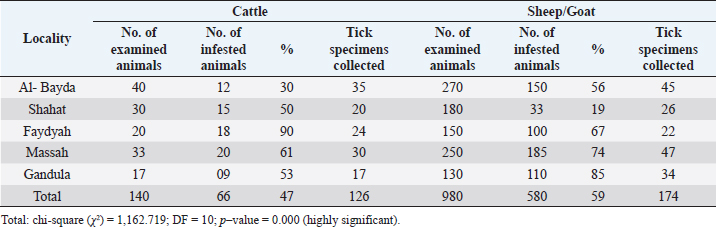
Morphological identificationThe method described by Farid et al. (2021) was adopted for the preparation of the tick specimens for light microscopy. Tick specimens preserved in an alcohol–glycerol mixture were taken and manually teased by forceps to remove any adherent host tissues. Blood from ticks was removed using a needle syringe. To dissolve more chitinous and other undesirable materials, the specimen was treated with 10% sodium hydroxide. After cleaning, the specimen was washed several times with water and subsequently dehydrated in a serial dilution of alcohol (25%, 50%, 75%, and 100% ethyl alcohol). To ensure better clearance, the specimen was kept in xylene for 15–30 minutes. The specimen was mounted on a glass slide using Canada balsam and covered by a cover slip, left to be dried in a hot oven at 40°C–50°C, and later examined under a light microscope (10× magnification). The specimens were morphologically identified according to Walker (2003). Molecular identification of ticksDNA extractionThe tick specimens preserved in 70% ethanol were processed for extraction of DNA. DNA was extracted utilizing QIAamp DNA Mini Kit (Qiagen), as per the protocol laid down by the manufacturer. Genomic DNA was stored at −20°C until use. Molecular identification of tick samples based on both 16S rDNA genesThe tick 16S rDNA was amplified from each specimen studied using conventional PCR methodologies and primers 5′-TTGGGCAAGAAGACCCTATGAA-3′ and 5′-CCGGGTTTGAACTCAGATCAAGT-3′, previously described by Black and Piesman (1994). The thermo-cycling cycling conditions were: initial denaturation at 95°C for 5 minutes. followed by 10 cycles of 92°C for 1 minute, 48°C for 1 minute, and 72°C for 90 seconds. This step was followed by an additional 32 cycles of 92°C for 1 minute, 54°C for 35 seconds, and 72°C for 90 seconds., this was followed by a final extension at 72°C for 7 minutes. The amplification products from 16S rDNA PCR were made to electrophoretically migrate on a 1.6% agarose gel containing 0.4 µg/ml of ethidium bromide at 90 volts for 40–60 minutes. Sequence analysisThe amplified PCR products were excised from the gel, purified, and sent for sequencing. Sanger sequencing was performed by Solgent Co. Ltd (South Korea). Sequences were then analyzed using BLAST® (Johnson et al., 2008). Phylogenetic analysisPhylogenetic analysis and estimates of evolutionary divergence between sequences were performed based on the 16S rDNA gene sequences of several closely related tick species. They were constructed in MEGAX (Kumar et al., 2018). Ethical approvalThis study was approved by the Ethics Committee of the Suez Canal University. All animal experiments were conducted following the guidelines of the Guide for the Care and Use of Laboratory Animals, Faculty of Veterinary Medicine Science, Suez Canal University, Egypt with approval number (2018123). ResultsPrevalence of tick infestationIn Ismailia, Egypt, 23.9% of the 230 animals (cattle and buffaloes) examined for tick infestations had one species of hard tick, Rhipicephalus annulatus. Table 2 shows the prevalence of R. annulatus infection in the studied animals across different locations in Ismailia province. In the Libyan province of Al Gabal Al Akhdar, the prevalence of tick infestation in cattle, both sheep and goats, was 47% (66/140) and 59% (580/980), respectively. Rhipicephalus annulatus is the identified tick species for cattle. Hyalomma marginatum and Rhipicephalus bursa are the identified tick species for the infested sheep and goats. Table 3 shows the percentage of animals with tick infection among those investigated at various locations throughout Libya’s Al Gabal Al Akhdar province. The morphology of the collected tick species using a light microscopeHyalomma marginatum rufipes (Koch, 1844): the scutum color was dark (Fig. 1), the male had adenal and accessory adenal shields (Fig. 2), and the basis capitulum had medium angular lateral margins. Palp articles 2 were longer than articles 1 and 3 (Fig. 3), and festoons were absent in the male and un-engorged female (Fig. 3). The spiracular plate was large and posterior to the legs (Fig. 4). The genital aperture anterior groove was deep, and the genital aperture posterior lips had a V shape (Fig. 2). Table 3. Prevalence of tick infestation on animals across different localities of Al Gabal Al Akhdar, Libya.
Fig. 2. (A): Ventral view of H. marginatum male. (B): Dorsal view of H. marginatum, male. (C): Dorsal view of H. marginatum, engorged female. (D): Ventral view of H. marginatum, engorged female.
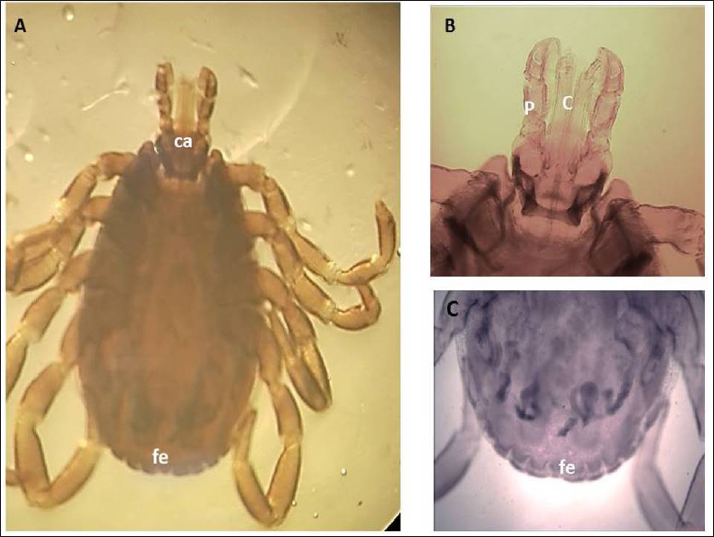
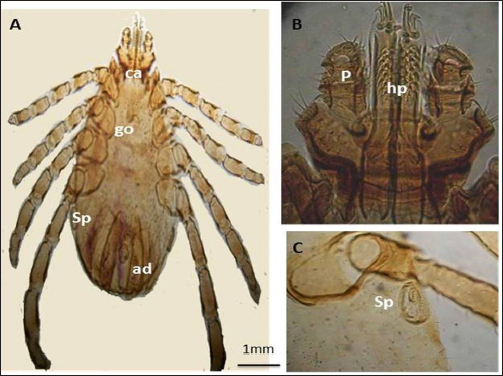
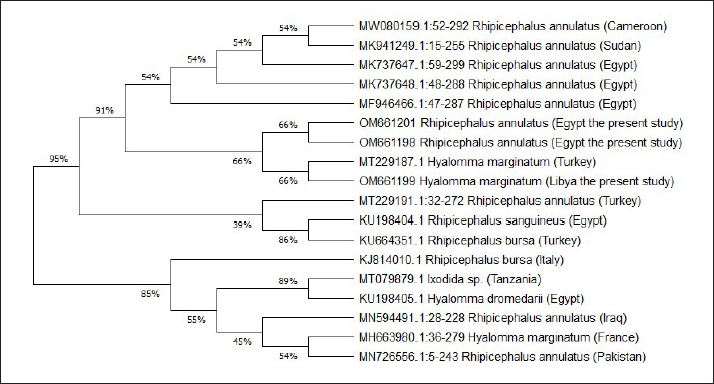
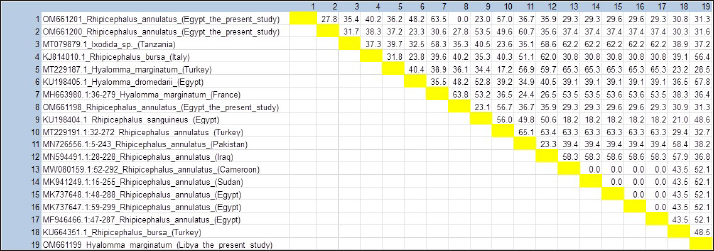
Fig. 3. LM of permanent slide of H. marginatum, male. (A): Dorsal view. (B): Anterior part. (C): Posterior part. (Sp): Spicular plate; (fe): festoon; (ca): capituli; (c): Chelicera; (P): palpai. Rhipicephalus annulatus (Say, 1821): was characterized by a weakly indistinct anal groove. The male had adenal and accessory adenal shields without a caudal process (Fig. 4). The basis capitulum was hexagonal in shape and laterally produced. Palpi were very short, consisting of four segments. Mouth parts were as long as the basis capitulum. The second segment of the palpi was as long as it was wide and was not produced laterally. Caudal appendages and festoons were absent (Fig. 4). The spiracular plate had an oval shape and was made up of stigma and peritreme. Coxa 2, 3, and 4 had no external spurs, while coxa 1 was broad with internal and external spurs. The genital aperture posterior lips had a broad U shape (Fig. 5). Rhipicephalus bursa (Canestrini and Fanzago, 1878): was characterized by dark-colored scutum (Fig. 6). The male had broad adenal and small accessory shields (Fig. 6). The basis capitulum lateral angles were sharply produced. Palp pedicles were short and festoons were present (Fig. 6). The spiracular plate was comma shaped and consisted of a stigma, peritreme, and a tail. In Coxa 1 anterior spurs were visible dorsally, and the genital aperture posterior lips had a narrow V shape (Fig. 7). Molecular identification of tick speciesA total of 100 tick samples were analyzed (Fig. 8) which were collected from Egypt and Libya. They were identified based on the16S rDNA PCR products as H. marginatum, R. bursa, and R. annulatus, the GenBank accession numbers are OM661198, OM661199, OM661200, and OM661201. Phylogenetic analysisThe phylogenetic analysis based on the 16S rDNA gene was performed using MEGA X10.1 software and the trees were constructed using neighbor-joining (NJ) methods (Fig. 9). There is a low degree of sequence variations was observed within most of the species of R. annulatus from Egypt, Sudan, and Cameroon since they all clustered into one original branch, and low variation between the present R. annulatus samples from Egypt (Fig.10). While H. marginatum sequence of our study had a close relationship and clustered with the same species from Turkey (Figs. 9 and 10). DiscussionIn Ismailia, Egypt, the overall prevalence of tick infestation was 23.9% in cattle and buffaloes, whereas in Al Gabal Al Akhdar, Libya, the overall prevalence of tick infestation was 47% in cattle, sheep, and goats. The difference in tick distribution may be attributed to the difference in environmental conditions, host density, host susceptibility, grazing habits, and pasture herd management (Pegram et al., 1981). In addition, the highest prevalence of tick infestation was observed in sheep and goats (59%). This may be attributed to the fact that tick infestation is facilitated by a large livestock population and herd size, as ticks can easily gain access to hosts and complete their life cycle quickly. Poor veterinary service and herders’ disregard for animal management practices may also contribute to tick infestation (Alessandra and Santo, 2012).
Fig. 4. (A): Dorsal view of R. annulatus, engorged female. (B): Ventral view of R. annulatus, engorged female. (C): Dorsal view of R. annulatus, male. (D): Ventral view of R. annulatus, male.
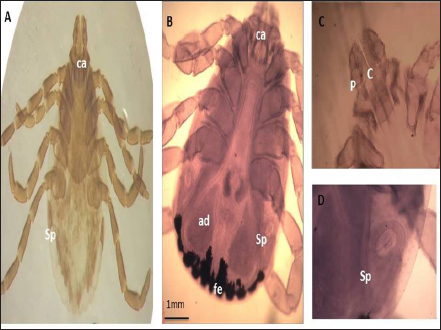
Fig. 5. LM of permanent slide of R. annulatus male. (A): Dorsal view. (B): Anterior part. (C): Specular plate. (Sp): Spicular plate; (go): genital opening; (ca): capituli; (hp): hypostome; (P): palpai; (ad): adenal shield.
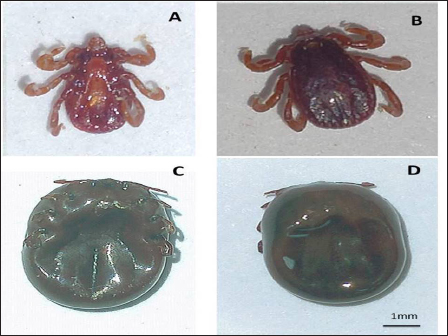
Fig. 6. (A): Ventral view of R. bursa, male. (B): Dorsal view of R. bursa, male. (C): Ventral view of R. bursa, engorged female. (D): Dorsal view of R. bursa, engorged female.
Fig. 7. LM of permanent slide. (A): Ventral view of R. bursa female. (B): Ventral view of R. bursa, male. (C): Anterior part. (D): Specular plate. (Sp): Spicular plate; (ca): capituli; (C): chelicera; (P): palpai; (ad): adenal shield. The difference in the prevalence of tick species may be due to the different environmental conditions and nature of the land as well as the management style and season of sample collection. It is reported that tick activity can be influenced by rainfall, altitude, season, and atmospheric relative humidity (Pegram et al., 1981). The collected ticks from cattle, sheep, and goats were identified as Hyalomma sp. and Rhipicephalus sp. at Al Gabal Al Akhdar in Libya. The higher prevalence of R. sanguineus in this study may be attributed, in our opinion, to high temperatures in these localities, as they are the greatest and most important factor for the development of this tick species (Dantas-Torres et al., 2010). Furthermore, there is no significant difference between the infestation rate and the changes in localities of R. sanguineus. This may be due to its adaptation to the environment in each locality. This corroborates well with Dantas-Torres et al. (2010), who stated that the wide geographical distribution of the brown dog tick; R. sanguineus was related to the cosmopolitan distribution of its primary host and to its adaptability to different environments, under variable climatic conditions.
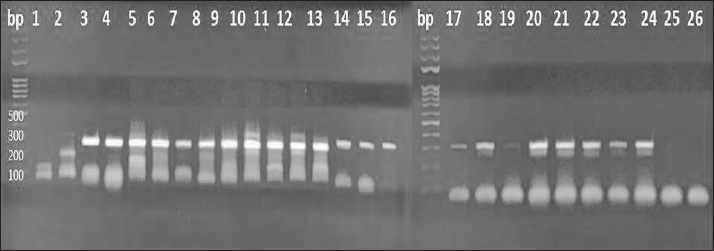
Fig. 8. PCR amplification utilizing tick samples of different species using: 16S rDNA Gene, DNA ladder is located on the left sides of the gel, fragment sizes are represented in base pairs (bp), 1: 25 tick samples, and 26: negative control.
Fig. 9. The phylogenetic analysis was constructed using NJ methods, to construct the tick phylogenetic tree of some of hard tick species sequences from the Genbank, and our sequences samples are included based on 16S r DNA sequences. The current investigations seek to resolve the issues and challenges connected with morphological identification by applying molecular approaches of identification based on DNA sequences to corroborate morphological identification and solve the problems and difficulties associated with morphological identification. For instance, certain tick species are categorized as species complexes, such as the R. sanguineus group, which has a total of 17 different tick species (Dantas-Torres et al., 2013). As a result, the amplification and sequencing of a 16S rDNA fragment served as the study technique of tick genetic identification.
Fig. 10. Estimates of evolutionary divergence between this study tick sequences and sequences of tick spp. 16S r DNA. On the other hand, concerning phylogeny, mtDNA sequences are a good phylogenetic marker for groups of organisms that have diverged relatively, based on their higher rate of base substitution than most nuclear markers (Parola and Raoult, 2001). Even though Black and Piesman (1994) reported that errors in the construction of phylogenies can happen by mistake when the mitochondrial gene transfers to the nucleus as mitochondrial pseudogenes (called numts), which are the problem of the mitochondrial gene. ConclusionIn this study, the 16S rDNA gene sequencing and phylogenetic analysis of sample species from Egypt and Libya proved instrumental in overcoming the difficulties associated with morphological identification techniques. Therefore, using mitochondrial genes is a good marker in tick species identification. The changes in environmental conditions affect tick development. Therefore, this idea will help in devising a better strategy for the control of ticks among livestock by providing useful insights into the epidemiology of these parasites. Conflict of interestThe authors have no conflicts of interest relevant to this article. Availability of data and materialAll data generated or analyzed during this study are included in this article. FundingNo funding was received for this study. Authors’ contributionAll authors contributed to the preparation of the manuscript. ReferencesAbouelhassan, E.M., El-Gawady, H.M., Abdel-Aal, A.A., El-Gayar, A.K. and Esteve-Gassent, M.D. 2019. Comparison of some molecular markers for tick species identification. J. Arthropod. Borne. Dis. 13, 153. Alessandra, T. and Santo, C. 2012. Tick-borne diseases in sheep and goats: clinical and diagnostic aspects. Small. Rum. Res. 106, S6–S11. Black, W. and Piesman, J. 1994. Phylogeny of hard-and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. In: Proceedings of the National Academy of Sciences, 91, pp 10034–10038. Dantas-Torres, F. 2018. Species concepts: what about ticks? Trends. Parasitol. 34, 1017–1026. Dantas-Torres, F., Giannelli, A., Figueredo, L.A. and Otranto, D. 2010. Effects of prolonged exposure to low temperature on eggs of the brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae). Vet. Parasitol. 171, 327–330. Dantas-Torres, F., Latrofa, M.S., Annoscia, G., Giannelli, A., Parisi, A. and Otranto, D. 2013. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the new and old worlds. Parasit. Vectors. 6, 1–17. Farid, D., Abouelhassan, E., El-Sebae, A., Enany, M. and Youssef, A. 2021. Ectoparasite fauna of commensal rodents collected from the North Sinai governorate, Egypt and its public health significance. Adv. Anim. Vet. Sci. 9, 563–570. Gebre, S., Nigist, M. and Kassa, B. 2001. Seasonal variation of ticks on calves at Sebeta in western Shewa Zone. Ethiop. Vet. J. 7, 17–30. Johnson, M., Zaretskaya, I., Raytselis, Y., Merezhuk, Y., McGinnis, S. and Madden, T.L. 2008. NCBI BLAST: a better web interface. Nucleic. Acids. Res. 36, W5–W9. Kumar, S., Stecher, G., Li, M., Knyaz, C. and Tamura, K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547. Makala, L.H., Mangani, P., Fujisaki, K. and Nagasawa, H. 2003. The current status of major tick borne diseases in Zambia. Vet. Res. 34, 27–45. Marufu, M.C. 2008. Prevalence of ticks and tick-borne diseases in cattle on communal rangelands in the highland areas of the Eastern Cape province, South Africa. Alice, South Africa: University of Fort Hare. Parola, P. and Raoult, D. 2001. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 32, 897–928. Pegram, R.G., Hoogstraal, H. and Wassef, H.Y. 1981. Ticks (Acari: Ixodoidea) of Ethiopia. I. distribution, ecology and host relationships of species infesting livestock. Bull. Entomol. Res. 71, 339–359. Rajput, Z., Hu, S.H., Arijo, A., Habib, M. and Khalid, M. 2005. Comparative study of Anaplasma parasites in tick carrying buffaloes and cattle. J. Zhejiang. Univ. Sci. B. 6, 1057. Uilenberg, G. 1995: International collaborative research: significance of tick-borne hemoparasitic diseases to world animal health. Vet. Parasitol. 57, 19–41. Walker, A.R. 2003. Ticks of domestic animals in Africa: a guide to identification of species. Biosci. Rep. Edinb. 3, 210. | ||
| How to Cite this Article |
| Pubmed Style Eljadar MS, Sallam NH, Soltan MA, Gawady HME, Abouelhassan EM. Morphological and Molecular studies on tick species in Ismailia Governorate in Egypt and Al Gabal al Akhdar in Libya. Open Vet J. 2022; 12(6): 985-994. doi:10.5455/OVJ.2022.v12.i6.27 Web Style Eljadar MS, Sallam NH, Soltan MA, Gawady HME, Abouelhassan EM. Morphological and Molecular studies on tick species in Ismailia Governorate in Egypt and Al Gabal al Akhdar in Libya. https://www.openveterinaryjournal.com/?mno=85146 [Access: July 03, 2025]. doi:10.5455/OVJ.2022.v12.i6.27 AMA (American Medical Association) Style Eljadar MS, Sallam NH, Soltan MA, Gawady HME, Abouelhassan EM. Morphological and Molecular studies on tick species in Ismailia Governorate in Egypt and Al Gabal al Akhdar in Libya. Open Vet J. 2022; 12(6): 985-994. doi:10.5455/OVJ.2022.v12.i6.27 Vancouver/ICMJE Style Eljadar MS, Sallam NH, Soltan MA, Gawady HME, Abouelhassan EM. Morphological and Molecular studies on tick species in Ismailia Governorate in Egypt and Al Gabal al Akhdar in Libya. Open Vet J. (2022), [cited July 03, 2025]; 12(6): 985-994. doi:10.5455/OVJ.2022.v12.i6.27 Harvard Style Eljadar, M. S., Sallam, . N. H., Soltan, . M. A., Gawady, . H. M. E. & Abouelhassan, . E. M. (2022) Morphological and Molecular studies on tick species in Ismailia Governorate in Egypt and Al Gabal al Akhdar in Libya. Open Vet J, 12 (6), 985-994. doi:10.5455/OVJ.2022.v12.i6.27 Turabian Style Eljadar, Mohamed S, Nahla H Sallam, Mohamed A Soltan, Hamdy M El Gawady, and Eman M Abouelhassan. 2022. Morphological and Molecular studies on tick species in Ismailia Governorate in Egypt and Al Gabal al Akhdar in Libya. Open Veterinary Journal, 12 (6), 985-994. doi:10.5455/OVJ.2022.v12.i6.27 Chicago Style Eljadar, Mohamed S, Nahla H Sallam, Mohamed A Soltan, Hamdy M El Gawady, and Eman M Abouelhassan. "Morphological and Molecular studies on tick species in Ismailia Governorate in Egypt and Al Gabal al Akhdar in Libya." Open Veterinary Journal 12 (2022), 985-994. doi:10.5455/OVJ.2022.v12.i6.27 MLA (The Modern Language Association) Style Eljadar, Mohamed S, Nahla H Sallam, Mohamed A Soltan, Hamdy M El Gawady, and Eman M Abouelhassan. "Morphological and Molecular studies on tick species in Ismailia Governorate in Egypt and Al Gabal al Akhdar in Libya." Open Veterinary Journal 12.6 (2022), 985-994. Print. doi:10.5455/OVJ.2022.v12.i6.27 APA (American Psychological Association) Style Eljadar, M. S., Sallam, . N. H., Soltan, . M. A., Gawady, . H. M. E. & Abouelhassan, . E. M. (2022) Morphological and Molecular studies on tick species in Ismailia Governorate in Egypt and Al Gabal al Akhdar in Libya. Open Veterinary Journal, 12 (6), 985-994. doi:10.5455/OVJ.2022.v12.i6.27 |